Occurrence and Risk Assessment of Personal PM2.5-Bound Phthalates Exposure for Adults in Hong Kong
Abstract
:1. Introduction
2. Materials and Methods
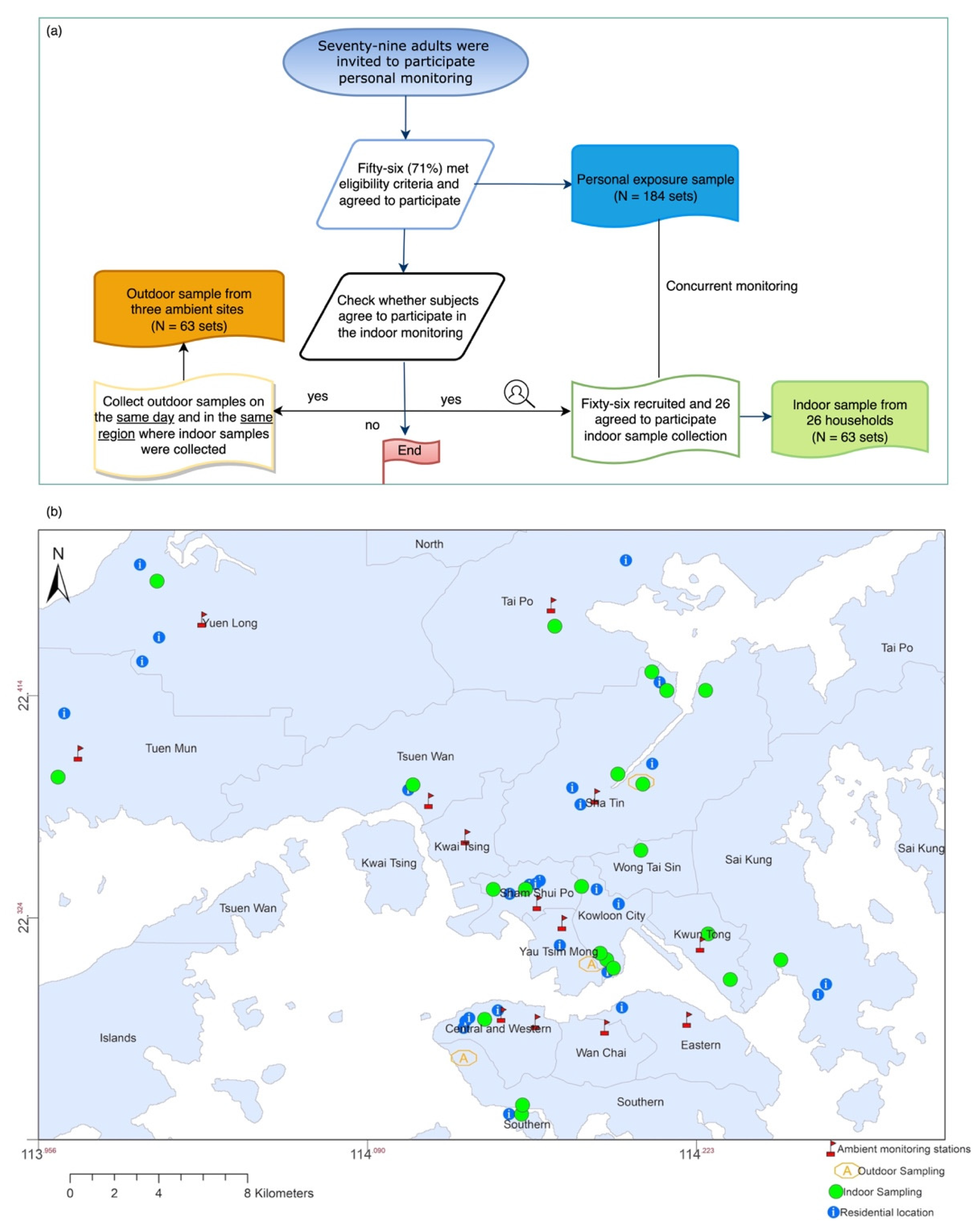
2.1. Participants and Study Design
2.2. Sample Collection
2.3. Phthalates Analysis
2.4. Risk Assessment of Inhalation Exposure to PAEs
2.5. Literature Review and Studies Comparison
2.6. Source Identification
2.7. Statistical Analysis
3. Results
3.1. Characteristics of PAEs in Personal PM2.5
3.2. Characteristics of PAEs in Residential Indoor PM2.5
3.3. Residential Indoor/Outdoor and Personal Exposure/Outdoor Relationships
3.4. Source Identification
3.5. Variations in Exposure Intake of PAEs via Inhalation
3.6. Health Risk Assessment of Inhalation Exposure to Targeted PAE Congeners
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eichler, C.M.A.; Hubal, E.A.C.; Little, J.C. Assessing Human Exposure to Chemicals in Materials, Products and Articles: The International Risk Management Landscape for Phthalates. Environ. Sci. Technol. 2019, 53, 13583–13597. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2097–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsikantami, I.; Sifakis, S.; Tzatzarakis, M.N.; Vakonaki, E.; Kalantzi, O.I.; Tsatsakis, A.M.; Rizos, A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016, 97, 212–236. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group. Some Industrial Chemicals; IARC: World Health Organization International Agency for Research on Cancer: Lyon, France, 2000. [Google Scholar]
- Net, S.; Sempere, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Eales, J.; Bethel, A.; Galloway, T.; Hopkinson, P.; Morrissey, K.; Short, R.E.; Garside, R. Human health impacts of exposure to phthalate plasticizers: An overview of reviews. Environ. Int. 2022, 158, 106903. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyu, L.; Tao, Y.; Ju, H.; Chen, J. Health risks of phthalates: A review of immunotoxicity. Environ. Pollut. 2022, 313, 120173. [Google Scholar] [CrossRef]
- Caldwell, J.C. DEHP: Genotoxicity and potential carcinogenic mechanisms—A review. Mutat. Res. 2012, 751, 82–157. [Google Scholar] [CrossRef]
- U.S. EPA. Butyl Benzyl Phthalate (BBP) (CASRN 85-68-7); U.S. Environmental Protection Agency, Environmental Protection Agency: Washington, DC, USA, 1987.
- Kim, S.H.; Park, M.J. Phthalate exposure and childhood obesity. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, B.; Zhu, Q.; Zhao, Y.; Ge, W.; Zhao, Y.; Song, Q.; Zhou, Y.; Shi, H.; Zhang, Y. Phthalate exposure and childhood overweight and obesity: Urinary metabolomic evidence. Environ. Int. 2018, 121, 159–168. [Google Scholar] [CrossRef]
- You, H.H.; Song, G. Review of endocrine disruptors on male and female reproductive systems. Comp. Biochem. Physiol. 2021, 244, 109002. [Google Scholar] [CrossRef]
- Radke, E.G.; Braun, J.M.; Meeker, J.D.; Cooper, G.S. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 2018, 121, 764–793. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cai, W.; Liu, H.; Jiang, H.; Bi, Y.; Wang, H. The Association of Bisphenol A and Phthalates with Risk of Breast Cancer: A Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 2375. [Google Scholar] [CrossRef] [PubMed]
- Ahern, T.P.; Broe, A.; Lash, T.L.; Cronin-Fenton, D.P.; Ulrichsen, S.P.; Christiansen, P.M.; Cole, B.F.; Tamimi, R.M.; Sørensen, H.T.; Damkier, P. Phthalate exposure and breast cancer incidence: A Danish nationwide cohort study. J. Clin. Oncol. 2019, 37, 1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Sun, C.; Lu, R.; Zou, Z.; Liu, W.; Huang, C. Associations between phthalic acid esters in household dust and childhood asthma in Shanghai, China. Environ. Res. 2021, 200, 111760. [Google Scholar] [CrossRef]
- Li, M.C.; Chen, C.H.; Guo, Y.L. Phthalate esters and childhood asthma: A systematic review and congener-specific meta-analysis. Environ. Pollut. 2017, 229, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Strum, M.; Scheffe, R. National review of ambient air toxics observations. J. Air Waste Manag. Assoc. 2016, 66, 120–133. [Google Scholar] [CrossRef]
- Yang, Y.; Pun, V.C.; Sun, S.; Lin, H.; Mason, T.G.; Qiu, H. Particulate matter components and health: A literature review on exposure assessment. J. Public Health Emerg. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Wang, G. Airborne particulate endocrine disrupting compounds in China: Compositions, size distributions and seasonal variations of phthalate esters and bisphenol A. Atmos. Res. 2015, 154, 138–145. [Google Scholar] [CrossRef]
- Rudel, R.A.; Dodson, R.E.; Perovich, L.J.; Morello-Frosch, R.; Camann, D.E.; Zuniga, M.M.; Yau, A.Y.; Just, A.C.; Brody, J.G. Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two northern California communities. Environ. Sci. Technol. 2010, 44, 6583–6590. [Google Scholar] [CrossRef]
- van Drooge, B.L.; Rivas, I.; Querol, X.; Sunyer, J.; Grimalt, J.O. Organic Air Quality Markers of Indoor and Outdoor PM2.5 Aerosols in Primary Schools from Barcelona. Int. J. Environ. Res. Public Health 2020, 17, 3685. [Google Scholar] [CrossRef]
- Otake, T.; Yoshinaga, J.; Yanagisawa, Y. Exposure to phthalate esters from indoor environment. J. Expo. Anal. Environ. Epidemiol. 2004, 14, 524–528. [Google Scholar] [CrossRef]
- Kang, Y.; Man, Y.B.; Cheung, K.C.; Wong, M.H. Risk assessment of human exposure to bioaccessible phthalate esters via indoor dust around the Pearl River Delta. Environ. Sci. Technol. 2012, 46, 8422–8430. [Google Scholar] [CrossRef] [PubMed]
- Clausen, P.A.; Liu, Z.; Kofoed-Sorensen, V.; Little, J.; Wolkoff, P. Influence of temperature on the emission of di-(2-ethylhexyl)phthalate (DEHP) from PVC flooring in the emission cell FLEC. Environ. Sci. Technol. 2012, 46, 909–915. [Google Scholar] [CrossRef]
- Giovanoulis, G.; Bui, T.; Xu, F.; Papadopoulou, E.; Padilla-Sanchez, J.A.; Covaci, A.; Haug, L.S.; Cousins, A.P.; Magner, J.; Cousins, I.T.; et al. Multi-pathway human exposure assessment of phthalate esters and DINCH. Environ. Int. 2018, 112, 115–126. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.C.; Ward, T.J.; Ho, K.F.; Sarkar, C.; Webster, C. Characteristics and health risks of personal exposure to particle-bound PAHs for Hong Kong adult residents: From ambient pollution to indoor exposure. Indoor Air 2022, 32, 12956. [Google Scholar] [CrossRef]
- Fan, Z.L.; Pun, V.C.; Chen, X.C.; Hong, Q.; Tian, L.W.; Ho, S.S.H.; Lee, S.C.; Tse, L.A.; Ho, K.F. Personal exposure to fine particles (PM 2.5) and respiratory inflammation of common residents in Hong Kong. Environ. Res. 2018, 164, 24–31. [Google Scholar] [CrossRef]
- Ho, S.S.; Yu, J.Z.; Chow, J.C.; Zielinska, B.; Watson, J.G.; Sit, E.H.; Schauer, J.J. Evaluation of an in-injection port thermal desorption-gas chromatography/mass spectrometry method for analysis of non-polar organic compounds in ambient aerosol samples. J. Chromatogr. A 2008, 1200, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ward, T.J.; Sarkar, C.; Ho, K.F.; Webster, C. Health risks of adults in Hong Kong related to inhalation of particle-bound heavy metal(loid)s. Air Qual. Atmos. Health 2022, 15, 691–706. [Google Scholar] [CrossRef]
- Phillips, L.; Moya, J. The evolution of EPA’s Exposure Factors Handbook and its future as an exposure assessment resource. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 13–21. [Google Scholar] [CrossRef]
- U.S. EPA. Exposure Factors Handbook 2011; US Environmental Protection Agency: Washington, DC, USA, 2011.
- OEHHA. Air Toxics Hot Spots Program Technical Support Document for Cancer Potencies, Appendix B. In Chemical-Specific Summaries of the Informaiton Used to Derive Unit Risk and Cancer Potency Values; California Office of Environmental Health Hazard Assessment: Sacramento, CA, USA, 2009. [Google Scholar]
- Chen, Y.; Lv, D.; Li, X.; Zhu, T. PM2.5-bound phthalates in indoor and outdoor air in Beijing: Seasonal distributions and human exposure via inhalation. Environ. Pollut. 2018, 241, 369–377. [Google Scholar] [CrossRef]
- Chen, X.C.; Jahn, H.J.; Engling, G.; Ward, T.J.; Kraemer, A.; Ho, K.F.; Yim, S.H.L.; Chan, C.Y. Chemical characterization and sources of personal exposure to fine particulate matter (PM 2.5) in the megacity of Guangzhou, China. Environ. Pollut. 2017, 231, 871–881. [Google Scholar] [CrossRef]
- Chen, X.C.; Ward, T.J.; Cao, J.J.; Lee, S.C.; Chow, J.C.; Lau, G.N.C.; Yim, S.H.L.; Ho, K.F. Determinants of personal exposure to fine particulate matter (PM2.5) in adult subjects in Hong Kong. Sci. Total Environ. 2018, 628–629, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Chuang, H.C.; Ward, T.J.; Tian, L.; Cao, J.J.; Ho, S.S.; Lau, N.C.; Hsiao, T.C.; Yim, S.H.; Ho, K.F. Indoor, outdoor, and personal exposure to PM2.5 and their bioreactivity among healthy residents of Hong Kong. Environ. Res. 2020, 188, 109780. [Google Scholar] [CrossRef]
- Xu, H.; Léon, J.-F.; Liousse, C.; Guinot, B.; Yoboué, V.; Akpo, A.B.; Adon, J.; Ho, K.F.; Ho, S.S.H.; Li, L.; et al. Personal exposure to PM 2.5 emitted from typical anthropogenic sources in southern West Africa: Chemical characteristics and associated health risks. Atmos. Chem. Phys. 2019, 19, 6637–6657. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Xu, H.; Feng, R.; Shen, Z.; Li, Y.; Zhang, Y.; Sun, J.; Zhang, Q.; Zhang, T.; Yang, L.; et al. Characteristics of indoor and personal exposure to particulate organic compounds emitted from domestic solid fuel combustion in rural areas of northwest China. Atmos. Res. 2021, 248, 105181. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Wang, J.; Ho, S.S.H.; He, K.; Shen, Z.; Ning, Z.; Sun, J.; Li, L.; Lei, R.; et al. Personal exposure to PM2.5-bound organic species from domestic solid fuel combustion in rural Guanzhong Basin, China: Characteristics and health implication. Chemosphere 2019, 227, 53–62. [Google Scholar] [CrossRef]
- Huang, L.; Qiao, Y.; Deng, S.; Zhou, M.; Zhao, W.; Yue, Y. Airborne phthalates in indoor environment: Partition state and influential built environmental conditions. Chemosphere 2020, 254, 126782. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Ji, Y.; Jiao, J.; Zou, D.; Liu, L.; Shan, C.; Bai, Z.; Sun, Z. Phthalate esters (PAEs) in indoor PM10/PM2.5 and human exposure to PAEs via inhalation of indoor air in Tianjin, China. Atmos. Environ. 2014, 85, 139–146. [Google Scholar] [CrossRef]
- Rakkestad, K.E.; Dye, C.J.; Yttri, K.E.; Holme, J.A.; Hongslo, J.K.; Schwarze, P.E.; Becher, R. Phthalate levels in Norwegian indoor air related to particle size fraction. J. Environ. Monit. 2007, 9, 1419–1425. [Google Scholar] [CrossRef]
- Song, M.; Chi, C.; Guo, M.; Wang, X.; Cheng, L.; Shen, X. Pollution levels and characteristics of phthalate esters in indoor air of offices. J. Environ. Sci. 2015, 28, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.G.; Morello-Frosch, R.; Zota, A.; Brown, P.; Perez, C.; Rudel, R.A. Linking exposure assessment science with policy objectives for environmental justice and breast cancer advocacy: The northern California household exposure study. Am. J. Public Health 2009, 99 (Suppl. 3), S600–S609. [Google Scholar] [CrossRef]
- Wang, J.; Guinot, B.; Dong, Z.; Li, X.; Xu, H.; Xiao, S.; Ho, S.S.H.; Liu, S.; Cao, J. PM2.5-bound polycyclic aromatic hydrocarbons (PAHs), oxygenated-PAHs and phthalate esters (PAEs) inside and outside middle school classrooms in Xi’an, China: Concentration, characteristics and health risk assessment. Aerosol Air Qual. Res. 2017, 17, 1811–1824. [Google Scholar] [CrossRef]
- Ouyang, X.; Xia, M.; Shen, X.; Zhan, Y. Pollution characteristics of 15 gas-and particle-phase phthalates in indoor and outdoor air in Hangzhou. J. Environ. Sci. 2019, 86, 107–119. [Google Scholar] [CrossRef]
- Bjerregaard, R. Commission regulation (EC) No 2268/95 of 27 September 1995 concerning the 2nd list of priority substances as foreseen under Council Regulation (EEC) No 793/93. Off. J. Eur. Union L 1995, 231, 18–19. [Google Scholar]
- Wang, Y.; Ding, D.; Shu, M.; Wei, Z.; Wang, T.; Zhang, Q.; Ji, X.; Zhou, P.; Dan, M. Characteristics of indoor and outdoor fine phthalates during different seasons and haze periods in Beijing. Aerosol Air Qual. Res. 2019, 19, 364–374. [Google Scholar] [CrossRef]
- Johns, L.E.; Cooper, G.S.; Galizia, A.; Meeker, J.D. Exposure assessment issues in epidemiology studies of phthalates. Environ. Int. 2015, 85, 27–39. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wang, Q.; He, W.; Xu, F. Phthalate esters (PAEs) in atmospheric particles around a large shallow natural lake (Lake Chaohu, China). Sci. Total Environ. 2019, 687, 297–308. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for di (2-Ethylhexyl) Phthalate; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2002. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp9.pdf (accessed on 12 January 2022).
- Zhou, X.; Lian, J.; Cheng, Y.; Wang, X. The gas/particle partitioning behavior of phthalate esters in indoor environment: Effects of temperature and humidity. Environ. Res. 2021, 194, 110681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Qiu, T.; Tang, S.; Li, J.; Giesy, J.P.; Zhu, Y.; Hu, X.; Xu, D. PM2.5 bound phthalates in four metropolitan cities of China: Concentration, seasonal pattern and health risk via inhalation. Sci. Total Environ. 2019, 696, 133982. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Zeng, Y.; Wang, T.; Chen, S.J.; Guan, Y.F.; Mai, B.X. PM2.5-bound phthalates and phthalate substitutes in a megacity of southern China: Spatioseasonal variations, source apportionment, and risk assessment. Environ. Sci. Pollut. Res. Int. 2022, 29, 37737–37747. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, T.; Su, K.; Harner, T. A first look at atmospheric concentrations and temporal trends of phthalates in distinct urban sectors of the Greater Toronto Area. Atmos. Pollut. Res. 2021, 12, 173–182. [Google Scholar] [CrossRef]
- Aylward, L.L.; Hays, S.M.; Gagné, M.; Krishnan, K. Derivation of Biomonitoring Equivalents for di (2-ethylhexyl) phthalate (CAS No. 117-81-7). Regul. Toxicol. Pharmacol. 2009, 55, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef]
- Wang, L.; Gong, M.; Xu, Y.; Zhang, Y. Phthalates in dust collected from various indoor environments in Beijing, China and resulting non-dietary human exposure. Build. Environ. 2017, 124, 315–322. [Google Scholar] [CrossRef]
- Bu, Z.; Mmereki, D.; Wang, J.; Dong, C. Exposure to commonly-used phthalates and the associated health risks in indoor environment of urban China. Sci. Total Environ. 2019, 658, 843–853. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Koch, H.M. Refined reference doses and new procedures for phthalate mixture risk assessment focused on male developmental toxicity. Int. J. Hyg. Environ. Health 2020, 224, 113428. [Google Scholar] [CrossRef]
- Zhu, Z.; Ji, Y.; Zhang, S.; Zhao, J.; Zhao, J. Phthalate ester concentrations, sources, and risks in the ambient air of Tianjin, China. Aerosol Air Qual. Res. 2016, 16, 2294–2301. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Wang, L.; Tao, W.; Liu, M.; Zhang, P.; Zhang, S.; Li, X.; Lu, X. Phthalate esters in atmospheric PM2.5 and PM10 in the semi-arid city of Xi’an, Northwest China: Pollution characteristics, sources, health risks, and relationships with meteorological factors. Chemosphere 2020, 242, 125226. [Google Scholar] [CrossRef]
- Elzein, A.; Stewart, G.J.; Swift, S.J.; Nelson, B.S.; Crilley, L.R.; Alam, M.S.; Reyes-Villegas, E.; Gadi, R.; Harrison, R.M.; Hamilton, J.F.; et al. A comparison of PM 2.5-bound polycyclic aromatic hydrocarbons in summer Beijing (China) and Delhi (India). Atmos. Chem. Phys. 2020, 20, 14303–14319. [Google Scholar] [CrossRef]
- Shivani; Gadi, R.; Sharma, S.K.; Mandal, T.K.; Kumar, R.; Mona, S.; Kumar, S. Levels and sources of organic compounds in fine ambient aerosols over National Capital Region of India. Environ. Sci. Pollut. Res. 2018, 25, 31071–31090. [Google Scholar] [CrossRef] [PubMed]
- Gadi, R.; Shivani; Sharma, S.K.; Mandal, T.K. Source apportionment and health risk assessment of organic constituents in fine ambient aerosols (PM2.5): A complete year study over National Capital Region of India. Chemosphere 2019, 221, 583–596. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Watanabe, T.; Horimoto, Y.; Ishii, K.; Naito, S. Measurements of 50 non-polar organic compounds including polycyclic aromatic hydrocarbons, n-alkanes and phthalate esters in fine particulate matter (PM2.5) in an industrial area of Chiba prefecture, Japan. Asian J. Atmos. Environ. 2018, 12, 274–288. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, G.H.; Wang, M.X.; Cao, J.J.; Sun, T.; Cheng, C.L.; Meng, J.J.; Hu, T.F.; Liu, S.X. Abundance, composition and source of atmospheric PM2.5 at a remote site in the Tibetan Plateau, China. Tellus B Chem. Phys. Meteorol. 2013, 65, 20281. [Google Scholar]
- Kong, S.; Ji, Y.; Liu, L.; Chen, L.; Zhao, X.; Wang, J.; Bai, Z.; Sun, Z. Spatial and temporal variation of phthalic acid esters (PAEs) in atmospheric PM10 and PM2.5 and the influence of ambient temperature in Tianjin, China. Atmos. Environ. 2013, 74, 199–208. [Google Scholar] [CrossRef]
- Larsen, J.C.; Larsen, P.B.; Hester, R.E.; Harrison, R.M. (Eds.) Chemical Carcinogens. In Air Pollution and Health; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 33–56. [Google Scholar]
- Li, P.-H.; Jia, H.-Y.; Wang, Y.; Li, T.; Wang, L.; Li, Q.-Q.; Yang, M.-M.; Yue, J.-J.; Yi, X.-L.; Guo, L.-Q. Characterization of PM2.5-bound phthalic acid esters (PAEs) at regional background site in northern China: Long-range transport and risk assessment. Sci. Total Environ. 2019, 659, 140–149. [Google Scholar] [CrossRef]
- Li, X.; An, Z.; Shen, Y.; Yuan, Y.; Duan, F.; Jiang, J. Dynamic variations of phthalate esters in PM2.5 during a pollution episode. Sci. Total Environ. 2022, 810, 152269. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhu, Z. Pollution characteristics, sources, and health risk of atmospheric phthalate esters in a multi-function area of Hangzhou, China. Environ. Sci. Pollut. Res. Int. 2021, 28, 8615–8625. [Google Scholar] [CrossRef]
- Lu, S.; Kang, L.; Liao, S.; Ma, S.; Zhou, L.; Chen, D.; Yu, Y. Phthalates in PM2.5 from Shenzhen, China and human exposure assessment factored their bioaccessibility in lung. Chemosphere 2018, 202, 726–732. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.-L.; Guo, Y.; Wu, Q.; Yang, M.; Wu, M.-H.; Kannan, K. Phthalate diesters in Airborne PM2.5 and PM10 in a suburban area of Shanghai: Seasonal distribution and risk assessment. Sci. Total Environ. 2014, 497, 467–474. [Google Scholar] [CrossRef]
- Nisbet, I.C.; Lagoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- OEHHA: Technical Support Document for Cancer Potency Factors 2009: Appendix H. Exposure Routes and Studies Used to Derive Cancer Unit Risks and Slope Factors. California Office of Environmental Health Hazard Assessment, California Environmental Protection Agency. Available online: https://oehha.ca.gov/air/crnr/technical-support-document-cancer-potency-factors-2009 (accessed on 6 October 2022).
- Quintana-Belmares, R.O.; Krais, A.M.; Esfahani, B.K.; Rosas-Pérez, I.; Mucs, D.; López-Marure, R.; Bergman, Å.; Alfaro-Moreno, E. Phthalate esters on urban airborne particles: Levels in PM10 and PM2.5 from Mexico City and theoretical assessment of lung exposure. Environ. Res. 2018, 161, 439–445. [Google Scholar] [CrossRef]
- Wang, J.; Dong, Z.; Li, X.; Gao, M.; Ho, S.S.H.; Wang, G.; Xiao, S.; Cao, J. Intra-urban levels, spatial variability, possible sources and health risks of PM2.5 bound phthalate esters in Xi’an. Aerosol Air Qual. Res. 2018, 18, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Wang, S.L.; Fan, C.Q. Atmospheric distribution of particulate-and gas-phase phthalic esters (PAEs) in a Metropolitan City, Nanjing, East China. Chemosphere 2008, 72, 1567–1572. [Google Scholar] [CrossRef]
- Yang, F.; Kawamura, K.; Chen, J.; Ho, K.; Lee, S.; Gao, Y.; Cui, L.; Wang, T.; Fu, P. Anthropogenic and biogenic organic compounds in summertime fine aerosols (PM2.5) in Beijing, China. Atmos. Environ. 2016, 124, 166–175. [Google Scholar] [CrossRef]
- Zhen, Z.; Yin, Y.; Chen, K.; Zhang, X.; Kuang, X.; Jiang, H.; Wang, H.; Cui, Y.; He, C.; Ezekiel, A.O. Phthalate esters in atmospheric PM2.5 at Mount Tai, north China plain: Concentrations and sources in the background and urban area. Atmos. Environ. 2019, 213, 505–514. [Google Scholar] [CrossRef]

| ng/m3 | Concurrent | Total | Total vs. Concurrent | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD a | Median | 95th g | Min–Max b | N c | Mean ± SD | Median | 95th g | Min–Max | N | σ2b (%) d | σ2w (%) e | p-Value f | |
| DMP | 0.10 ± 0.07 | 0.08 | 0.28 | 0.00–0.32 | 56 | 0.11 ± 0.09 | 0.08 | 0.23 | 0.003–0.61 | 170 | 24.5 | 75.5 | 0.62 |
| DEP | 2.10 ± 2.49 | 1.58 | 6.52 | 0.01–15.85 | 58 | 2.13 ± 2.25 | 1.57 | 6.22 | 0.01–15.85 | 176 | 22.3 | 77.7 | 0.94 |
| DnBP | 20.8 ± 20.7 | 13.8 | 72.9 | 0.02–81.6 | 59 | 21.7 ± 21.8 | 14.8 | 63.1 | 0.02–107.0 | 180 | 1.1 | 98.9 | 0.76 |
| BBP | 95.6 ± 153.0 | 7.9 | 420.1 | 0.03–598.1 | 54 | 93.4 ± 143.4 | 11.1 | 394.7 | 0.02–678.3 | 165 | 11.5 | 88.5 | 0.93 |
| DEHP | 579.6 ±719.2 | 390.3 | 2201.6 | 0.27–3002.3 | 57 | 605.7 ± 786.1 | 361.4 | 2433.0 | 0.16–5134.5 | 175 | 14.5 | 85.5 | 0.82 |
| DnOP | 32.9 ± 70.1 | 6.7 | 211.4 | 0.13–353.7 | 52 | 40.1 ± 104.2 | 6.3 | 195.7 | 0.04–929.9 | 153 | 25.5 | 74.5 | 0.58 |
| ∑6PAEs h | 699.4 ± 899.9 | 364.3 | 2978.4 | 0.10–3599.9 | 59 | 732.4 ± 970.2 | 409.7 | 3057.1 | 0.10–6146.7 | 180 | 11.0 | 89.0 | 0.81 |
| Exposure Category | Site (Study Area) | Season | DMP | DEP | DnBP | BBP | DEHP | DnOP | Mean (SD) | Range (Min–Max) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoor (residential) | Beijing, China | Summer | 39.5 | 21.1 | 1879.1 | 11.6 | 341.9 | 5.8 | 2299.0 | nr | Huang et al. (2020) [42] |
| 55.4 | 8.0 | 2973.2 | 2.2 | 1304.9 | 93.1 | 4436.8 | nr | ||||
| Indoor (residential) | Tianjin, China | Mixed | 2.99 | 0.75 | 130.7 | 0.32 | 44.4 | 0.099 | 179.2 | 7.3–1244 | Zhang et al. (2014) [43] |
| Indoor | Norwegian, Norway | nr | — | — | 74 | 11 | 12 | — | 97 | na | Rakkestad et al. (2007) [44] |
| Indoor (office) | Hangzhou, China | Mixed | 223.6 | 269.7 | 321.2 | 153 | 408.3 | nd | 1375.8 | 774–2050 | Song et al. (2015) [45] |
| Indoor (office) | Beijing, China | Spring | 5.2 | 1.6 | 936.2 | 0.4 | 344 | 0.6 | 1288.0 | na | Wang et al. (2019) [27] |
| Beijing, China | Winter | 44.7 | 2.7 | 529.8 | 1.1 | 277.6 | 0.8 | 856.6 | na | ||
| Outdoor | Beijing, China | Spring | 52.3 | 2.6 | 179.7 | 3.1 | 217.4 | 0.7 | 455.8 | na | |
| Beijing, China | Winter | 203.7 | 3.2 | 363.3 | 0.1 | 244.4 | 0.6 | 815.2 | na | ||
| Outdoor | Northern California, US | Mixed | — | — | — | — | 17 | — | na | na | Brody et al. (2009) [46] |
| Northern California, US | Mixed | — | — | — | — | <MDL | — | na | na | ||
| Indoor | Northern California, US | Mixed | — | — | — | — | 79 | — | na | na | |
| Northern California, US | Mixed | — | — | — | — | 56 | — | na | na | ||
| Indoor (school) | Barcelona, Spain | nr | — | — | 270 | — | — | — | na | na | Van Drooge et al. (2020) [22] |
| Outdoor | Barcelona, Spain | nr | — | — | 12 | — | — | — | na | na | |
| Indoor (school) | Barcelona, Spain | nr | — | — | 92 | — | — | — | na | na | |
| Outdoor | Barcelona, Spain | nr | — | — | 9 | — | — | — | na | na | |
| Indoor (school) | Xi’an, China | May | 32.45 | 68.15 | 282 | 43 | 328.2 | 35.15 | 788.9 | nr | Wang et al. (2017) [47] |
| Outdoor | Xi’an, China | May | 34.5 | 41.4 | 186.9 | 41.4 | 236.4 | 33.8 | 574.4 | nr | |
| Indoor (dormitory) | Beijing, China | Mixed | 4.33 | 7.02 | 368 | 7.36 | 65 | 15.9 | 468 | nr | Chen et al. (2018) [35] |
| Indoor (office) | Beijing, China | Mixed | 5.92 | 6.16 | 118 | 10.9 | 123 | 16.3 | 280 | nr | |
| Indoor (residential) | Beijing, China | Mixed | 3.54 | 7.38 | 350 | 41.2 | 84.1 | 11.9 | 498 | nr | |
| Outdoor | Beijing, China | Mixed | 6.15 | 6.8 | 43.5 | 10.1 | 39 | 19.3 | 125 | nr | |
| Indoor (newly decorated residence/ordinary/office) | Hangzhou, China | Mixed | 135.1 | 207.1 | 190.43 | 222.9 | 522.6 | 298.6 | 1576.7 | nr | Ouyang et al. (2019) [48] |
| Outdoor | Hangzhou, China | Mixed | 15.6 | 32.9 | 40 | 45.4 | 104 | 82.9 | 320.8 | nr | |
| Personal | Hong Kong, China | Summer | ++ | ++ | ++ | ++ | ++ | ++ | 471 | 0.1–3800 | Fan et al. (2018) [29] |
| Personal | Abidjan, West Africa | Mixed | 2.2 | 8.3 | 224.8 | 13.8 | 566.4 | 40.9 | 856.4 | nr | Xu et al. (2019) [39] |
| Personal | Abidjan, West Africa | Mixed | 9.6 | 146.5 | 440.7 | 248.2 | 8.1 | 376.3 | 1229.4 | nr | |
| Personal | Cotonou, West Africa | Mixed | 1.9 | 6.8 | 248.2 | 8.1 | 376.3 | 33 | 674.3 | nr | |
| Outdoor, indoor, personal | Hong Kong | Mixed | ++ | ++ | ++ | ++ | ++ | ++ | 606 | 0.2–5135 | Chen et al. (2020) [38] |
| Indoor | Rural Xian, China | Winter | <MDL | <MDL | 2.4 | — | 2.1 | bd | na | na | Li et al. (2019) [41] |
| Outdoor | Winter | <MDL | <MDL | 2 | — | 1.8 | bd | na | na | ||
| Personal | Winter | <MDL | <MDL | 2.8 | — | 3.3 | 0.12 | na | na | ||
| Indoor a | Rural Xian, China | Winter | 4.9 | 1.7 | 0.57 | 1.6 | 25 | 42 | 75.8 | nr | He et al. (2021) [40] |
| Indoor b | Rural Xian, China | Winter | 16 | 4.4 | 0.57 | 3.9 | 16 | 20 | 60.9 | nr | |
| Personal a | Rural Xian, China | Winter | 0 | 0.77 | 0.82 | 0.59 | 35 | 59 | 96.2 | nr | |
| Personal b | Rural Xian, China | Winter | 0.02 | 1.1 | 0.76 | 0.99 | 29 | 32 | 63.9 | nr |
| Indoor (ng/m3) | I/O Ratio (No Unit) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD a | Median | 95th h | Min–Max b | N c | σ2b (%) e | σ2w (%) f | Mean ± SD | Median | Q1–Q3 g | N d | |
| DMP | 0.17 ± 0.17 | 0.12 | 0.43 | 0.01–1.08 | 61 | 30.3 | 69.7 | 1.9 ± 4.3 | 0.81 | 0.52–1.46 | 57 |
| DEP | 3.22 ± 3.07 | 2.21 | 9.25 | 0.05–16.11 | 62 | 23.7 | 76.3 | 2.3 ± 5.8 | 0.84 | 0.36–1.75 | 60 |
| DnBP | 27.1 ± 24.9 | 21.2 | 71.10 | 0.1–129.0 | 63 | 0 | 100 | 4.8 ± 15.2 | 0.69 | 0.34–1.93 | 57 |
| BBP | 65.5 ± 122.5 | 12.9 | 315.6 | 0.2–654.4 | 53 | 0 | 100 | 1.9 ± 3.5 | 0.27 | 0.03–1.71 | 41 |
| DEHP | 582.2 ± 604.8 | 409.5 | 2007.4 | 0.4–2330.6 | 59 | 3.7 | 96.3 | 1.8 ± 3.3 | 0.46 | 0.16–1.75 | 50 |
| DnOP | 20.5 ± 50.1 | 6.4 | 98.9 | 0.1–243.0 | 50 | 1.6 | 98.4 | 2.8 ± 7.5 | 0.45 | 0.06–3.04 | 40 |
| ∑6PAEs i | 646.9 ± 734.1 | 471.8 | 2495.5 | 0.8–3245.4 | 63 | 7.2 | 92.8 | 3.2 ± 11.6 | 0.46 | 0.16–1.76 | 55 |
| Personal Exposure (P) | DMP | DEP | DnBP | BBP | DEHP | DnOP |
|---|---|---|---|---|---|---|
| DMP | 1 | 0.74 ** | 0.51 ** | −0.05 | 0.24 ** | 0.005 |
| DEP | 1 | 0.30 ** | −0.21 | 0.09 | −0.16 | |
| DnBP | 1 | 0.63 ** | 0.81 ** | 0.58 ** | ||
| BBP | 1 | 0.87 ** | 0.90 ** | |||
| DEHP | 1 | 0.90 ** | ||||
| DnOP | 1 | |||||
| Residential indoor (I) | ||||||
| DMP | 1 | 0.72 ** | 0.30 * | −0.003 | 0.30 * | 0.05 |
| DEP | 1 | 0.07 | −0.16 | 0.03 | −0.04 | |
| DnBP | 1 | 0.73 ** | 0.88 ** | 0.76 ** | ||
| BBP | 1 | 0.83 ** | 0.80 ** | |||
| DEHP | 1 | 0.87 ** | ||||
| DnOP | 1 | |||||
| Outdoor (O) | ||||||
| DMP | 1 | 0.68 ** | 0.09 | 0.11 | 0.11 | 0.07 |
| DEP | 1 | −0.0005 | −0.16 | −0.11 | −0.21 | |
| DnBP | 1 | 0.77 ** | 0.90 ** | 0.81 ** | ||
| BBP | 1 | 0.90 ** | 0.93 ** | |||
| DEHP | 1 | 0.93 ** | ||||
| DnOP | 1 | |||||
| P-O | −0.05 | −0.12 | −0.10 | 0.14 | −0.14 | −0.03 |
| I-O | 0.14 | 0.02 | 0.03 | −0.15 | −0.13 | −0.16 |
| P-I | 0.23 | 0.17 | −0.18 | 0.07 | −0.03 | −0.02 |
| Personal Exposure | Residential Indoor | Outdoor | |||||
|---|---|---|---|---|---|---|---|
| Species | PC a 1 | PC2 | PC1 | PC2 | PC3 | PC1 | PC2 |
| DMP | b | 0.93 | b | b | 0.86 | b | 0.87 |
| DEP | b | 0.87 | b | b | 0.91 | b | 0.87 |
| DnBP | 0.50 | 0.52 | b | 0.96 | b | 0.74 | b |
| BBP | 0.91 | b | 0.90 | b | b | 0.90 | b |
| DEHP | 0.89 | b | 0.58 | 0.76 | b | 0.93 | b |
| DnOP | 0.83 | b | 0.93 | b | b | 0.87 | b |
| Eigenvalue | 2.58 | 1.91 | 2.67 | 1.61 | 1.00 | 3.00 | 1.54 |
| % of variance c | 43.0 | 31.9 | 33.5 | 28.0 | 26.8 | 50.0 | 25.7 |
| Personal Exposure | Measured | Estimated | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD a | Median | 95th c | Mean | SD a | Median | 95th c | p-Value b | |
| DMP (μg/kg-day) | 2.2 × 10−5 | 1.8 × 10−5 | 1.6 × 10−5 | 5.5 × 10−5 | 3.8 × 10−5 | 3.9 × 10−5 | 2.9 × 10−5 | 9.5 × 10−5 | 0.006 |
| DEP (μg/kg-day) | 4.6 × 10−4 | 5.1 × 10−4 | 3.1 × 10−4 | 1.5 × 10−3 | 7.6 × 10−4 | 7.0 × 10−4 | 5.0 × 10−4 | 2.3 × 10−3 | 0.01 |
| DnBP (μg/kg-day) | 4.3 × 10−3 | 4.2 × 10−3 | 3.1 × 10−3 | 1.2 × 10−2 | 7.2 × 10−3 | 5.7 × 10−3 | 5.6 × 10−3 | 1.7 × 10−2 | 0.003 |
| BBP (μg/kg-day) | 1.8 × 10−2 | 3.0 × 10−2 | 1.5 × 10−3 | 2.3 × 10−2 | 2.3 × 10−2 | 2.0 × 10−2 | 5.8 × 10−3 | 5.8 × 10−2 | 0.48 |
| DEHP (μg/kg-day) | 1.2 × 10−1 | 1.6 × 10−1 | 7.3 × 10−2 | 5.0 × 10−1 | 1.6 × 10−1 | 1.3 × 10−1 | 1.1 × 10−1 | 4.3 × 10−1 | 0.21 |
| DnOP (μg/kg-day) | 6.0 × 10−3 | 1.3 × 10−2 | 1.1 × 10−3 | 3.9 × 10−2 | 4.7 × 10−3 | 7.8 × 10−3 | 2.1 × 10−3 | 2.0 × 10−2 | 0.55 |
| ∑6PAEs d (μg/kg-day) | 1.4 × 10−1 | 2.0 × 10−1 | 7.6 × 10−2 | 6.2 × 10−1 | 1.7 × 10−1 | 1.6 × 10−1 | 1.2 × 10−1 | 4.9 × 10−1 | 0.35 |
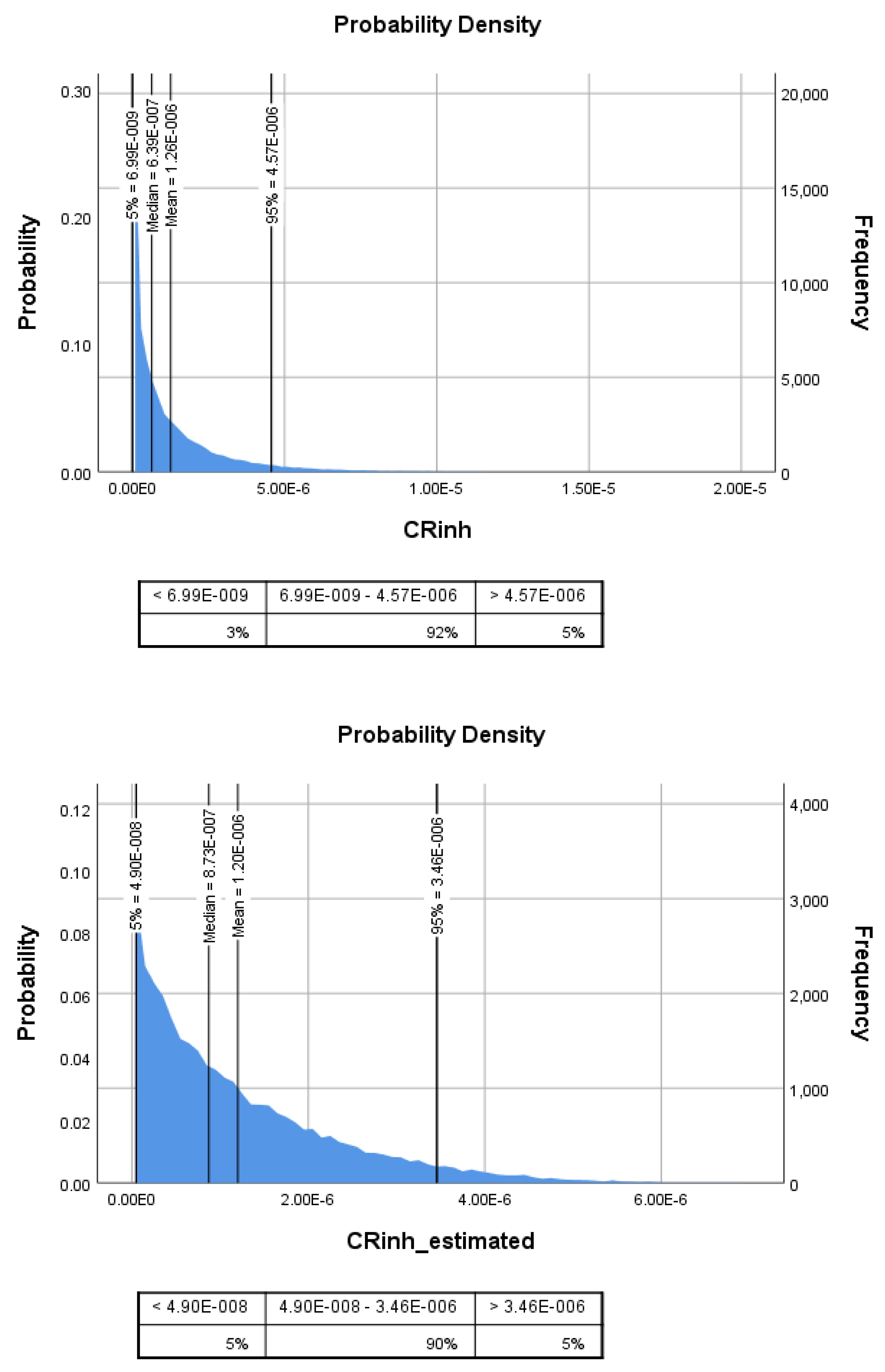
| CRinh for DEHP e | 1.3 × 10−6 | 1.8 × 10−6 | 6.6 × 10−7 | 4.9 × 10−6 | 1.2 × 10−6 | 1.1 × 10−6 | 7.1 × 10−7 | 3.3 × 10−6 | 0.76 |
| CR for DEHP f | 2.1 × 10−5 | 3.0 × 10−5 | 1.6 × 10−5 | 8.0 × 10−5 | 2.0 × 10−5 | 13.8 × 10−5 | 1.2 × 10−5 | 5.5 × 10−5 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Ward, T.J.; Ho, S.S.H.; Ho, K.F. Occurrence and Risk Assessment of Personal PM2.5-Bound Phthalates Exposure for Adults in Hong Kong. Int. J. Environ. Res. Public Health 2022, 19, 13425. https://doi.org/10.3390/ijerph192013425
Chen J, Ward TJ, Ho SSH, Ho KF. Occurrence and Risk Assessment of Personal PM2.5-Bound Phthalates Exposure for Adults in Hong Kong. International Journal of Environmental Research and Public Health. 2022; 19(20):13425. https://doi.org/10.3390/ijerph192013425
Chicago/Turabian StyleChen, Jiayao, Tony J. Ward, Steven Sai Hang Ho, and Kin Fai Ho. 2022. "Occurrence and Risk Assessment of Personal PM2.5-Bound Phthalates Exposure for Adults in Hong Kong" International Journal of Environmental Research and Public Health 19, no. 20: 13425. https://doi.org/10.3390/ijerph192013425






