Non-Linear and Sex-Specific Effect of Maternal Pre-Pregnancy BMI on Emotional and Behavioral Development of Preschool Children: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
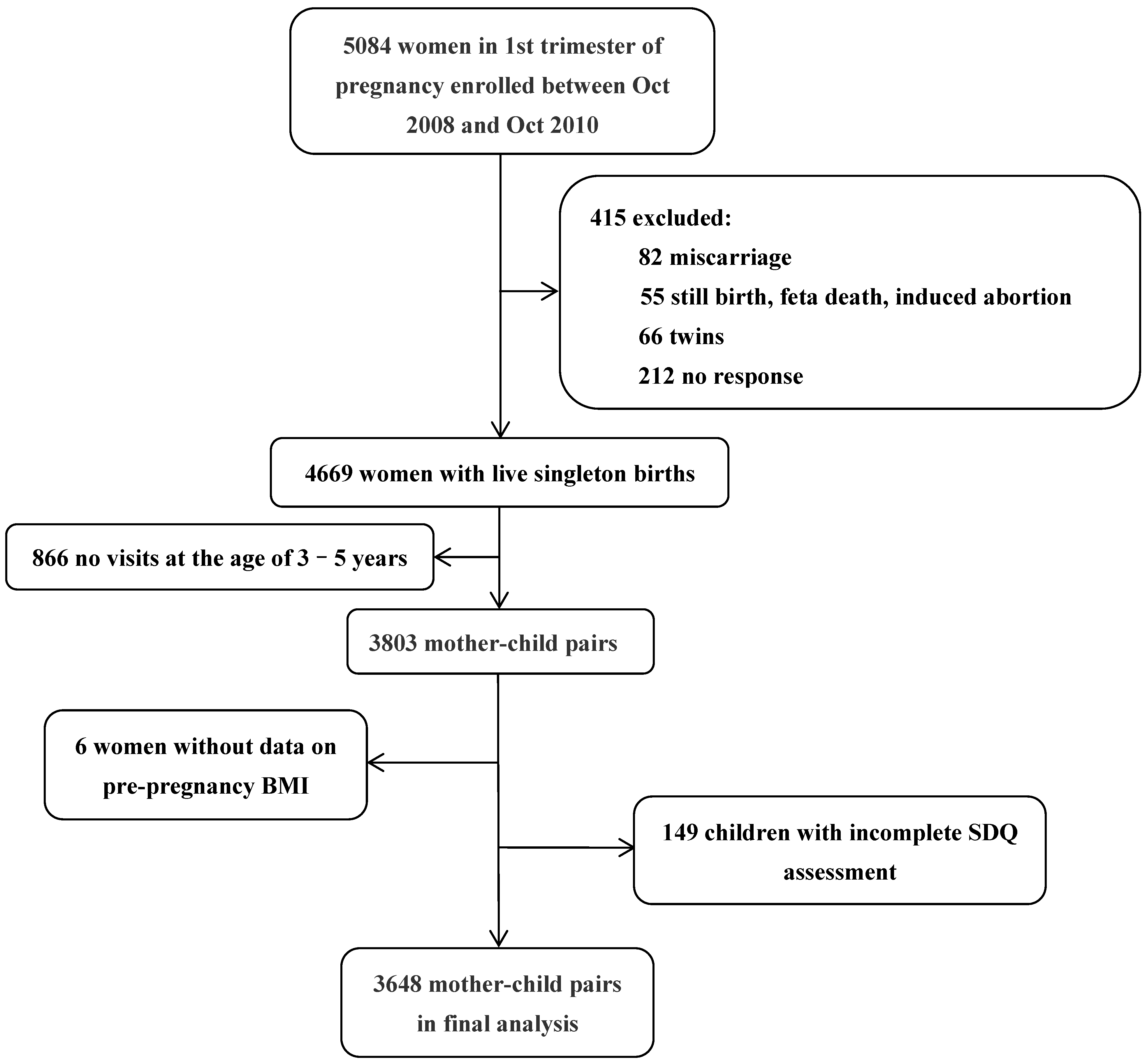
2.1. Participants
2.2. Maternal Pre-Pregnancy BMI
2.3. Assessment of Children’s Emotional and Behavioral Development
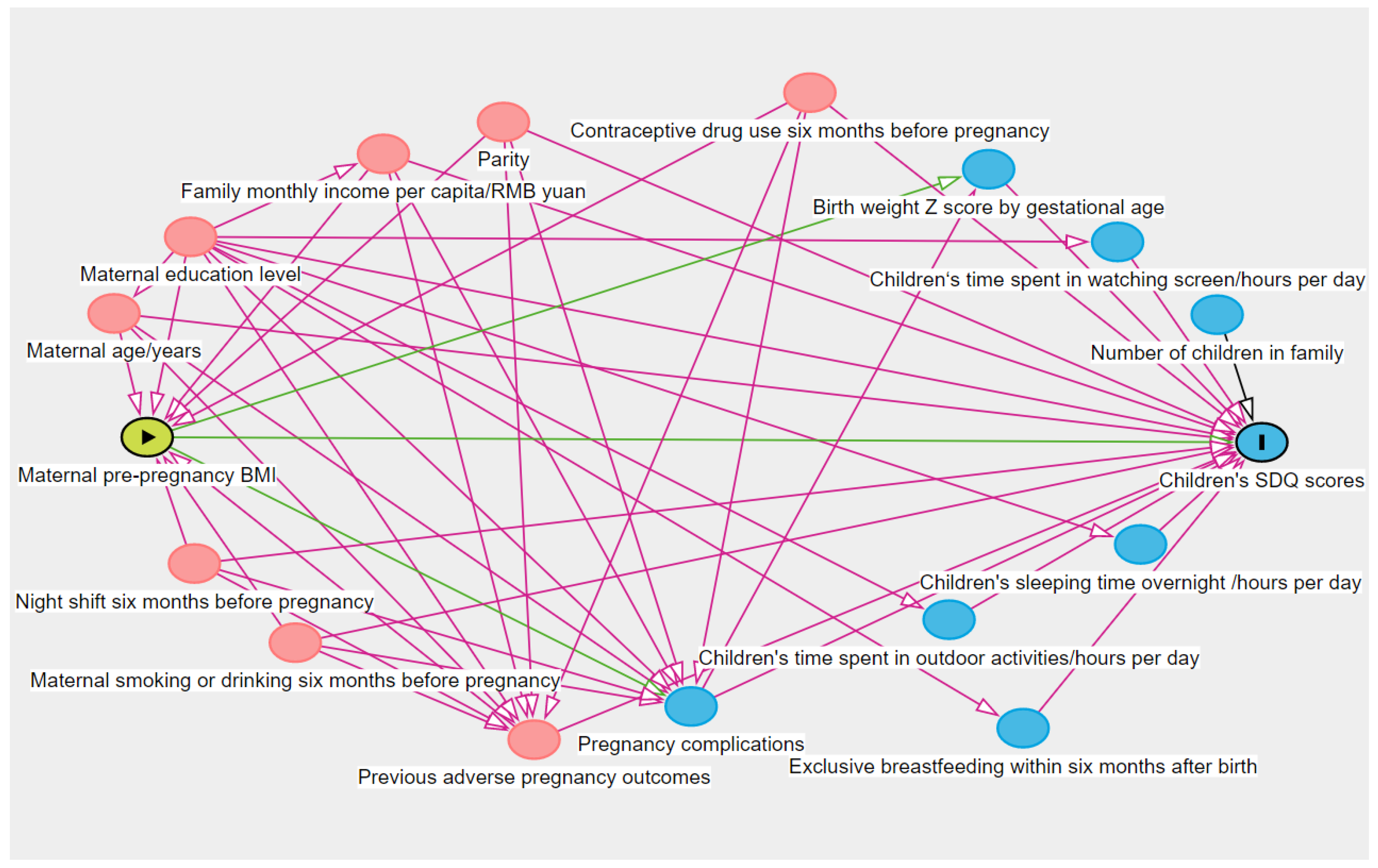
3. Covariates
Statistical Analysis
4. Results
4.1. Baseline Maternal and Children’s Characteristics
4.2. Prevalence of Children’s Abnormal SDQ Dimensions in Different Maternal Pre-Pregnancy BMI Categories
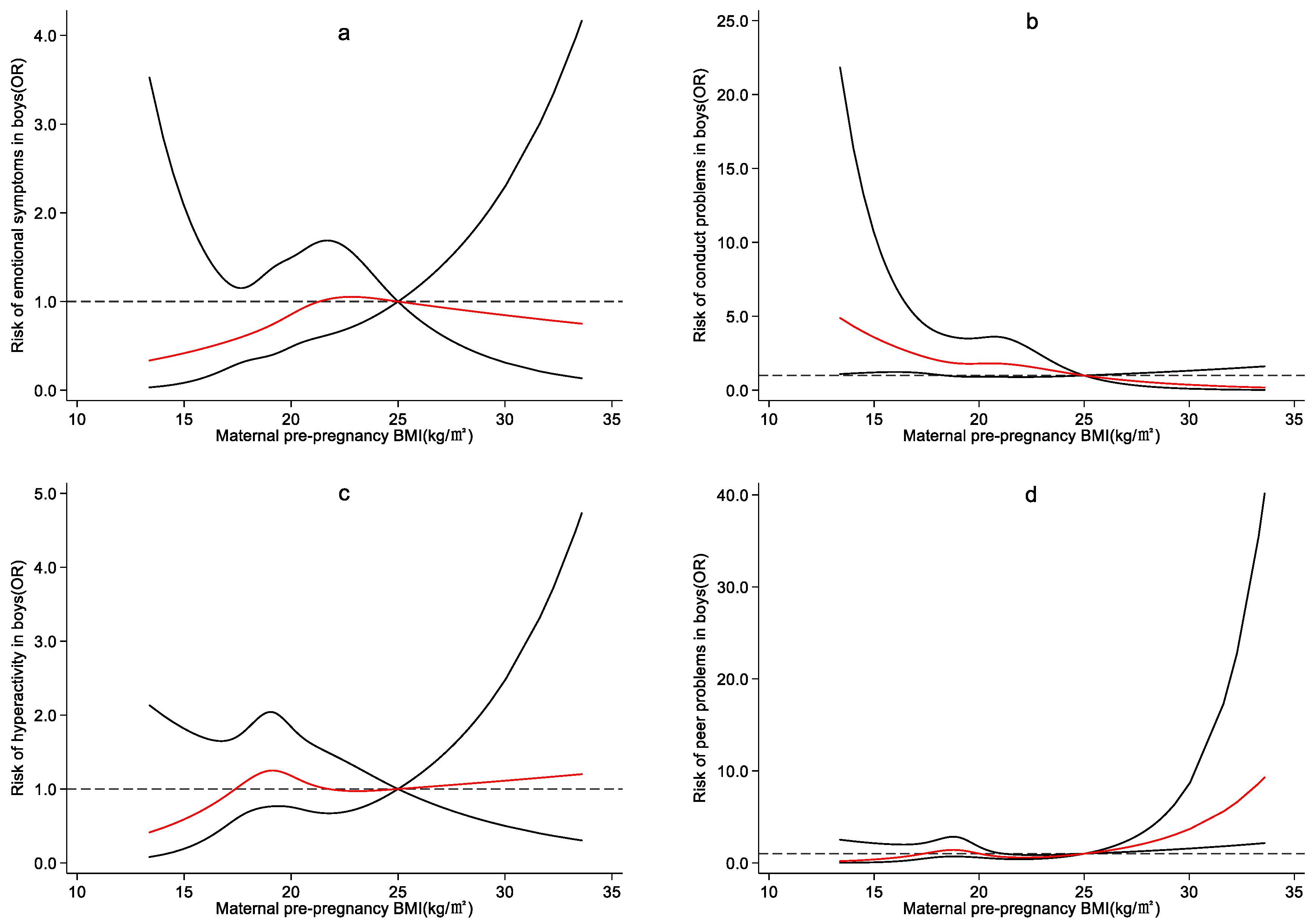
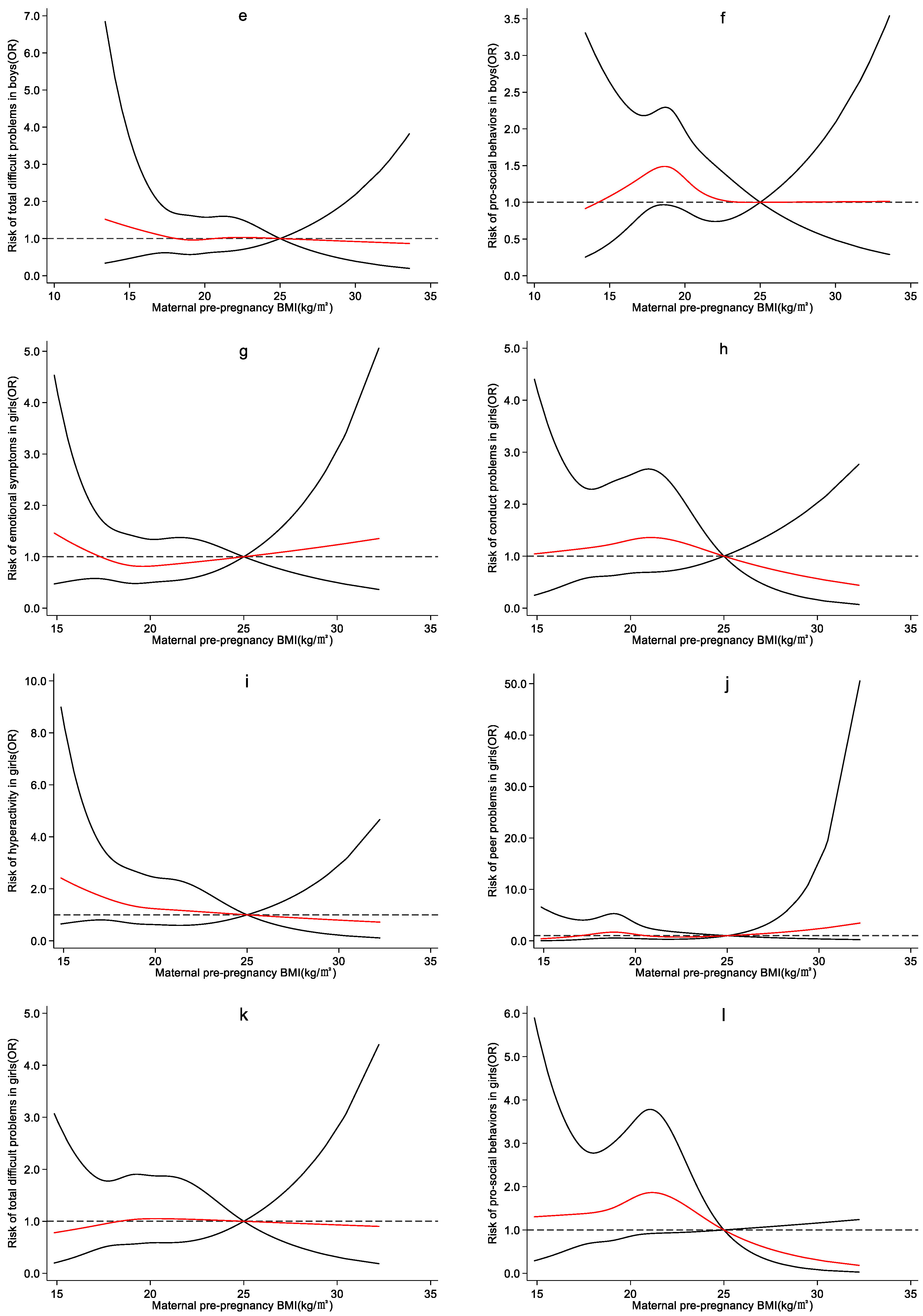
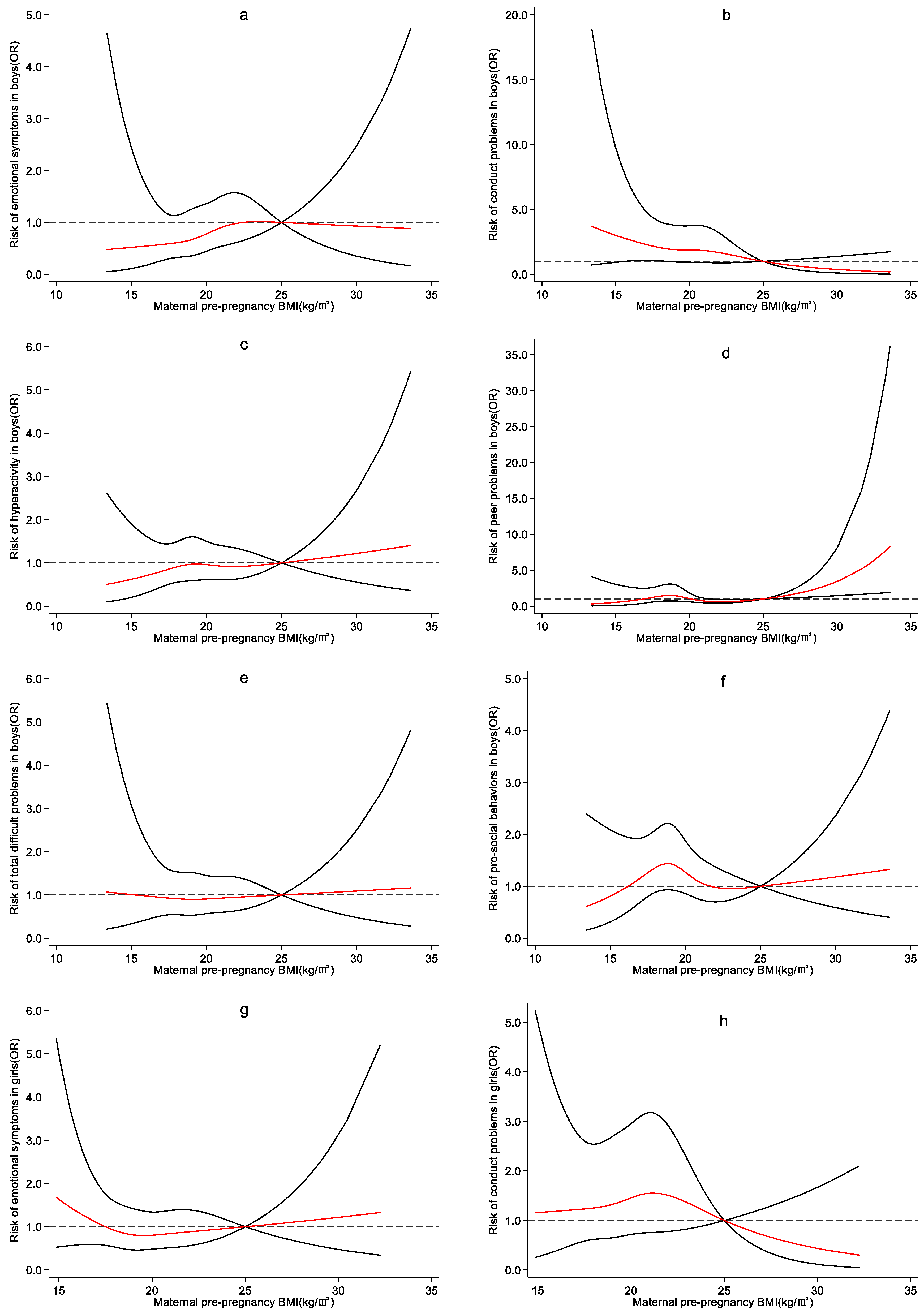
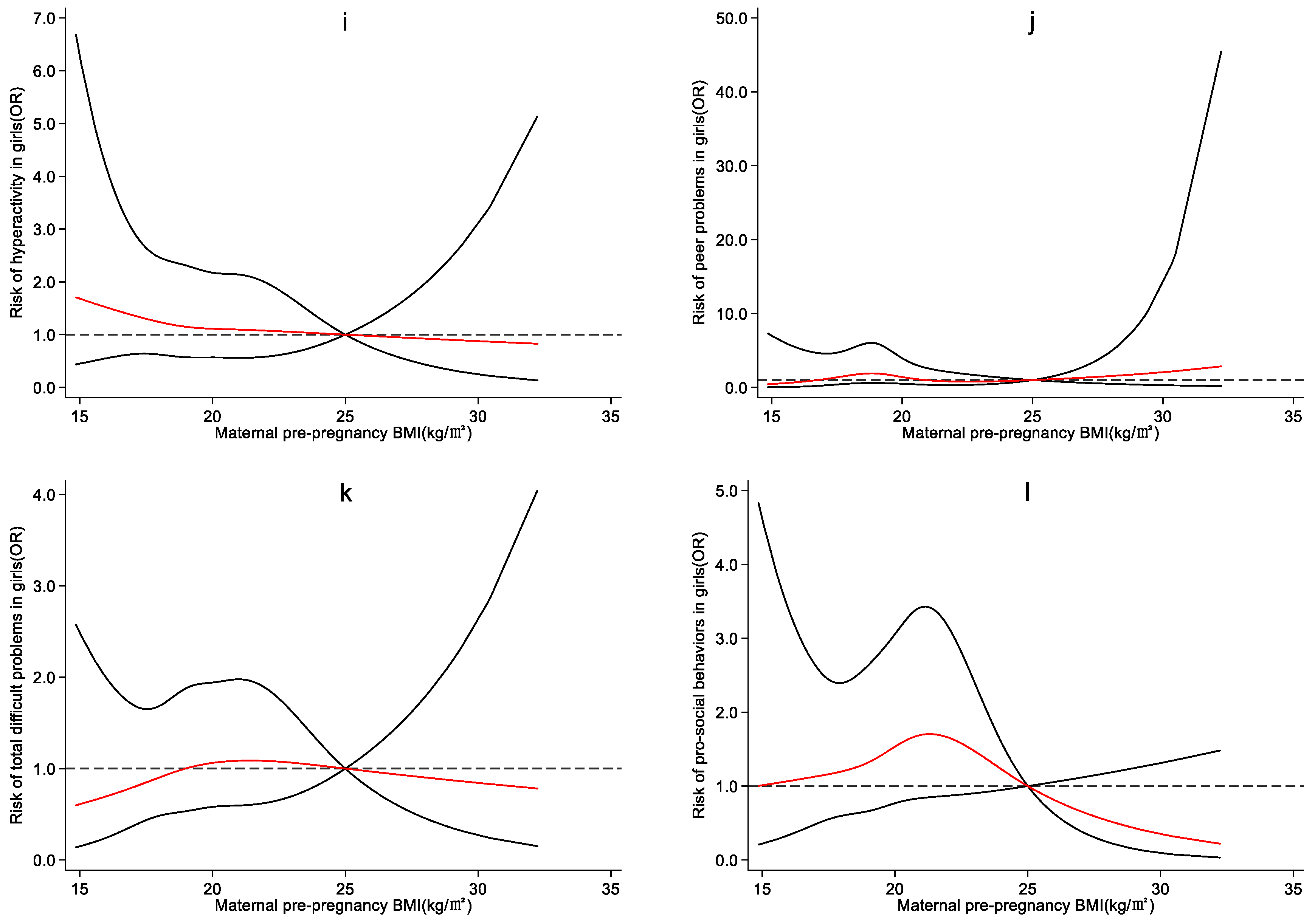
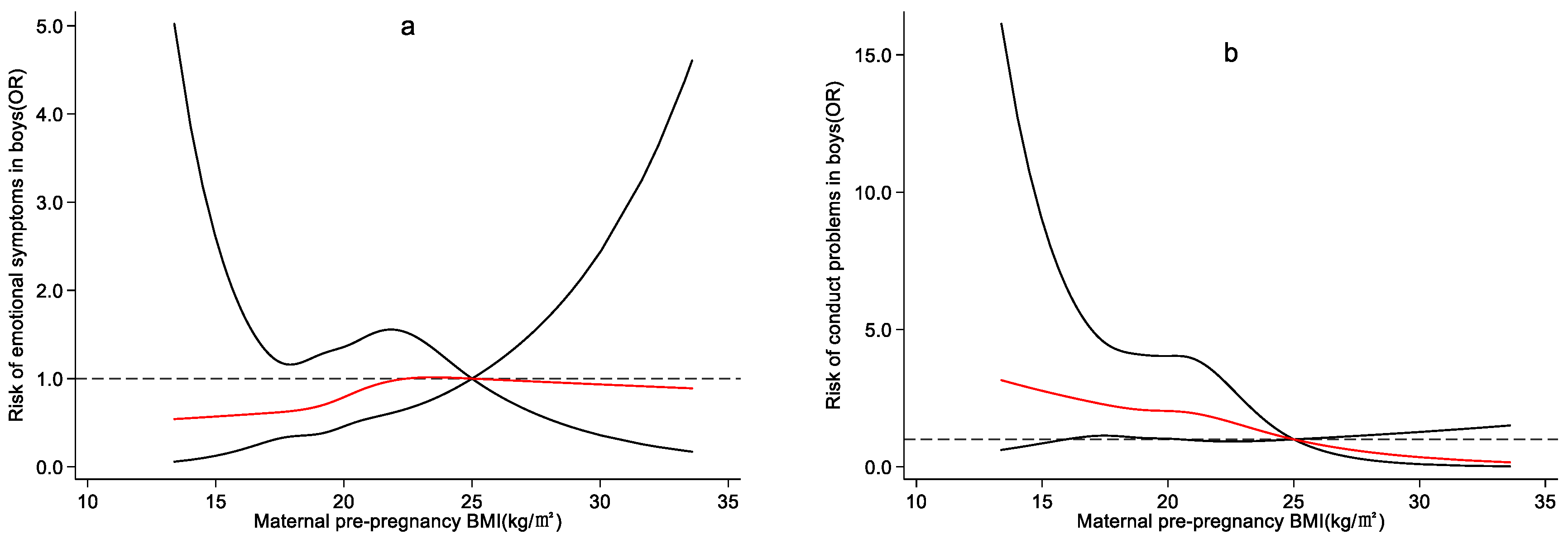
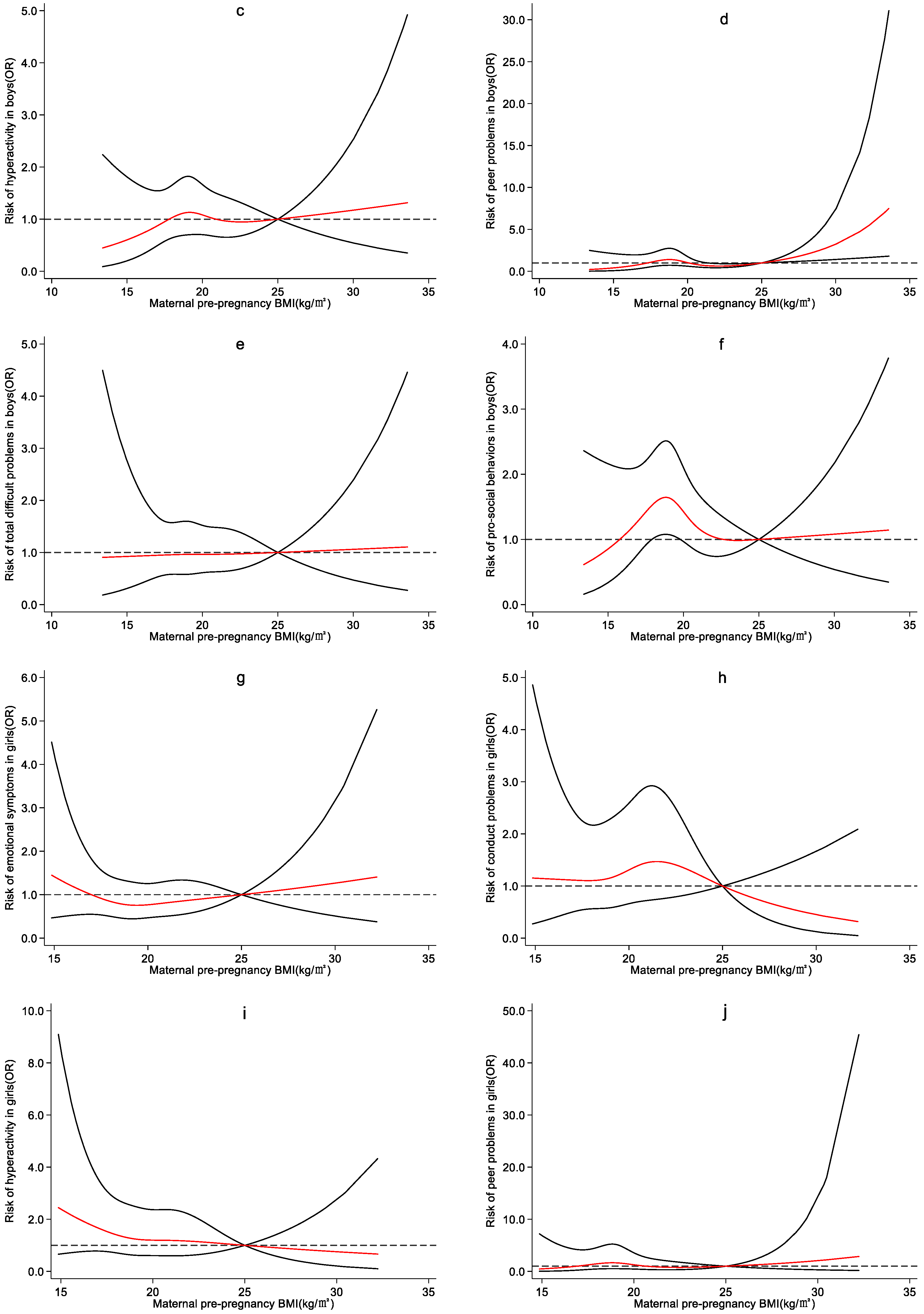
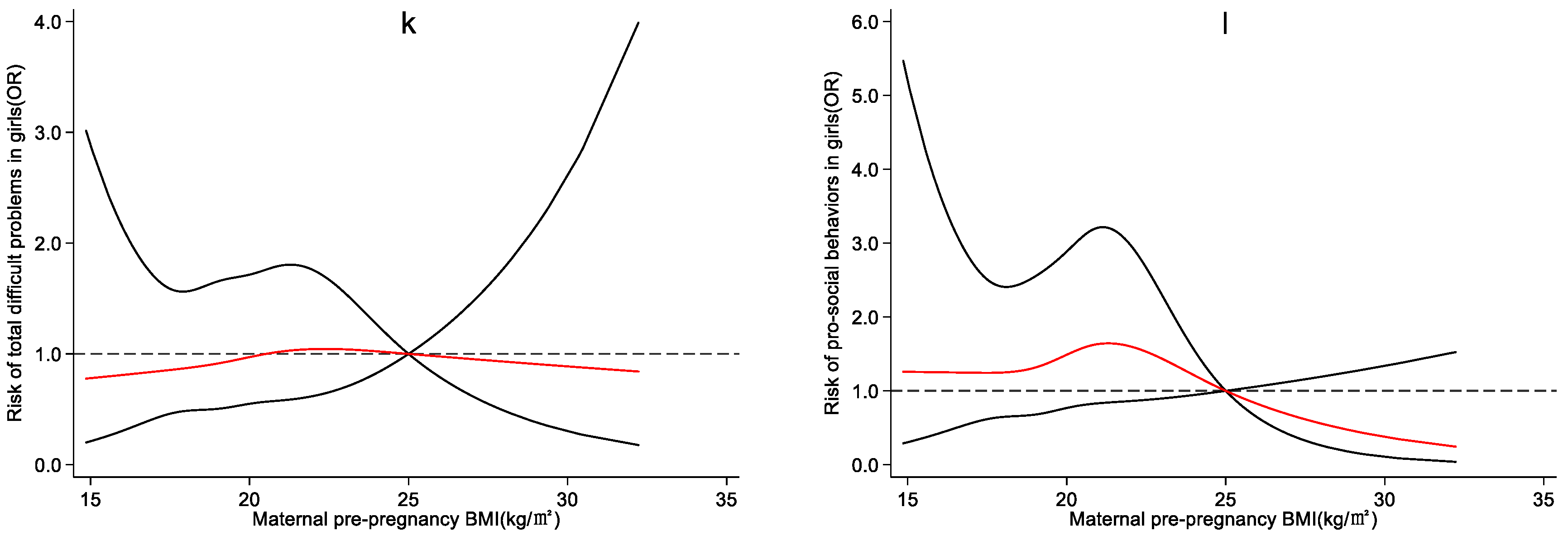
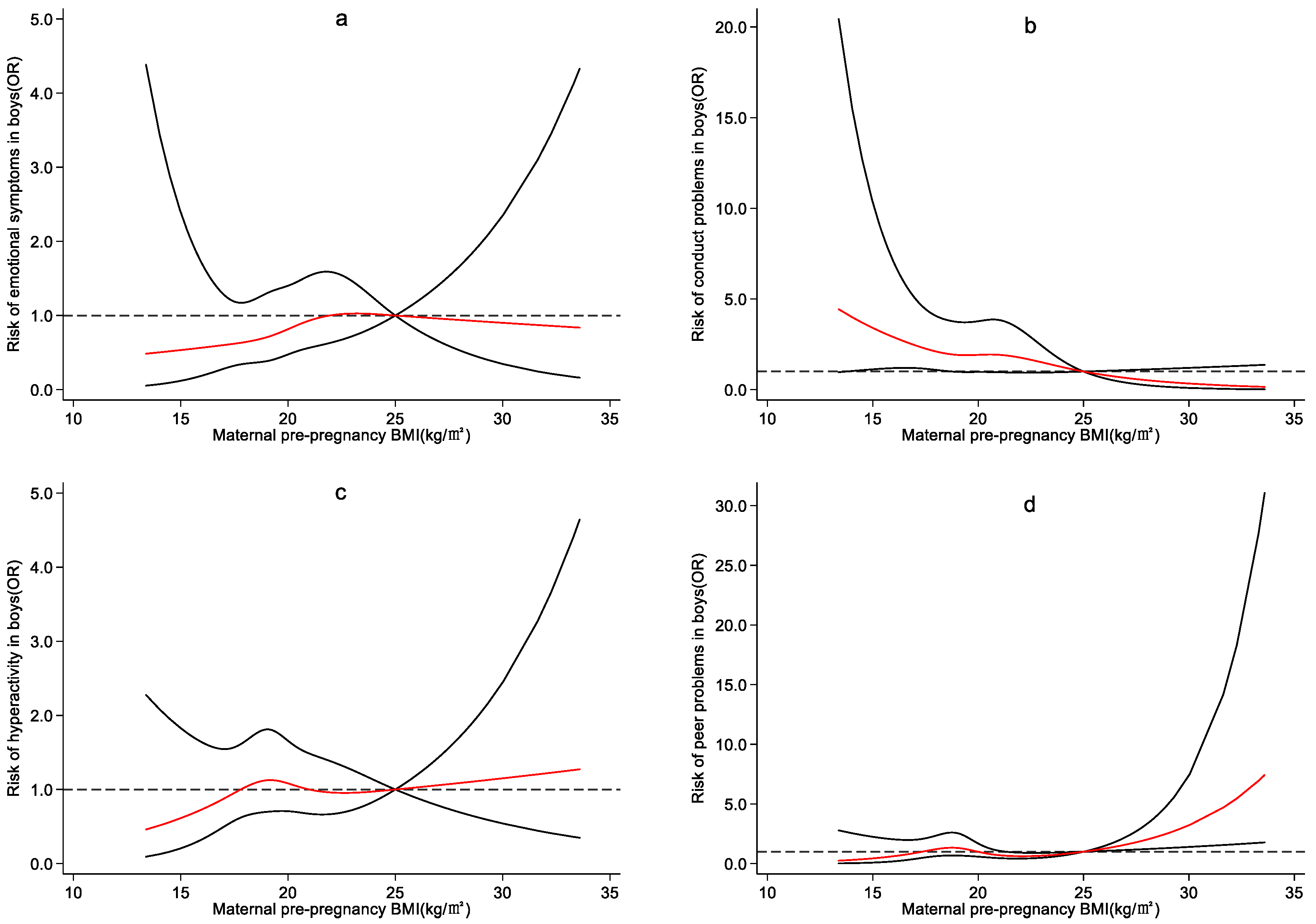
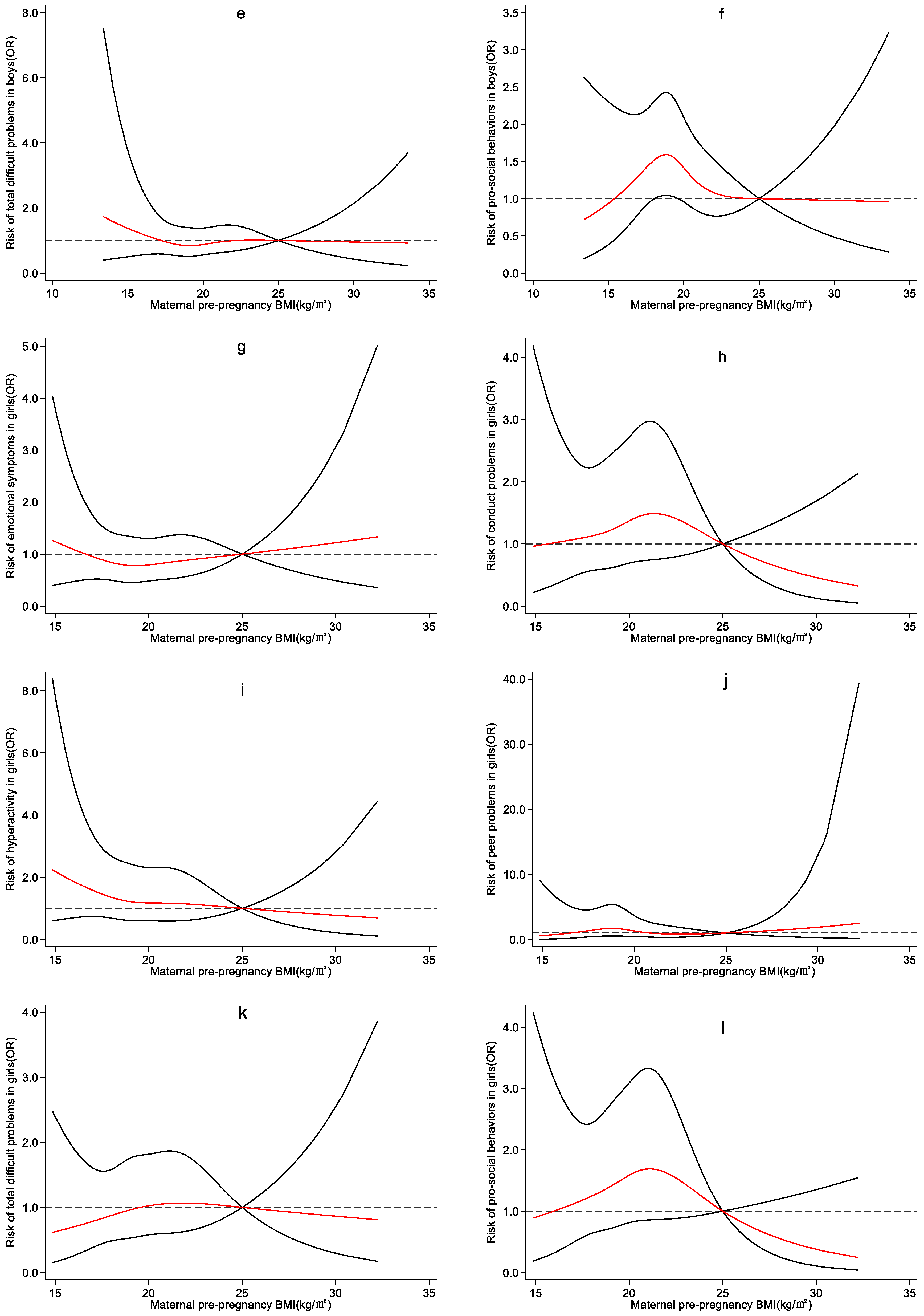
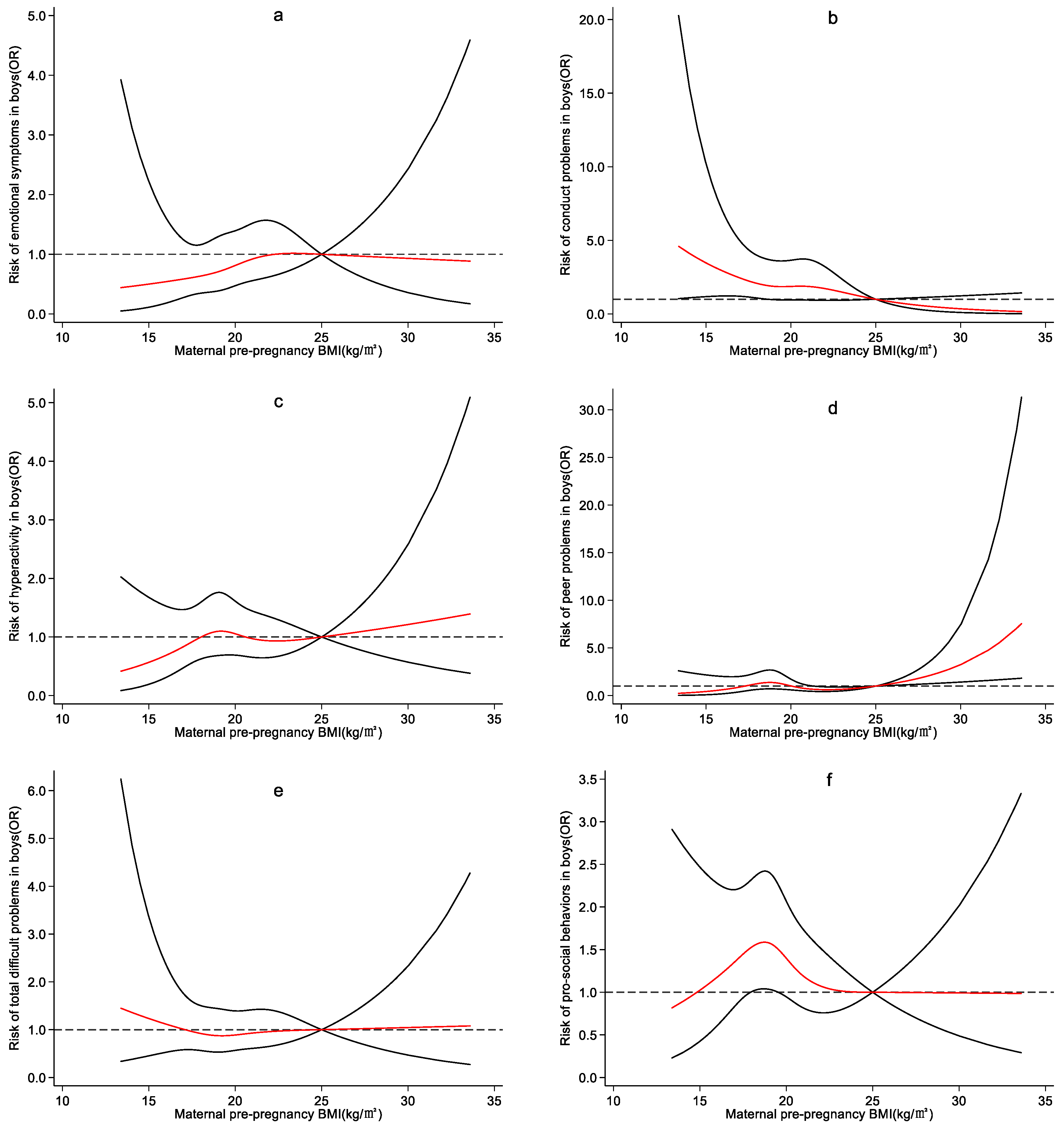
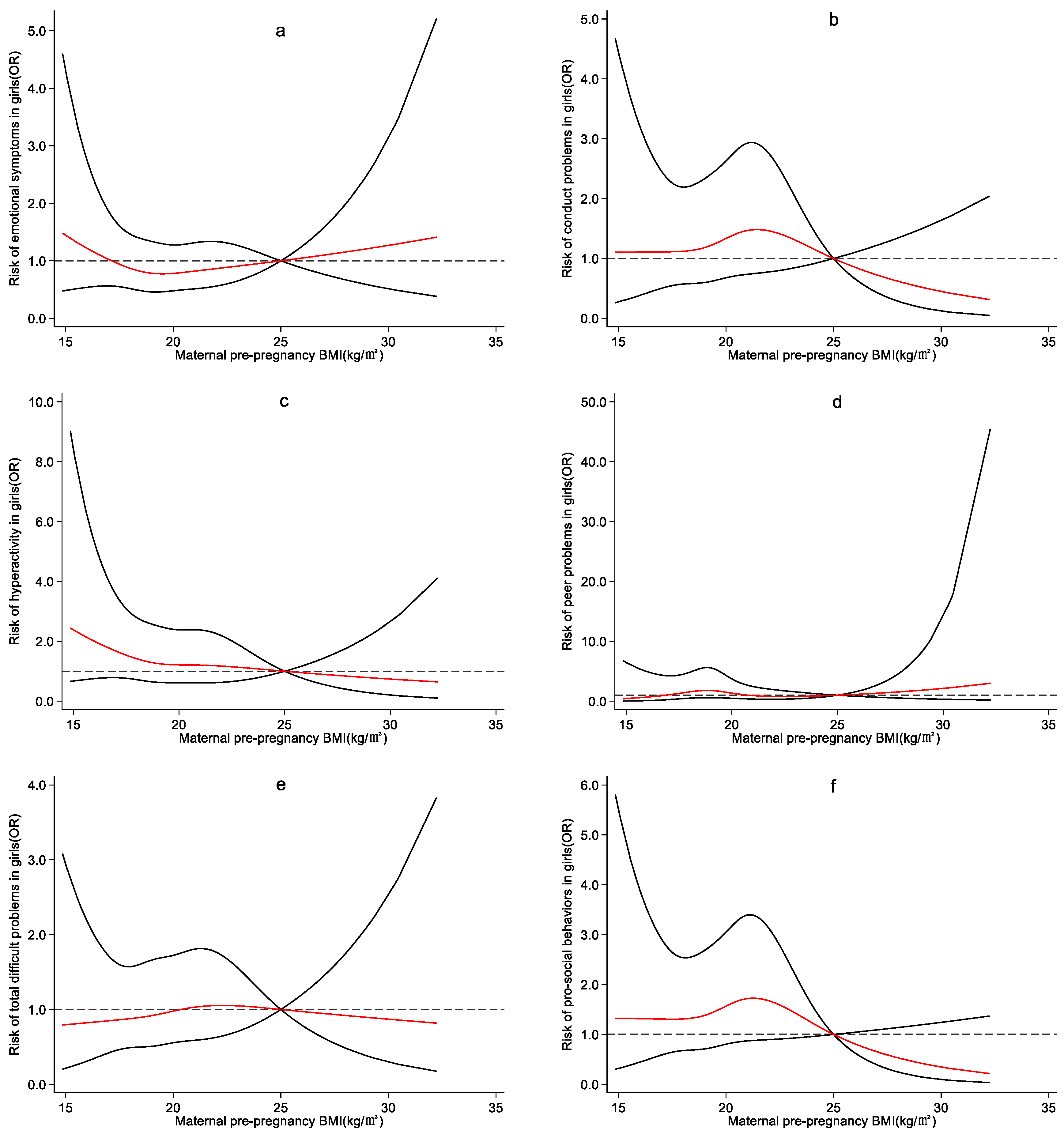
4.3. Effect of Maternal Pre-Pregnancy BMI on Preschool Children’s Emotional and Behavioral Development
5. Discussion
Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Idris, I.B.; Barlow, J.; Dolan, A. A Longitudinal Study of Emotional and Behavioral Problems among Malaysian School Children. Ann. Glob. Health 2019, 85, 30. [Google Scholar] [CrossRef] [PubMed]
- Ghandour, R.M.; Sherman, L.J.; Vladutiu, C.J.; Ali, M.M.; Lynch, S.E.; Bitsko, R.H.; Blumberg, S.J. Prevalence and Treatment of Depression, Anxiety, and Conduct Problems in US Children. J. Pediatr. 2019, 206, 256–267.e3. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Wu, X.Y.; Tao, S.M.; Ding, P.; Geng, M.L.; Tao, F.B. Emotional and behavioral problems associated with health-risk behaviors in preschool children. Zhonghua Yu Fang Yi Xue Za Zhi 2020, 54, 1255–1260. [Google Scholar] [PubMed]
- Magai, D.N.; Malik, J.A.; Koot, H.M. Emotional and Behavioral Problems in Children and Adolescents in Central Kenya. Child Psychiatry Hum. Dev. 2018, 49, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Salam, R.A. Global nutrition epidemiology and trends. Ann. Nutr. Metab. 2012, 61 (Suppl. S1), 19–27. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Dean, S.V.; Lassi, Z.S.; Imam, A.M.; Bhutta, Z.A. Preconception care: Nutritional risks and interventions. Reprod. Health 2014, 11 (Suppl. S3), S3. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, J.; Smith, L.H.; Petito, L.; Kim, H.; Abrams, B.F. Maternal Prepregnancy Weight and Children’s Behavioral and Emotional Outcomes. Am. J. Prev. Med. 2017, 53, 432–440. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef]
- Saikia, D.; Ahmed, S.J.; Saikia, H.; Sarma, R. Body mass index and body fat percentage in assessing obesity: An analytical study among the adolescents of Dibrugarh, Assam. Indian J. Public Health 2018, 62, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.C.; Dumith, S.C.; Orlandi, S.P.; Assunção, M.C.F. Diet and body fat in adolescence and early adulthood: A systematic review of longitudinal studies. Cienc. Saude Coletiva 2017, 22, 1539–1552. [Google Scholar] [CrossRef][Green Version]
- Getz, K.D.; Anderka, M.T.; Werler, M.M.; Jick, S.S. Maternal Pre-pregnancy Body Mass Index and Autism Spectrum Disorder among Offspring: A Population-Based Case-Control Study. Paediatr. Perinat. Epidemiol. 2016, 30, 479–487. [Google Scholar] [CrossRef]
- Minatoya, M.; Itoh, S.; Araki, A.; Tamura, N.; Yamazaki, K.; Nishihara, S.; Miyashita, C.; Kishi, R. Associated factors of behavioural problems in children at preschool age: The Hokkaido study on environment and children’s health. Child Care Health Dev. 2017, 43, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Terhaag, S.; Fitzsimons, E.; Daraganova, G.; Patalay, P. Sex, ethnic and socioeconomic inequalities and trajectories in child and adolescent mental health in Australia and the UK: Findings from national prospective longitudinal studies. J. Child Psychol. Psychiatry 2021, 62, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.B.; Hao, J.H.; Huang, K.; Su, P.Y.; Cheng, D.J.; Xing, X.Y.; Huang, Z.H.; Zhang, J.L.; Tong, S.L. Cohort Profile: The China-Anhui Birth Cohort Study. Int. J. Epidemiol. 2013, 42, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Kou, J.; Coghill, D. The validity, reliability and normative scores of the parent, teacher and self report versions of the Strengths and Difficulties Questionnaire in China. Child Adolesc. Psychiatry Ment. Health 2008, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, M.H.; Vogels, A.G.; de Wolff, M.S.; Reijneveld, S.A. Characteristics of the strengths and difficulties questionnaire in preschool children. Pediatrics 2013, 131, e446–e454. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal diagrams for epidemiologic research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahab, B.; Lanes, A.; Feldman, M.; Tamim, H. Prevalence and predictors of 6-month exclusive breastfeeding among Canadian women: A national survey. BMC Pediatr. 2010, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, Z.; Zhan, Y.; Wang, Y.; Ma, S.; Zhang, S.; Liu, J.; Wu, S.; Feng, Y.; Chen, Y.; et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth 2020, 20, 390. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xie, J.; Jiao, X.C.; Ma, S.S.; Liu, Y.; Yin, W.J.; Tao, R.X.; Hu, H.L.; Zhang, Y.; Chen, X.X.; et al. Maternal Glycemia During Pregnancy and Early Offspring Development: A Prospective Birth Cohort Study. J. Clin. Endocrinol. Metab. 2021, 106, 2279–2290. [Google Scholar] [CrossRef]
- Maher, G.M.; O’Keeffe, G.W.; Kenny, L.C.; Kearney, P.M.; Dinan, T.G.; Khashan, A.S. Hypertensive disorders of pregnancy and risk of neurodevelopmental disorders in the offspring: A systematic review and meta-analysis protocol. BMJ Open 2017, 7, e018313. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Z.; Neiderhiser, J.M.; Uchiyama, M.; Okawa, M. Low birth weight, developmental milestones, and behavioral problems in Chinese children and adolescents. Psychiatry Res. 2001, 101, 115–129. [Google Scholar] [CrossRef]
- Johnson, S.; Marlow, N. Preterm birth and childhood psychiatric disorders. Pediatr. Res. 2011, 69 Pt 2, 11r–18r. [Google Scholar] [CrossRef]
- Wallenborn, J.T.; Levine, G.A.; Carreira Dos Santos, A.; Grisi, S.; Brentani, A.; Fink, G. Breastfeeding, Physical Growth, and Cognitive Development. Pediatrics 2021, 147, e2020008029. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Torres Pacheco, M.; Orsati, F.T.; Laurence, P.G.; da Cunha Gonçalves, H.M.; da Silva Ribeiro, T.G.; Pacheco, M.B.; Vantini, M.A.B.; da Silva, P.B.; Tomás, R.C.; de Abreu, P.E.; et al. Low weight, socioeconomics and behavioral issues: Examining a population in the Northeast of Brazil. Heliyon 2019, 5, e02399. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, X.; Huang, K.; Yan, S.; Ma, L.; Cao, H.; Gan, H.; Tao, F. Early childhood screen time as a predictor of emotional and behavioral problems in children at 4 years: A birth cohort study in China. Environ. Health Prev. Med. 2021, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Morales-Muñoz, I.; Lemola, S.; Saarenpää-Heikkilä, O.; Kylliäinen, A.; Pölkki, P.; Paunio, T.; Broome, M.R.; Paavonen, E.J. Parent-reported early sleep problems and internalising, externalising and dysregulation symptoms in toddlers. BMJ Paediatr. Open 2020, 4, e000622. [Google Scholar] [CrossRef] [PubMed]
- Natamba, B.K.; Sanchez, S.E.; Gelaye, B.; Williams, M.A. Concordance between self-reported pre-pregnancy body mass index (BMI) and BMI measured at the first prenatal study contact. BMC Pregnancy Childbirth 2016, 16, 187. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.E.; Carlson, J.M.; Kebede, N.; Werler, M.M.; Janulewicz, P.A. Pre-pregnancy body mass index and parent and teacher-reported behavioral outcomes among offspring in childhood. Neurotoxicol. Teratol. 2022, 89, 107049. [Google Scholar] [CrossRef] [PubMed]
- Menting, M.D.; van de Beek, C.; de Rooij, S.R.; Painter, R.C.; Vrijkotte, T.G.M.; Roseboom, T.J. The association between pre-pregnancy overweight/obesity and offspring’s behavioral problems and executive functioning. Early Hum. Dev. 2018, 122, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, T.; Schulze-Edinghausen, M.; Turnwald, E.M.; Janoschek, R.; Bae-Gartz, I.; Zentis, P.; Handwerk, M.; Wohlfarth, M.; Schauss, A.; Hucklenbruch-Rother, E.; et al. Effect of Maternal Obesity in Mice on IL-6 Levels and Placental Endothelial Cell Homeostasis. Nutrients 2020, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.M.; Rasmussen, J.M.; Rudolph, M.D.; Heim, C.M.; Gilmore, J.H.; Styner, M.; Potkin, S.G.; Entringer, S.; Wadhwa, P.D.; Fair, D.A.; et al. Maternal Systemic Interleukin-6 During Pregnancy Is Associated With Newborn Amygdala Phenotypes and Subsequent Behavior at 2 Years of Age. Biol. Psychiatry 2018, 83, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.D.; Graham, A.M.; Feczko, E.; Miranda-Dominguez, O.; Rasmussen, J.M.; Nardos, R.; Entringer, S.; Wadhwa, P.D.; Buss, C.; Fair, D.A. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 2018, 21, 765–772. [Google Scholar] [CrossRef]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Mire, E. Maternal obesity and developmental programming of neuropsychiatric disorders: An inflammatory hypothesis. Brain Neurosci. Adv. 2021, 5, 23982128211003484. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.R.; Kim, D.W.; Glendining, K.A.; Jasoni, C.L. Maternal obesity and IL-6 lead to aberrant developmental gene expression and deregulated neurite growth in the fetal arcuate nucleus. Endocrinology 2014, 155, 2566–2577. [Google Scholar] [CrossRef]
- Hieronimus, B.; Ensenauer, R. Influence of maternal and paternal pre-conception overweight/obesity on offspring outcomes and strategies for prevention. Eur. J. Clin. Nutr. 2021, 75, 1735–1744. [Google Scholar] [CrossRef]
- Liu, P.; Xu, L.; Wang, Y.; Zhang, Y.; Du, Y.; Sun, Y.; Wang, Z. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes. Rev. 2016, 17, 1091–1102. [Google Scholar] [CrossRef]
- Duncan, A.F.; Matthews, M.A. Neurodevelopmental Outcomes in Early Childhood. Clin. Perinatol. 2018, 45, 377–392. [Google Scholar] [CrossRef]
- Bartal, T.; Adams, M.; Natalucci, G.; Borradori-Tolsa, C.; Latal, B. Behavioral problems in very preterm children at five years of age using the Strengths and Difficulties Questionnaire: A multicenter cohort study. Early Hum. Dev. 2020, 151, 105200. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Gall, J.G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J. Mol. Biol. 1978, 120, 33–53. [Google Scholar] [CrossRef]
- Bosquet Enlow, M.; Bollati, V.; Sideridis, G.; Flom, J.D.; Hoxha, M.; Hacker, M.R.; Wright, R.J. Sex differences in effects of maternal risk and protective factors in childhood and pregnancy on newborn telomere length. Psychoneuroendocrinology 2018, 95, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.S.; Plusquin, M.; Gyselaers, W.; De Vivo, I.; Nawrot, T.S. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016, 14, 148. [Google Scholar] [CrossRef]
- Costa Dde, S.; Rosa, D.V.; Barros, A.G.; Romano-Silva, M.A.; Malloy-Diniz, L.F.; Mattos, P.; de Miranda, D.M. Telomere length is highly inherited and associated with hyperactivity-impulsivity in children with attention deficit/hyperactivity disorder. Front. Mol. Neurosci. 2015, 8, 28. [Google Scholar] [CrossRef]
- Henje Blom, E.; Han, L.K.; Connolly, C.G.; Ho, T.C.; Lin, J.; LeWinn, K.Z.; Simmons, A.N.; Sacchet, M.D.; Mobayed, N.; Luna, M.E.; et al. Peripheral telomere length and hippocampal volume in adolescents with major depressive disorder. Transl. Psychiatry 2015, 5, e676. [Google Scholar] [CrossRef]
- Li, Z.; Tang, J.; Li, H.; Chen, S.; He, Y.; Liao, Y.; Wei, Z.; Wan, G.; Xiang, X.; Xia, K.; et al. Shorter telomere length in peripheral blood leukocytes is associated with childhood autism. Sci. Rep. 2014, 4, 7073. [Google Scholar] [CrossRef]
- Wojcicki, J.M.; Heyman, M.B.; Elwan, D.; Shiboski, S.; Lin, J.; Blackburn, E.; Epel, E. Telomere length is associated with oppositional defiant behavior and maternal clinical depression in Latino preschool children. Transl. Psychiatry 2015, 5, e581. [Google Scholar] [CrossRef]
- Graf, A.E.; Lallier, S.W.; Waidyaratne, G.; Thompson, M.D.; Tipple, T.E.; Hester, M.E.; Trask, A.J.; Rogers, L.K. Maternal high fat diet exposure is associated with increased hepcidin levels, decreased myelination, and neurobehavioral changes in male offspring. Brain Behav. Immun. 2016, 58, 369–378. [Google Scholar] [CrossRef]
- Guilloty, N.I.; Soto, R.; Anzalota, L.; Rosario, Z.; Cordero, J.F.; Palacios, C. Diet, Pre-pregnancy BMI, and Gestational Weight Gain in Puerto Rican Women. Matern. Child Health J. 2015, 19, 2453–2461. [Google Scholar] [CrossRef]
- Motoki, N.; Inaba, Y.; Shibazaki, T.; Misawa, Y.; Ohira, S.; Kanai, M.; Kurita, H.; Tsukahara, T.; Nomiyama, T. Insufficient maternal gestational weight gain and infant neurodevelopment at 12 months of age: The Japan Environment and Children’s Study. Eur. J. Pediatr. 2022, 181, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Dong, J.; Ma, X.; He, Y. Prevalence and risk factors of underweight, overweight and obesity in women of reproductive age in China. Zhonghua Lin Chuang Ying Yang Za Zhi 2022, 30, 79–86. [Google Scholar]
- Kuruczova, D.; Klanova, J.; Jarkovsky, J.; Pikhart, H.; Bienertova-Vasku, J. Socioeconomic characteristics, family structure and trajectories of children’s psychosocial problems in a period of social transition. PLoS ONE 2020, 15, e0234074. [Google Scholar] [CrossRef] [PubMed]
- Gete, D.G.; Waller, M.; Mishra, G.D. Pre-pregnancy diet quality and its association with offspring behavioral problems. Eur. J. Nutr. 2021, 60, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Volk, R.J.; Mendoza, T.R.; Hoover, D.S.; Nishi, S.P.E.; Choi, N.J.; Bevers, T.B. Reliability of self-reported smoking history and its implications for lung cancer screening. Prev. Med. Rep. 2020, 17, 101037. [Google Scholar] [CrossRef]
- Murray, A.L.; Speyer, L.G.; Hall, H.A.; Valdebenito, S.; Hughes, C. Teacher Versus Parent Informant Measurement Invariance of the Strengths and Difficulties Questionnaire. J. Pediatr. Psychol. 2021, 46, 1249–1257. [Google Scholar] [CrossRef]
| Maternal and Children’s Characteristics | Boys (n = 1944) | Girls (n = 1704) | Statistical Value | p-Values |
|---|---|---|---|---|
| Mater nal characteristic | ||||
| Maternal age/years (M ± SD) | 26.9 ± 3.4 | 26.8 ± 3.5 | 1.147 | 0.273 |
| Maternal education level (n/%) | ||||
| Junior high school and below | 459/23.6 | 408/23.9 | 1.128 | 0.770 |
| High school or technical secondary school | 561/28.9 | 465/27.3 | ||
| Junior college | 507/26.1 | 455/26.7 | ||
| Bachelor degree and above | 417/21.4 | 376/22.1 | ||
| Family monthly income per capita/RMB yuan (n/%) a | 4.759 | 0.093 | ||
| <2000 | 1102/56.7 | 1022/60.0 | ||
| 2000–4000 | 703/36.2 | 558/32.8 | ||
| >4000 | 139/7.1 | 123/7.2 | ||
| Parity (n/%) | 2.077 | 0.150 | ||
| Primiparity | 1837/94.50 | 1628/95.5 | ||
| Multiparity | 107/5.5 | 76/4.5 | ||
| Previous adverse pregnancy outcomes (n/%) | 0.524 | 0.469 | ||
| Did not have | 1000/51.4 | 897/52.6 | ||
| Had | 944/48.6 | 807/47.4 | ||
| Pregnancy complications (n/%) a | 0.138 | 0.710 | ||
| Did not have | 1607/84.8 | 1415/85.3 | ||
| Had | 287/15.2 | 244/14.7 | ||
| Night shift six months before pregnancy (n/%) | 1.107 | 0.293 | ||
| Yes | 325/16.7 | 263/15.4 | ||
| No | 1619/83.3 | 1441/84.6 | ||
| Contraceptive drug use six months before pregnancy (n/%) a | 0.245 | 0.621 | ||
| Yes | 195/10.1 | 163/9.6 | ||
| No | 1741/89.9 | 1538/90.4 | ||
| Maternal smoking six months before pregnancy (n/%) | 0.701 | 0.403 | ||
| Yes | 80/4.1 | 61/3.6 | ||
| No | 1864/95.9 | 1643/96.4 | ||
| Maternal drinking six months before pregnancy (n/%) | 0.013 | 0.908 | ||
| Yes | 75/3.9 | 67/3.9 | ||
| No | 1869/96.1 | 1637/96.1 | ||
| Chil dren’s characteristics | ||||
| Gestational age/week (M±SD) a | 39.0 ± 1.3 | 39.1 ± 1.3 | 3.760 | 0.020 |
| Birth weight/kg (M±SD) a | 3.5 ± 0.5 | 3.3 ± 0.4 | 2.411 | <0.001 |
| Exclusive breastfeeding within six months after birth (n/%) a | 0.081 | 0.777 | ||
| Yes | 372/19.5 | 333/19.9 | ||
| No | 1532/80.5 | 1339/80.1 | ||
| Number of children in family (n/%) | 4.991 | 0.025 | ||
| One child | 1791/92.1 | 1534/90.0 | ||
| More than one child | 153/7.9 | 170/10.0 | ||
| Time spent in watching screen/hours per day (M ± SD) | 0.7 ± 0.4 | 0.6 ± 0.4 | 3.313 | <0.001 |
| Time spent in outdoor activities/hours per day (M ± SD) | 1.8 ± 1.0 | 1.6 ± 1.0 | 0.032 | <0.001 |
| Sleeping time overnight/hours per day a (M ± SD) | 8.9 ± 0.8 | 9.0 ± 0.8 | 2.953 | 0.142 |
| Sex | Groups of Maternal Pre-Pregnancy BMI | Emotional Symptoms | Conduct Problems | Hyperactivity | Peer Problems | Total Difficult Problems | Pro-Social Behaviors |
|---|---|---|---|---|---|---|---|
| Boys (n = 1944) | ≤18.64 | 20 (4.1) | 47 (9.7) | 44 (9.1) | 21 (4.3) | 42 (8.6) | 71 (14.6) |
| 18.65–19.90 | 18 (3.7) | 41 (8.4) | 49 (10.0) | 19 (3.9) | 37 (7.6) | 72 (14.8) | |
| 19.91–21.48 | 33 (6.6) | 40 (8.0) | 35 (7.0) | 17 (3.4) | 35 (7.0) | 72 (14.3) | |
| ≥21.49 | 25 (5.4) | 29 (6.2) | 46 (9.9) | 16 (3.4) | 39 (8.4) | 49 (10.5) | |
| Girls (n = 1704) | ≤18.55 | 38 (8.8) | 30 (7.0) | 33 (7.7) | 8 (1.9) | 30 (7.0) | 28 (6.5) |
| 18.56–19.81 | 29 (6.9) | 28 (6.6) | 23 (5.4) | 10 (2.4) | 26 (6.1) | 33 (7.8) | |
| 19.82–21.48 | 32 (7.2) | 30 (6.8) | 32 (7.2) | 7 (1.6) | 39 (8.8) | 43 (9.7) | |
| ≥21.49 | 34 (8.4) | 29 (7.1) | 16 (3.9) | 5 (1.2) | 29 (7.1) | 30 (7.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Hao, X.; Zhu, L.; Guo, Y.; Wu, X.; Hao, J.; Tao, F.; Huang, K. Non-Linear and Sex-Specific Effect of Maternal Pre-Pregnancy BMI on Emotional and Behavioral Development of Preschool Children: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 13414. https://doi.org/10.3390/ijerph192013414
Lu J, Hao X, Zhu L, Guo Y, Wu X, Hao J, Tao F, Huang K. Non-Linear and Sex-Specific Effect of Maternal Pre-Pregnancy BMI on Emotional and Behavioral Development of Preschool Children: A Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(20):13414. https://doi.org/10.3390/ijerph192013414
Chicago/Turabian StyleLu, Jingru, Xuemei Hao, Linlin Zhu, Yufan Guo, Xiaoyan Wu, Jiahu Hao, Fangbiao Tao, and Kun Huang. 2022. "Non-Linear and Sex-Specific Effect of Maternal Pre-Pregnancy BMI on Emotional and Behavioral Development of Preschool Children: A Population-Based Cohort Study" International Journal of Environmental Research and Public Health 19, no. 20: 13414. https://doi.org/10.3390/ijerph192013414
APA StyleLu, J., Hao, X., Zhu, L., Guo, Y., Wu, X., Hao, J., Tao, F., & Huang, K. (2022). Non-Linear and Sex-Specific Effect of Maternal Pre-Pregnancy BMI on Emotional and Behavioral Development of Preschool Children: A Population-Based Cohort Study. International Journal of Environmental Research and Public Health, 19(20), 13414. https://doi.org/10.3390/ijerph192013414






