LESS-PHARMA Study: Identifying and Deprescribing Potentially Inappropriate Medication in the Elderly Population with Excessive Polypharmacy in Primary Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Recruitment
2.3. Data Collection and Procedure
2.4. Primary and Secondary Outcomes
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Potentially Inappropriate Medication
3.3. Feasibility of the Tools
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández-Rodríguez, M.Á.; Sempere-Verdú, E.; Vicens-Caldentey, C.; González-Rubio, F.; Miguel-García, F.; Palop-Larrea, V.; Orueta-Sánchez, R.; Esteban-Jiménez, Ó.; Sempere-Manuel, M.; Arroyo-Aniés, M.P.; et al. Drug Prescription Profiles in Patients with Polypharmacy in Spain: A Large-Scale Pharmacoepidemiologic Study Using Real-World Data. Int. J. Environ. Res. Public Health 2021, 18, 4754. [Google Scholar] [CrossRef] [PubMed]
- Ioakeim-Skoufa, I.; Clerencia-Sierra, M.; Moreno-Juste, A.; Elías de Molins Peña, C.; Poblador-Plou, B.; Aza-Pascual-Salcedo, M.; González-Rubio, F.; Prados-Torres, A.; Gimeno-Miguel, A. Multimorbidity Clusters in the Oldest Old: Results from the EpiChron Cohort. Int. J. Environ. Res. Public Health 2022, 19, 10180. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Pérez, M.; López de Andrés, A.; Hernández-Barrera, V.; Jiménez-García, R.; Jiménez-Trujillo, I.; Palacios-Ceña, D.; Carrasco-Garrido, P. Prevalencia de polifarmacia en la población mayor de 65 años en España: Análisis de las Encuestas Nacionales de Salud 2006 y 2011/12. Rev. Esp. Geriatr. Gerontol. 2017, 52, 2–8. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.Á.; Sempere-Verdú, E.; Vicens-Caldentey, C.; González-Rubio, F.; Miguel-García, F.; Palop-Larrea, V.; Orueta-Sánchez, R.; Esteban-Jiménez, Ó.; Sempere-Manuel, M.; Arroyo-Aniés, M.P.; et al. Evolution of polypharmacy in a spanish population (2005–2015): A database study. Pharmacoepidemiol. Drug Saf. 2020, 29, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Midão, L.; Giardini, A.; Menditto, E.; Kardas, P.; Costa, E. Polypharmacy prevalence among older adults based on the survey of health, ageing and retirement in Europe. Arch. Gerontol. Geriatr. 2018, 78, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Field, T.S.; Gurwitz, J.H.; Avorn, J.; McCormick, D.; Jain, S.; Eckler, M.; Benser, M.; Bates, D.W. Risk factors for adverse drug events among nursing home residents. Arch. Intern. Med. 2001, 161, 1629–1634. [Google Scholar] [CrossRef] [Green Version]
- Field, T.S.; Gurwitz, J.H.; Harrold, L.R.; Rothschild, J.; DeBellis, K.R.; Seger, A.C.; Auger, J.C.; Garber, L.A.; Cadoret, C.; Fish, L.S.; et al. Risk factors for adverse drug events among older adults in the ambulatory setting. J. Am. Geriatr. Soc. 2004, 52, 1349–1354. [Google Scholar] [CrossRef]
- Chan, D.; Chen, J.; Kuo, H.; We, C.; Lu, I.; Chiu, L. Drug-related problems (DRPs) identified from geriatric medication safety review clinics. Arch. Gerontol. Geriatr. 2012, 1, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Leelakanok, N.; Holcombe, A.L.; Lund, B.C.; Gu, X.; Schweizer, M.L. Association between polypharmacy and death: A systematic review and meta-analysis. J. Am. Pharm. Assoc. 2017, 57, 729–738. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Marquina, I.; Olabarri, A.; Miranda, G.; Rubiera, G.; Baena, M. Negative results associated with medication in the emergency department of a hospital. Farm. Hosp. 2008, 3, 157–162. [Google Scholar] [CrossRef]
- Ernst, F.; Grizzle, A.J. Drug-related morbidity and mortality: Updating the cost-of-illness model. J. Am. Pharm Assoc. 2001, 2, 192–199. [Google Scholar] [CrossRef]
- Scottish Government. Realistic Medicine, NHS Scotland. In Polypharmacy Guidance, Realistic Prescribing, 3rd ed.; Scottish Government: Edinburgh, UK, 2018. Available online: https://www.therapeutics.scot.nhs.uk/wp-content/uploads/2018/04/Polypharmacy-Guidance-2018.pdf (accessed on 10 October 2022).
- Opondo, D.; Eslami, S.; Visscher, S.; de Rooij, S.E.; Verheij, R.; Korevaar, J.C.; Abu-Hanna, A. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: A systematic review. PLoS ONE 2012, 7, e43617. [Google Scholar] [CrossRef] [PubMed]
- Storms, H.; Marquet, K.; Aertgeerts, B.; Claes, N. Prevalence of inappropriate medication use in residential long-term care facilities for the elderly. A systematic review. Eur. J. Gen. Pract. 2017, 23, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, K.M.; Hajjar, E.R.; Kumar, C.; Hwang, C.; Salzman, B. Deprescribing: A simple method for reducing polypharmacy. J. Fam. Pract. 2017, 66, 436–445. [Google Scholar]
- Page, A.T.; Clifford, R.M.; Potter, K.; Schwartz, D.; Etherton-Beer, C. The feasibility and effect of deprescribing in older adults on mortality and health: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2016, 82, 583–623. [Google Scholar] [CrossRef] [Green Version]
- MedStopper. Available online: Medstopper.com (accessed on 22 August 2022).
- CheckTheMeds. Available online: https://www.checkthemeds.com/index.php?lang=en (accessed on 22 August 2022).
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.; Schmiedl, S.; Thürmann, P.A. Potentially Inapropiate Medication in the Elderly: The PRISCUS list. Deutches Arztebl. Int. 2010, 107, 543–551. [Google Scholar]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inapropiate prescribing in older people. Age Ageing 2015, 44, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Pérez, A.; Alfaro-Lara, E.R.; Albiñana-Perez, S.; Nieto-Martín, M.D.; Díez-Manglano, J.; Pérez-Guerrero, C.; Santos-Ramos, B. Novel tool for deprescribing in chronic patients with multimorbidity: List of Evidence-Based Deprescribing for Chronic Patients criteria. Geriatr. Gerontol. Int. 2017, 17, 2200–2207. [Google Scholar] [CrossRef] [Green Version]
- Earl, T.R.; Katapodis, N.D.; Schneiderman, S.R.; Shoemake-Hunt, S. Using deprescribing practices and the screening tool of older persons’ potentially inappropriate prescriptions criteria to reduce harm and preventable adverse drug events in older adults. J. Patient Saf. 2020, 16, S23–S35. [Google Scholar] [CrossRef] [PubMed]
- Monteiroc, L.; Maricoto, T.; Solha, I.; Ribeiro-Vaz, I.; Martins, C.; Monteiro-Soares, M. Reducing Potentially Inappropriate Prescriptions for Older Patients Using Computerized Decision Support Tools: Systematic Review. J. Med. Internet Res. 2019, 21, e15385. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Geraghty, O.C.; Mehta, Z.; Rothwell, P.M.; Oxford Vascular Study. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: A population-based cohort study. Lancet 2017, 390, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Motter, F.R.; Fritzen, J.S.; Hilmer, S.N.; Paniz, É.V.; Paniz, V.M.V. Potentially inappropriate medication in the elderly: A systematic review of validated explicit criteria. Eur. J. Clin. Pharmacol. 2018, 74, 679–700. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total, n/N (%) |
|---|---|
| Age 1 | 82 (78–85) |
| Female | 97/168 (57.7) |
| Number of drugs 2 | 12.24 (±2.17) |
| PIMs per patient 3 | 3.86 (±2.56) |
| Comorbidities 4 | 8 (6–10) |
| Hypertension | 157/168 (91.8) |
| Dyslipidemia | 123/168 (73.2) |
| Diabetes | 93/168 (54.4) |
| Depression/Anxiety | 82/168 (48) |
| Chronic musculoskeletal disease | 68/168 (40.5) |
| Urinary incontinence | 61/168 (36.3) |
| Respiratory disease | 60/168 (35.7) |
| Arrhythmias | 56/168 (32.7) |
| Chronic kidney failure | 54/168 (32.1) |
| Cardiac insufficiency | 54/168 (32.1) |
| Ischemic cardiomyopathy | 49/168 (28.7) |
| Prostatic hyperplasia | 48/71 (67.6) a |
| Insomnia | 43/168 (25.1) |
| Hyperuricemia | 40/168 (23.4) |
| Chronic dermatologic disease | 30/168 (17.9) |
| Chronic ophthalmologic disease | 30/168 (17.9) |
| Heart valvular disease | 28/168 (16.4) |
| Peripheral artery disease | 26/168 (15.5) |
| Thyroid disease | 25/168 (14.9) |
| Cerebrovascular disease | 24/168 (14.3) |
| Gastroesophageal reflux disease | 23/168 (13.7) |
| Rheumatologic disease | 21/168 (12.3) |
| Dementia | 17/169 (10.1) |
| Gastroduodenal ulcer | 10/168 (6) |
| Liver disease | 5/168 (3) |
| Other 5 | 51/168 (30.4) |
| Clinical Feature (Unit) | Associated Diagnosis | N | Average 1,2 | Patients in Range | Nº of Drugs Per Diagnosis N (%) | |||
|---|---|---|---|---|---|---|---|---|
| Parameter 1 | N (%) | 1 | 2 | 3 | ||||
| BP (mmHg) | Hypertension | 157 | 132/72 | BP < 140/90 | 88 (56.1) | 14 (8.9) | 42 (26.8) | 100 (63.7) |
| BP < 150/90 | 117 (74.5) | |||||||
| HbA1C (%) | Diabetes | 93 | 7 | HbA1C < 8 | 69 (75) | 33 (35.5) | 27 (29) | 27 (29) |
| Total cholesterol (mg/dL) | Dyslipidemia | 123 | 175.5 | Total cholesterol < 200 and/or LDLc < 130 | 82 (77.4) | 108 (87.8) | 15 (12.2) | 0 (0) |
| LDL cholesterol (mg/dL) | 96.7 | |||||||
| Urate (mg/dL) | Hyperuricemia | 40 | 6.2 | Urate ≤ 6 | 15 (37.5) | |||
| BMI (Kg/m2) | 166 | 29.7 | BMI < 30 | 89 (53) | ||||
| GF (L/min) | 166 | 56.1 | GF ≥ 60 | 76 (45.2) | ||||
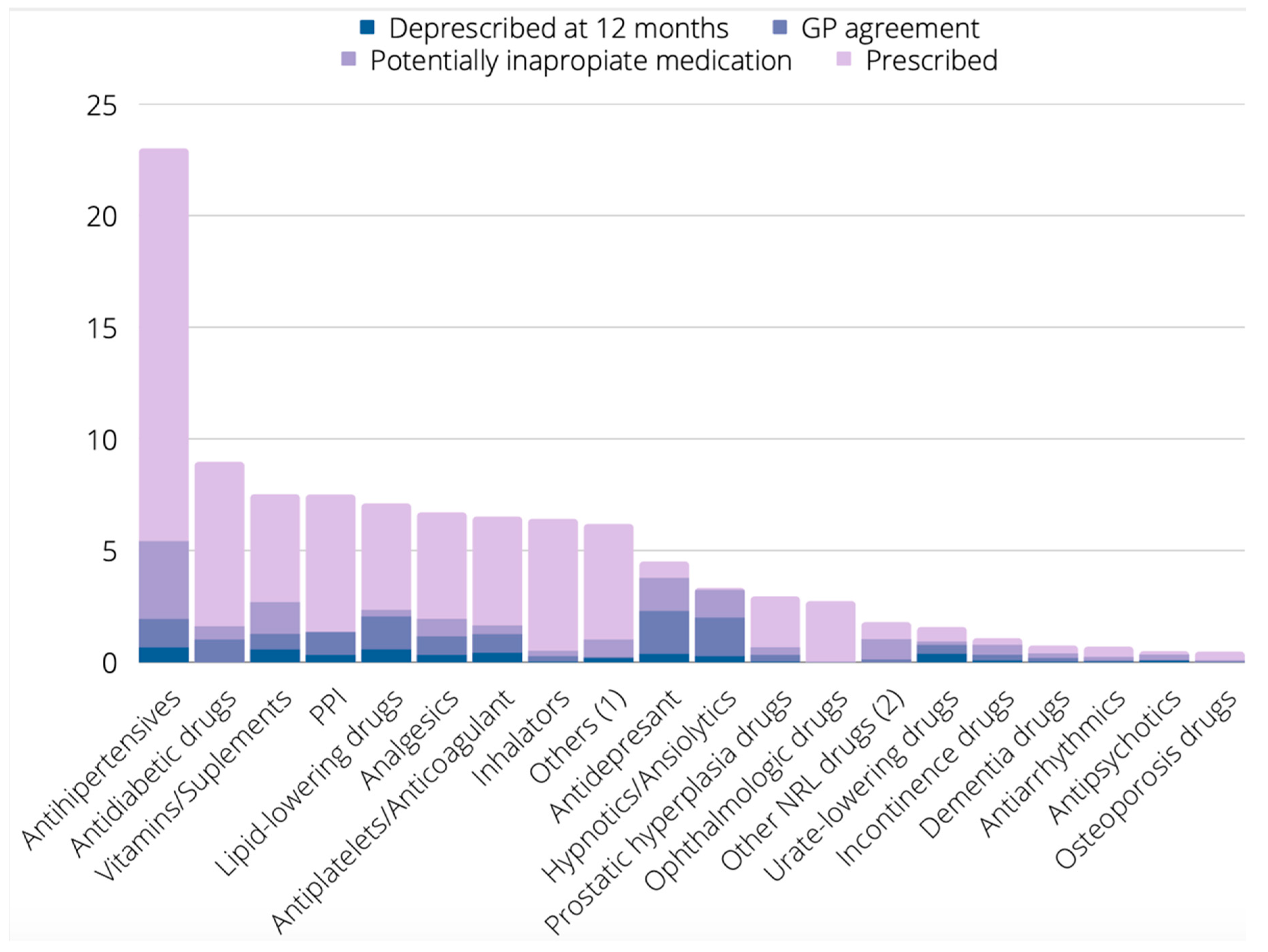
| Therapeutic Group | Prescribed N (%) | Proposed for Deprescribing by Any Tool N (%) | Proposed for Deprescribing by BEERS N (%) | Proposed for Deprescribing by LESS-CHRON N (%) | Proposed for Deprescribing by STOPP N (%) | Proposed for Deprescribing by PRISCUS N (%) |
|---|---|---|---|---|---|---|
| Antihypertensives | 468 (22.9) | 111 (23.7%) | 44 (9.4) | 58 (12.4) | 35 (7.5) | 1 (0.2) |
| Antidiabetic drugs | 182 (8.9) | 33 (18.1) | 6 (3.3) | 27 (14.8) | 6 (3.3) | 1 (0.5) |
| Vitamins/Supplements | 153 (7.5) | 55 (35.9) | 0 (0) | 16 (10.5) | 34 (22.2) | 0 (0) |
| PPI | 150 (7.3) | 25 (16.9) | 57 (38) | 1 (0.7) | 36 (24) | 0 (0) |
| Lipid-lowering drugs | 145 (7.1) | 48 (33.1) | 9 (6.2) | 47 (32.4) | 3 (2.1) | 0 (0) |
| Analgesics | 137 (6.7) | 40 (29.2) | 36 (26.3) | 1 (0.7 | 24 (17.5) | 0 (0) |
| Antiplatelets/Anticoagulants | 133 (6.5) | 34 (25.6) | 15 (11.3) | 28 (21.1) | 5 (3.8) | 0 (0) |
| Inhalators | 131 (6.4) | 11 (8.4) | 12 (9.2) | 0 (0) | 10 (7.6) | 0 (0) |
| Others 1 | 126 (6.2) | 21 (16.7) | 3 (3.2) | 0 (0) | 10 (7.9) | 1 (0.8) |
| Antidepressants | 92 (4.5) | 77 (83.7) | 49 (53.3) | 70 (76.1) | 11 (12) | 3 (3.3) |
| Hypnotics/Anxiolytics | 68 (3.3) | 66 (97.1) | 64 (94.1) | 60 (88.2) | 59 (86.8) | 37 (57.8) |
| Prostatic hyperplasia drugs | 60 (2.9) | 14 (23.3) | 5 (8.3) | 1 (1.7) | 11 (18.3) | 1 (1.7) |
| Ophthalmologic drugs | 55 (2.7) | 1 (1.8) | 0 (0) | 0 (0) | 1 (1.8) | 0 (0) |
| Other NRL drugs 2 | 36 (1.8) | 21 (58.3) | 12 (33.3) | 0 (0) | 2 (5.6) | 0 (0) |
| Urate-lowering drugs | 32 (1.6) | 19 (59.4) | 0 (0) | 19 (59.4) | 2 (6.3) | 0 (0) |
| Incontinence drugs | 22 (1.1) | 16 (72.7) | 6 (27.3) | 2 (9.1) | 9 (40.9) | 4 (18.2) |
| Dementia drugs | 15 (0.7) | 8 (53.3) | 1 (6.7) | 6 (40) | 3 (20) | 0 (0) |
| Antiarrhythmics | 14 (0.7) | 5 (35.7) | 5 (35.7) | 1 (7.1) | 3 (21.4) | 3 (21.4) |
| Antipsychotics | 10 (0.5) | 7 (70) | 5 (50) | 2 (40) | 5 (50) | 1 (20) |
| Osteoporosis drugs | 9 (0.4) | 2 (22.2) | 0 (0) | 0 (0) | 2 (22.2) | 0 (0) |
| All groups | 2038 (100) | 649 (31.8) | 329 (16.1) | 339 (16.6) | 271 (13.3) | 52 (2.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reus, X.; Sastre, M.L.; Leiva, A.; Sánchez, B.; García-Serra, C.; Ioakeim-Skoufa, I.; Vicens, C. LESS-PHARMA Study: Identifying and Deprescribing Potentially Inappropriate Medication in the Elderly Population with Excessive Polypharmacy in Primary Care. Int. J. Environ. Res. Public Health 2022, 19, 13241. https://doi.org/10.3390/ijerph192013241
Reus X, Sastre ML, Leiva A, Sánchez B, García-Serra C, Ioakeim-Skoufa I, Vicens C. LESS-PHARMA Study: Identifying and Deprescribing Potentially Inappropriate Medication in the Elderly Population with Excessive Polypharmacy in Primary Care. International Journal of Environmental Research and Public Health. 2022; 19(20):13241. https://doi.org/10.3390/ijerph192013241
Chicago/Turabian StyleReus, Xisco, Maria Lluisa Sastre, Alfonso Leiva, Belén Sánchez, Cristina García-Serra, Ignatios Ioakeim-Skoufa, and Caterina Vicens. 2022. "LESS-PHARMA Study: Identifying and Deprescribing Potentially Inappropriate Medication in the Elderly Population with Excessive Polypharmacy in Primary Care" International Journal of Environmental Research and Public Health 19, no. 20: 13241. https://doi.org/10.3390/ijerph192013241
APA StyleReus, X., Sastre, M. L., Leiva, A., Sánchez, B., García-Serra, C., Ioakeim-Skoufa, I., & Vicens, C. (2022). LESS-PHARMA Study: Identifying and Deprescribing Potentially Inappropriate Medication in the Elderly Population with Excessive Polypharmacy in Primary Care. International Journal of Environmental Research and Public Health, 19(20), 13241. https://doi.org/10.3390/ijerph192013241







