Patient-Reported Quality of Life versus Physical Examination in Treating Temporomandibular Disorders with Intra-Articular Platelet-Rich Plasma Injections: An Open-Label Clinical Trial
Abstract
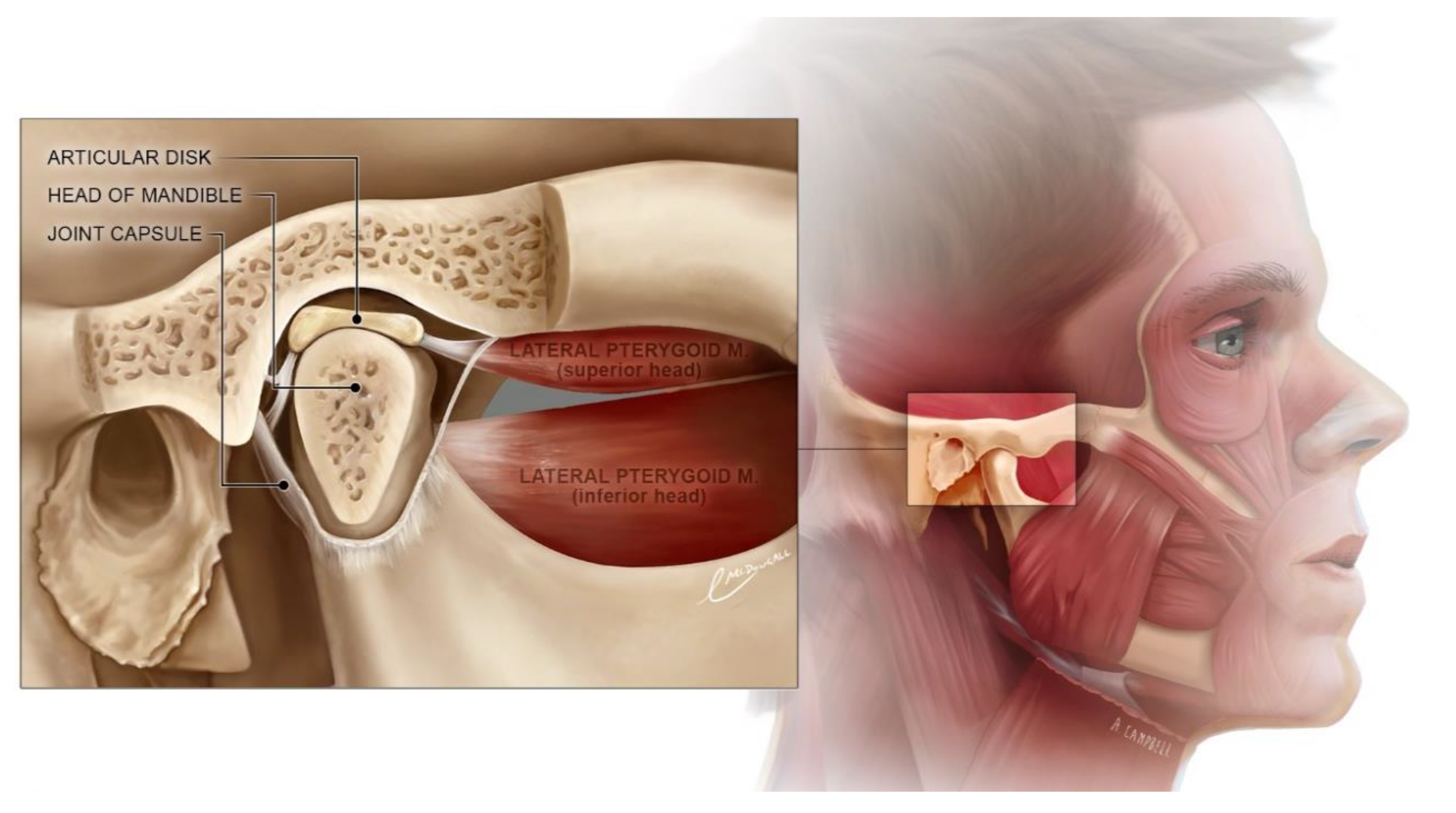
1. Introduction
Objectives
2. Materials and Methods
2.1. Study Design and Settings
2.2. Intervention
2.3. Participants
2.4. Variables and Data Sources
2.5. Bias
2.6. Study Size
2.7. Statistical Methods
3. Results
3.1. Participants and Descriptive Data
3.2. Outcome Data
3.3. Main Results
3.4. Other Analyses
4. Discussion
4.1. General Considerations
4.2. Key Results
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Visit | Questionnaire | Physical Examination | Intervention |
|---|---|---|---|
| 1 | Fonseca questionnaire Right and left TMJ acoustic symptoms on VAS Right and left TMJ spontaneous and provoked pain on VAS | Maximum mouth opening without pain Maximum mouth opening Maximum protrusive, right, and left mandibular movements Acoustic symptoms testing | Yes |
| 2 | Fonseca questionnaire Right and left TMJ acoustic symptoms on VAS Right and left TMJ spontaneous pain on VAS | Maximum mouth opening without pain Maximum mouth opening | Yes |
| 3 | Fonseca questionnaire Right and left TMJ acoustic symptoms on VAS Right and left TMJ spontaneous pain on VAS | Maximum mouth opening without pain Maximum mouth opening | Yes |
| 4 | Fonseca questionnaire Right and left TMJ acoustic symptoms on VAS Right and left TMJ spontaneous pain on VAS | Maximum mouth opening without pain Maximum mouth opening | Yes |
| 5 | Fonseca questionnaire Right and left TMJ acoustic symptoms on VAS Right and left TMJ spontaneous pain on VAS | Maximum mouth opening without pain Maximum mouth opening | Yes |
| 6 | Fonseca questionnaire Right and left TMJ acoustic symptoms on VAS Right and left TMJ spontaneous and provoked pain on VAS | Maximum mouth opening without pain Maximum mouth opening Maximum protrusive, right, and left mandibular movements Acoustic symptoms testing | No |
| Variable | Abbreviation | Possible Values |
|---|---|---|
| Fonseca questionnaire total score (P) | FT1-FT-5 | 0 (no symptoms)–20 (strong symptoms) |
| Visual Analog Scale Acoustic symptoms (P) | VASA-R, VASA-L | 0 (none)–10 (unbearable) |
| Visual Analog Scale Spontaneous Pain (P) | VASSP-R, VASSP-L | 0 (none)–10 (unbearable) |
| Visual Analog Scale Provoked Pain (P) | VASPP-R, VASPP-L | 0 (none)–10 (unbearable) |
| Maximum Mouth Opening without Pain (P,E) | MMOwoP | millimeters |
| Maximum Mouth Opening (E) | MMO | millimeters |
| Maximum Mandible Protrusion (E) | MMP | millimeters |
| Maximum Right-hand movement (E) | MR | millimeters |
| Maximum Light-hand movement (E) | ML | millimeters |
| Acoustic Symptoms for Opening (E) | AO-R, AO-L | 0—none, 1—mild, 2—strong |
| Acoustic Symptoms for Protrusion (E) | AP-R, AP-L | 0—none, 1—mild, 2—strong |
| Acoustic Symptoms for Right-hand movement (E) | AR-R, AR-L | 0—none, 1—mild, 2—strong |
| Acoustic Symptoms for Light-hand movement (E) | AL-R, AR-L | 0—none, 1—mild, 2—strong |
| Acoustic Symptoms for Opening from Protrusion (E) | AOP-R, AOP-L | 0—none, 1—mild, 2—strong |
References
- Gębska, M.; Dalewski, B.; Pałka, Ł.; Kołodziej, Ł.; Sobolewska, E. The Importance of Type D Personality in the Development of Temporomandibular Disorders (TMDs) and Depression in Students during the COVID-19 Pandemic. Brain Sci. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.E.; Stocum, D.L. Part II: Temporomandibular Joint (TMJ)-Regeneration, Degeneration, and Adaptation. Curr. Osteoporos. Rep. 2018, 16, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zou, L.; He, D.; Ellis, E. Comparison of Bone Adaptation after Modification in Biomet Standard Alloplastic Temporomandibular Joint Prostheses. J. Cranio Maxillo Fac. Surg. 2018, 46, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Poluha, R.L.; De la Torre Canales, G.; Bonjardim, L.R.; Conti, P.C.R. Who Is the Individual That Will Complain about Temporomandibular Joint Clicking? J. Oral Rehabil. 2022, 49, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, R.; Nishiyama, A.; Shimada, M. Creating a Quality of Life Index for Patients with Temporomandibular Disorders. Int. J. Dent. Oral Health 2017, 3. [Google Scholar] [CrossRef]
- Nowak, Z.; Chęciński, M.; Nitecka-Buchta, A.; Bulanda, S.; Ilczuk-Rypuła, D.; Postek-Stefańska, L.; Baron, S. Intramuscular Injections and Dry Needling within Masticatory Muscles in Management of Myofascial Pain. Systematic Review of Clinical Trials. Int. J. Environ. Res. Public Health 2021, 18, 9552. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Czerwińska-Niezabitowska, B.; Chęciński, M.A.; Sielski, M.; Chlubek, D. Short-Term Effects of Intra-Articular Hyaluronic Acid Administration in Patients with Temporomandibular Joint Disorders. J. Clin. Med. 2020, 9, 1749. [Google Scholar] [CrossRef]
- Sikora, M.; Sielski, M.; Chęciński, M.; Nowak, Z.; Czerwińska-Niezabitowska, B.; Chlubek, D. Repeated Intra-Articular Administration of Platelet-Rich Plasma (PRP) in Temporomandibular Disorders: A Clinical Case Series. J. Clin. Med. 2022, 11, 4281. [Google Scholar] [CrossRef] [PubMed]
- Trize, D.D.M.; Calabria, M.P.; Franzolin, S.D.O.B.; Cunha, C.O.; Marta, S.N. Is Quality of Life Affected by Temporomandibular Disorders? Einstein 2018, 16, eAO4339. [Google Scholar] [CrossRef]
- Karaman, A.; Sapan, Z. Evaluation of Temporomandibular Disorders, Quality of Life, and Oral Habits among Dentistry Students. Cranio J. Craniomandib. Pract. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bitiniene, D.; Zamaliauskiene, R.; Kubilius, R.; Leketas, M.; Gailius, T.; Smirnovaite, K. Quality of Life in Patients with Temporomandibular Disorders. A Systematic Review. Stomatologija 2018, 20, 3–9. [Google Scholar] [PubMed]
- De Resende, C.M.B.M.; Alves, A.C.D.M.; Coelho, L.T.; Alchieri, J.C.; Roncalli, Â.G.; Barbosa, G.A.S. Quality of Life and General Health in Patients with Temporomandibular Disorders. Braz. Oral Res. 2013, 27, 116–121. [Google Scholar] [CrossRef]
- Da Fonseca, D.M.; Bonfante, G.; do Valle, A.L.; de Freitas, S.F.T. Diagnóstico pela anamnese da disfunção craniomandibular. RGO Porto Alegre 1994, 1, 23–28. [Google Scholar]
- Manafikhi, M.; Ataya, J.; Heshmeh, O. Evaluation of the Efficacy of Platelet Rich Fibrin (I-PRF) Intra-Articular Injections in the Management of Internal Derangements of Temporomandibular Joints—A Controlled Preliminary Prospective Clinical Study. BMC Musculoskelet. Disord. 2022, 23, 454. [Google Scholar] [CrossRef]
- Gutiérrez, I.Q.; Sábado-Bundó, H.; Gay-Escoda, C. Intraarticular Injections of Platelet Rich Plasma and Plasma Rich in Growth Factors with Arthrocenthesis or Arthroscopy in the Treatment of Temporomandibular Joint Disorders: A Systematic Review. J. Stomatol. Oral Maxillofac. Surg. 2021, 123, e327–e335. [Google Scholar] [CrossRef]
- Chung, P.-Y.; Lin, M.-T.; Chang, H.-P. Effectiveness of Platelet-Rich Plasma Injection in Patients with Temporomandibular Joint Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 106–116. [Google Scholar] [CrossRef]
- Chęciński, M.; Sikora, M.; Chęcińska, K.; Nowak, Z.; Chlubek, D. The Administration of Hyaluronic Acid into the Temporomandibular Joints’ Cavities Increases the Mandible’s Mobility: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1901. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; Wolford, L.M.; Ellis, E.; Neff, A. The Hierarchy of Different Treatments for Arthrogenous Temporomandibular Disorders: A Network Meta-Analysis of Randomized Clinical Trials. J. Cranio Maxillo Fac. Surg. 2020, 48, 9–23. [Google Scholar] [CrossRef]
- Chęciński, M.; Chęcińska, K.; Turosz, N.; Kamińska, M.; Nowak, Z.; Sikora, M.; Chlubek, D. Autologous Stem Cells Transplants in the Treatment of Temporomandibular Joints Disorders: A Systematic Review and Meta-Analysis of Clinical Trials. Cells 2022, 11, 2709. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7405. [Google Scholar] [CrossRef] [PubMed]
- Ansar, A.S.; Munna, K.; Iqbal, A.; Mohammad, F.; Naved, A.; Shamimul, H. Prognostic Criteria for the Management of Temporomandibular Disorders Using Arthrocentesis with Normal Saline and Arthrocentesis with Normal Saline and Platelet-Rich Plasma. J. Med. Life 2022, 15, 698–704. [Google Scholar] [CrossRef]
- Bousnaki, M.; Bakopoulou, A.; Koidis, P. Platelet-Rich Plasma for the Therapeutic Management of Temporomandibular Joint Disorders: A Systematic Review. Int. J. Oral Maxillofac. Surg. 2018, 47, 188–198. [Google Scholar] [CrossRef]
- Haigler, M.C.; Abdulrehman, E.; Siddappa, S.; Kishore, R.; Padilla, M.; Enciso, R. Use of Platelet-Rich Plasma, Platelet-Rich Growth Factor with Arthrocentesis or Arthroscopy to Treat Temporomandibular Joint Osteoarthritis: Systematic Review with Meta-Analyses. J. Am. Dent. Assoc. 2018, 149, 940–952.e2. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Fernández-de-Retana, S.; Alkhraisat, M.H. Platelet Rich Plasma in Oral and Maxillofacial Surgery from the Perspective of Composition. Platelets 2021, 32, 174–182. [Google Scholar] [CrossRef]
- Liapaki, A.; Thamm, J.R.; Ha, S.; Monteiro, J.L.G.C.; McCain, J.P.; Troulis, M.J.; Guastaldi, F.P.S. Is There a Difference in Treatment Effect of Different Intra-Articular Drugs for Temporomandibular Joint Osteoarthritis? A Systematic Review of Randomized Controlled Trials. Int. J. Oral Maxillofac. Surg. 2021, 50, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Cömert Kiliç, S.; Güngörmüş, M.; Sümbüllü, M.A. Is Arthrocentesis Plus Platelet-Rich Plasma Superior to Arthrocentesis Alone in the Treatment of Temporomandibular Joint Osteoarthritis? A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2015, 73, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Chandra, L.; Goyal, M.; Srivastava, D. Minimally Invasive Intraarticular Platelet Rich Plasma Injection for Refractory Temporomandibular Joint Dysfunction Syndrome in Comparison to Arthrocentesis. J. Fam. Med. Prim. Care 2021, 10, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Hegab, A.F.; Ali, H.E.; Elmasry, M.; Khallaf, M.G. Platelet-Rich Plasma Injection as an Effective Treatment for Temporomandibular Joint Osteoarthritis. J. Oral Maxillofac. Surg. 2015, 73, 1706–1713. [Google Scholar] [CrossRef]
- Gokçe Kutuk, S.; Gökçe, G.; Arslan, M.; Özkan, Y.; Kütük, M.; Kursat Arikan, O. Clinical and Radiological Comparison of Effects of Platelet-Rich Plasma, Hyaluronic Acid, and Corticosteroid Injections on Temporomandibular Joint Osteoarthritis. J. Craniofac. Surg. 2019, 30, 1144–1148. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Kelly, A.M. The Minimum Clinically Significant Difference in Visual Analogue Scale Pain Score Does Not Differ with Severity of Pain. Emerg. Med. J. EMJ 2001, 18, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Holmlund, A.; Hellsing, G. Arthroscopy of the Temporomandibular Joint. An Autopsy Study. Int. J. Oral Surg. 1985, 14, 169–175. [Google Scholar] [CrossRef]
- Pihut, M.; Szuta, M.; Ferendiuk, E.; Zeńczak-Więckiewicz, D. Evaluation of Pain Regression in Patients with Temporomandibular Dysfunction Treated by Intra-Articular Platelet-Rich Plasma Injections: A Preliminary Report. BioMed Res. Int. 2014, 2014, 132369. [Google Scholar] [CrossRef]
- International Headache Society. International Classification of Orofacial Pain, 1st Edition (ICOP). Cephalalgia Int. J. Headache 2020, 40, 129–221. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Nitecka-Buchta, A.; Buchta, P.; Tabeńska-Bosakowska, E.; Walczyńska-Dragoń, K.; Baron, S. Myorelaxant Effect of Bee Venom Topical Skin Application in Patients with RDC/TMD Ia and RDC/TMD Ib: A Randomized, Double Blinded Study. BioMed Res. Int. 2014, 2014, 296053. [Google Scholar] [CrossRef]
- Cheng, J.; Abdi, S. Complications of Joint, Tendon, and Muscle Injections. Tech. Reg. Anesth. Pain Manag. 2007, 11, 141–147. [Google Scholar] [CrossRef]
- Lane, J.C.E.; Craig, R.S.; Rees, J.L.; Gardiner, M.D.; Shaw, A.V.; Spiteri, M.; Kuo, R.; Dean, B.F.; Green, J.; Prieto-Alhambra, D.; et al. Low Rate of Subsequent Surgery and Serious Complications Following Intra-Articular Steroid Injection for Base of Thumb Osteoarthritis: National Cohort Analysis. Rheumatol. Oxf. Engl. 2021, 60, 4262–4271. [Google Scholar] [CrossRef]
- Bjørnland, T.; Gjærum, A.A.; Møystad, A. Osteoarthritis of the Temporomandibular Joint: An Evaluation of the Effects and Complications of Corticosteroid Injection Compared with Injection with Sodium Hyaluronate. J. Oral Rehabil. 2007, 34, 583–589. [Google Scholar] [CrossRef]
- Parra, D.A.; Chan, M.; Krishnamurthy, G.; Spiegel, L.; Amaral, J.G.; Temple, M.J.; John, P.R.; Connolly, B.L. Use and Accuracy of US Guidance for Image-Guided Injections of the Temporomandibular Joints in Children with Arthritis. Pediatr. Radiol. 2010, 40, 1498–1504. [Google Scholar] [CrossRef]
- Isacsson, G.; Schumann, M.; Nohlert, E.; Mejersjö, C.; Tegelberg, Å. Pain Relief Following a Single-dose Intra-articular Injection of Methylprednisolone in the Temporomandibular Joint Arthralgia—A Multicentre Randomised Controlled Trial. J. Oral Rehabil. 2019, 46, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yu, G.; Yang, K.; Xiang, W.; Li, J.; Chen, H. Efficacy and Safety of Mesenchymal Stem Cell Transplantation in the Treatment of Autoimmune Diseases (Rheumatoid Arthritis, Systemic Lupus Erythematosus, Inflammatory Bowel Disease, Multiple Sclerosis, and Ankylosing Spondylitis): A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Stem Cells Int. 2022, 2022, 9463314. [Google Scholar] [CrossRef] [PubMed]
- Lodders, J.N.; van Baar, G.J.C.; Vergeer, M.R.; Jansen, F.; Schulten, E.A.J.M.; Lissenberg-Witte, B.I.; Verdonck-de Leeuw, I.M.; Forouzanfar, T.; Leusink, F.K.J. Implant-Based Dental Rehabilitation in Head and Neck Cancer Patients after Maxillofacial Reconstruction with a Free Vascularized Fibula Flap: The Effect on Health-Related Quality of Life. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2022, 30, 5411–5420. [Google Scholar] [CrossRef]
- Somoye, M.S.; Adetayo, A.M.; Adeyemo, W.L.; Ladeinde, A.L.; Gbotolorun, M.O. A Comparative Study of Quality of Life of Patients with Maxillofacial Fracture and Healthy Controls at Two Tertiary Healthcare Institutions. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 351–359. [Google Scholar] [CrossRef]
- Boffano, P.; Pau, A.; Dosio, C.; Ruslin, M.; Forouzanfar, T.; Rodríguez-Santamarta, T.; de Vicente, J.C.; Tarle, M.; Dediol, E.; Pechalova, P.; et al. Quality of Life Following Maxillofacial Trauma in the Elderly: A Multicenter, Prospective Study. Oral Maxillofac. Surg. 2022, 26, 383–392. [Google Scholar] [CrossRef]
- Koga, S.; Ogino, Y.; Fujikawa, N.; Ueno, M.; Kotaki, Y.; Koyano, K. Oral Health-Related Quality of Life and Oral Hygiene Condition in Patients with Maxillofacial Defects: A Retrospective Analysis. J. Prosthodont. Res. 2020, 64, 397–400. [Google Scholar] [CrossRef]
- Sikora, M.; Chlubek, M.; Grochans, E.; Jurczak, A.; Safranow, K.; Chlubek, D. Analysis of Factors Affecting Quality of Life in Patients Treated for Maxillofacial Fractures. Int. J. Environ. Res. Public. Health 2019, 17, 4. [Google Scholar] [CrossRef]
- Roman, M.B.; Cybulska, A.M.; Dowgierd, K.; Wójcik, G.; Stanisławska, M.; Grochans, E. Quality of Life of Young Adult Patients after Orthognathic Surgery. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7903–7912. [Google Scholar] [CrossRef]
- Xiao, Y.; Liang, Y.; Yang, L.; Yang, W.; Liao, G. Long-Term Quality of Life in Patients with Maxillofacial Malignancies Who Have Undergone Craniofacial Resection: A Cross-Sectional Survivorship Study. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2019, 77, 2573–2583. [Google Scholar] [CrossRef]
- Dings, J.P.J.; Merkx, M.A.W.; de Clonie Maclennan-Naphausen, M.T.P.; van de Pol, P.; Maal, T.J.J.; Meijer, G.J. Maxillofacial Prosthetic Rehabilitation: A Survey on the Quality of Life. J. Prosthet. Dent. 2018, 120, 780–786. [Google Scholar] [CrossRef]
- Tamme, J.A.; Rohnen, M.; Gaßling, V.; Ciesielski, R.; Fischer-Brandies, H.; Wiltfang, J.; Koos, B. Correlation of General and Oral Health-Related Quality of Life in Malocclusion Patients Treated with a Combined Orthodontic and Maxillofacial Surgical Approach. J. Cranio Maxillo Fac. Surg. 2017, 45, 1971–1979. [Google Scholar] [CrossRef]
- Jain, S.; Chourse, S.; Jain, D. Prevalence and Severity of Temporomandibular Disorders among the Orthodontic Patients Using Fonseca’s Questionnaire. Contemp. Clin. Dent. 2018, 9, 31–34. [Google Scholar] [CrossRef]
- Nomura, K.; Vitti, M.; Oliveira, A.S.D.; Chaves, T.C.; Semprini, M.; Siéssere, S.; Hallak, J.E.C.; Regalo, S.C.H. Use of the Fonseca’s Questionnaire to Assess the Prevalence and Severity of Temporomandibular Disorders in Brazilian Dental Undergraduates. Braz. Dent. J. 2007, 18, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Yarasca-Berrocal, E.; Huamani-Echaccaya, J.; Tolmos-Valdivia, R.; Tolmos-Regal, L.; López-Gurreonero, C.; Cervantes-Ganoza, L.A.; Cayo-Rojas, C.F. Predictability and Accuracy of the Short-Form Fonseca Anamnestic Index in Relation to the Modified Helkimo Index for the Diagnosis of Temporomandibular Disorders: A Cross-Sectional Study. J. Int. Soc. Prev. Community Dent. 2022, 12, 178–188. [Google Scholar] [CrossRef]
- Zagalaz-Anula, N.; Sánchez-Torrelo, C.M.; Acebal-Blanco, F.; Alonso-Royo, R.; Ibáñez-Vera, A.J.; Obrero-Gaitán, E.; Rodríguez-Almagro, D.; Lomas-Vega, R. The Short Form of the Fonseca Anamnestic Index for the Screening of Temporomandibular Disorders: Validity and Reliability in a Spanish-Speaking Population. J. Clin. Med. 2021, 10, 5858. [Google Scholar] [CrossRef]
- He, S.; Renne, A.; Argandykov, D.; Convissar, D.; Lee, J. Comparison of an Emoji-Based Visual Analog Scale with a Numeric Rating Scale for Pain Assessment. JAMA 2022, 328, 208–209. [Google Scholar] [CrossRef]
- Holdgate, A.; Asha, S.; Craig, J.; Thompson, J. Comparison of a Verbal Numeric Rating Scale with the Visual Analogue Scale for the Measurement of Acute Pain. Emerg. Med. 2003, 15, 441–446. [Google Scholar] [CrossRef]
- Modarresi, S.; Lukacs, M.J.; Ghodrati, M.; Salim, S.; MacDermid, J.C.; Walton, D.M. CATWAD Consortium Group A Systematic Review and Synthesis of Psychometric Properties of the Numeric Pain Rating Scale and the Visual Analog Scale for Use in People With Neck Pain. Clin. J. Pain 2021, 38, 132–148. [Google Scholar] [CrossRef]



| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| MMOwoP1 | MMOwoP2 | MMOwoP3 | MMOwoP4 | MMOwoP5 | MMOwoP6 | |
|---|---|---|---|---|---|---|
| MMO1 | 0.74 | |||||
| MMO2 | 0.59 | 0.73 | ||||
| MMO3 | 0.63 | 0.59 | 0.82 | |||
| MMO4 | 0.63 | 0.35 * | 0.55 | 0.81 | ||
| MMO5 | 0.59 | 0.59 | 0.68 | 0.65 | 0.82 | |
| MMO6 | 0.51 | 0.45 * | 0.57 | 0.66 | 0.57 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, M.; Sielski, M.; Chęciński, M.; Chęcińska, K.; Czerwińska-Niezabitowska, B.; Chlubek, D. Patient-Reported Quality of Life versus Physical Examination in Treating Temporomandibular Disorders with Intra-Articular Platelet-Rich Plasma Injections: An Open-Label Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 13299. https://doi.org/10.3390/ijerph192013299
Sikora M, Sielski M, Chęciński M, Chęcińska K, Czerwińska-Niezabitowska B, Chlubek D. Patient-Reported Quality of Life versus Physical Examination in Treating Temporomandibular Disorders with Intra-Articular Platelet-Rich Plasma Injections: An Open-Label Clinical Trial. International Journal of Environmental Research and Public Health. 2022; 19(20):13299. https://doi.org/10.3390/ijerph192013299
Chicago/Turabian StyleSikora, Maciej, Marcin Sielski, Maciej Chęciński, Kamila Chęcińska, Barbara Czerwińska-Niezabitowska, and Dariusz Chlubek. 2022. "Patient-Reported Quality of Life versus Physical Examination in Treating Temporomandibular Disorders with Intra-Articular Platelet-Rich Plasma Injections: An Open-Label Clinical Trial" International Journal of Environmental Research and Public Health 19, no. 20: 13299. https://doi.org/10.3390/ijerph192013299
APA StyleSikora, M., Sielski, M., Chęciński, M., Chęcińska, K., Czerwińska-Niezabitowska, B., & Chlubek, D. (2022). Patient-Reported Quality of Life versus Physical Examination in Treating Temporomandibular Disorders with Intra-Articular Platelet-Rich Plasma Injections: An Open-Label Clinical Trial. International Journal of Environmental Research and Public Health, 19(20), 13299. https://doi.org/10.3390/ijerph192013299









