Galvanic Skin Response Features in Psychiatry and Mental Disorders: A Narrative Review
Abstract
:1. Introduction
2. Electrodermal Activity (EDA)
3. Use of Galvanic Skin Response (GSR) in Psychiatry
4. Modulation of Electrodermal Activity Based on GSR Biofeedback
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sulzer, J.; Sitaram, R.; Blefari, M.; Kollias, S.; Birbaumer, N.; Stephan, K.E.; Luft, A.; Gassert, R. Neurofeedback-mediated self-regulation of the dopaminergic midbrain. NeuroImage 2013, 83, 817–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazarnik, A. Aktywność Eletektrodermalna w Metodzie Biofeedback—Zastosowanie Kliniczne na Przykładzie Padaczki [Electrodermal Activity in the Biofeedback Method—Clinical Use on the Example of Epilepsy]; Jagiellonian University: Kraków, Poland, 2016. [Google Scholar]
- Thompson, M.; Thompson, L. Neurofeedback. Wprowadzenie do Podstawowych Koncepcji Psychofizjologii Stosowanej [Neurofeedback. Introduction to the Basic Concepts of Applied Psychophysiology]; Biomed Neurotechnologie: Wrocław, Poland, 2013. [Google Scholar]
- Konturek, S. Fizjologia Człowieka [Human Phisiology]; Elsevier Urban & Partner: Wrocław, Poland, 2013. [Google Scholar]
- Horowitz, M.A.; Zunszain, P.A. Neuroimmune and neuroendocrine abnormalities in depression: Two sides of the same coin. Ann. N. Y. Acad. Sci. 2015, 1351, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.A.; Malla, A.K.; Norman, R.M. Cognitive functioning in stabilized first-episode psychosis patients. Psychiatry Res. 2001, 104, 118–131. [Google Scholar] [CrossRef]
- Riley, E.M.; McGovern, D.; Mockler, D.; Doku, V.C.K.; OCeallaigh, S.; Fannon, D.G.; Tennakoon, L.; Santamaria, M.; Soni, W.; Morris, R.G.; et al. Neuropsychological functioning in the first-episode psychosis—Evidence of specific deficits. Schizophr. Res. 2000, 43, 47–55. [Google Scholar] [CrossRef]
- Addington, D.; Addington, J.; Robinson, G. Attributional style, and depression in schizophrenia. Can. J. Psychiatry 1999, 44, 697–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, R.S. Why Kraepelin was right: Schizophrenia as a cognitive disorder. Neuropsychiatry Neurophysiol. 2014, 9, 41–47. [Google Scholar]
- Heaton, R.K.; Gladsjo, J.A.; Palmer, B.W.; Kuck, J.; Marcotte, T.D.; Jeste, D.V. Stability, and course of neuropsychological deficits in schizophrenia. Arch. Gen. Psychiatry 2001, 58, 24–32. [Google Scholar] [CrossRef]
- Citrome, L.; Bilder, R.M.; Volavka, J. Managing treatment-resistant schizophrenia: Evidence from randomized clinical trials. J. Psychiatr. Pract. 2002, 8, 205–215. [Google Scholar] [CrossRef]
- Kane, J.M.; Agid, O.; Baldwin, M.L.; Howes, O.; Lindenmayer, J.P.; Marder, S.; Olfson, M.; Potkin, S.G.; Correll, C.U. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J. Clin. Psychiatry 2019, 80, 2783. [Google Scholar] [CrossRef] [Green Version]
- Acheson, D.; Twamley, E.; Young, J.W. Reward learning as a potential target for pharmacological augmentation of cognitive remediation for schizophrenia: A roadmap for preclinical development. Front. Neurosci. 2013, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Lally, J.; Gaughran, F. Treatment resistant schizophrenia—Review and a call to action. Ir. J. Psychol. Med. 2019, 36, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Conjeti, S.; Banerjee, R. A comparative evaluation of neural network classifiers for stress level analysis of automotive drivers using physiological signals. Biomed. Signal Process. Control. 2013, 8, 740–754. [Google Scholar] [CrossRef]
- Zakeri, S.; Abbasi, A.; Goshvarpour, A. The effect of creativity test on the galvanic skin response signal and detection with vector machine. In Proceedings of the International Conference on Research in Engineering Science and Technology, Istanbul, Turkey, 21 July 2015. [Google Scholar]
- Villarejo, M.V.; Zapirain, B.G.; Zorrilla, A.M. A stress sensor based on galvanic skin response (GSR) controlled by ZigBee. Sensors 2012, 12, 6075–6101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumm, J.; Bachlin, M.; Setz, C.; Arnrich, B.; Roggen, D.; Troster, G. Effect of movements on the electrodermal response after a startle event. Methods Inf. Med. 2008, 47, 186–191. [Google Scholar] [CrossRef]
- Aleksander, D.M.; Trengove, C.; Johnston, P.; Cooper, T.; August, J.P.; Gordon, E. Separating individual conductance responses in a short interstimulus-interval paradigm. J. Neurosci. Methods 2005, 146, 116–123. [Google Scholar] [CrossRef]
- Vahey, R.; Bacerra, R. Galvanic skin response in mood disorders: A critical review. Int. J. Psychol. Psychol. Ther. 2015, 15, 275–394. [Google Scholar]
- Lagopoulos, J.; Malhi, G. Impairments in “top-down” processing in bipolar disorder: A simultaneous fMRI-GSR study. Psychiatry Res. Neuroimaging 2011, 192, 100–108. [Google Scholar] [CrossRef]
- Fontanella, L.; Ippoliti, L.; Merla, A. Multiresolution Karhunen Loeve analysis of galvanic skin response for psycho-physiological studies. Metrika 2012, 75, 287–309. [Google Scholar] [CrossRef]
- Braithwaite, J.; Watson, D.; Joner, R.; Rowe, M. Przewodnik do analizy aktywności elektotermicznej (EDA) i odpowiedzi przewodnictwa skóry (SCR) do eksperymentów psychologicznych [A guide fot analysis electrodermal activity (EDA) & skin conductance responses (SCRs) for psychological experiments]. Psychofizjologia 2013, 49, 1017–1034. [Google Scholar]
- Najafpour, E.; Asl-Aminabadi, N.; Nuroloyuni, S.; Jamali, Z.; Shirazi, S. Can galvanic skin conductance be used as an objective indicator of children’s anxiety in the dental setting? J. Clin. Exp. Dent. 2017, 9, e377–e383. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, P.K.; Bond, A.J.; Lader, M.H. Characteristics of galvanic skin response in anxiety states. J. Psychiatr. Res. 1975, 12, 265–270. [Google Scholar] [CrossRef]
- Crider, A. Personality and electrodermal response lability: An interpretation. Appl. Psychophysiol. Biofeedback 2008, 33, 141. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Iacono, W.; Remick, R. Electrodermal activity among subtypes of depression. Biol Psychiatry 1985, 20, 158–162. [Google Scholar] [CrossRef]
- Jandl, M.; Steyer, J.; Kaschka, W.P. Suicide risk markers in major depressive disorder: A study of electrodermal activity and event-related potentials. J. Affect. Disord. 2010, 123, 138–149. [Google Scholar] [CrossRef]
- Xinfang, D.; Xinxin, Y.; Rui, Z.; Cheng, B.; Dai, L.; Guizhong, Y. Classifying major depression patients and healthy controls using EEG, eye tracking and galvanic skin response data. J. Affect. Disord. 2019, 251, 156–161. [Google Scholar] [CrossRef]
- Naszariahi, M.N.; Khaleeda, K.A.; Mortar, A.M. The development of galvanic skin response for depressed people. AIP Conf. Proc. 2020, 2291, 020096. [Google Scholar] [CrossRef]
- Thorell, L.H.; Wolfersdorf, W.; Straub, R.; Steyer, J.; Hodgkinson, S.; Kaschka, W.P.; Jandl, M. Electrodermal hyporeactivity as a trait marker for suicidal propensity in uni- and bipolar depression. J. Psychiatr. Res. 2013, 47, 1925–1931. [Google Scholar] [CrossRef]
- Coryell, W.H. Clinical assessment of suicide risk in depressive disorder. CNS Spectr. 2006, 11, 455–461. [Google Scholar] [CrossRef]
- Iacono, W.G.; Lykken, D.T.; Peloquin, L.J.; Lumry, A.E.; Valentine, R.H.; Tuason, V.B. Electrodermal activity in euthymic unipolar and bipolar affective disorders. Arch. Gen. Psychiatry 1983, 40, 557–565. [Google Scholar] [CrossRef]
- Schneider, D.; Regenbogen, C.; Kellermann, T.; Finkelmeyer, A.; Kohn, N.; Derntl, B.; Schneider, F.; Habel, U. Empathic behavioral and physiological responses to dynamic stimuli in depression. Psychiatry Res. 2012, 200, 294–305. [Google Scholar] [CrossRef]
- Sarchiapone, M.; Gramaglia, C.; Iosue, M.; Carli, V.; Mandelli, L.; Serretti, A.; Marangon, D.; Zeppegno, P. The association between electrodermal activity (EDA) depression and suicidal behavior: A systematic review and narrative synthesis. BMC Psychiatry 2018, 18, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brekke, J.S.; Raine, A.; Ansel, M.; Lencz, T.; Bird, L. Neuropsychological and psychophysiological correlates of psychosocial functioning in schizophrenia. Schizophr. Bull. 1997, 23, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Schell, A.M.; Dawson, M.E.; Rissling, A.; Ventura, J.; Subotnik, K.L.; Gitlin, M.J.; Nuechterlein, K.H. Electrodermal predictors of functional outcome and negative symptoms in schizophrenia. Psychophysiology 2005, 42, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Campo, J.; Merkelbach, H.; Nijman, H.; Yeates-Frederikx, M.; Allertz, W. Skin conductance and schizophrenic symptomatology. Acta Neuropsychiatr. 2000, 12, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, R.; Dobrowolska, B. Initial results of tests using GSR biofeedback as a new neurorehabilitation technology complementing pharmacological treatment of patients with schizophrenia. BioMed. Res. Int. 2021, 2021, 5552937. [Google Scholar] [CrossRef]
- Malhi, G.S.; Lagopoulos, J.; Sachdev, P.S.; Ivanovski, B.; Shnier, R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord. 2005, 5, 58–69. [Google Scholar] [CrossRef]
- Nissen, C.; Holz, J.; Blechert, J.; Feige, B.; Riemann, D.; Voderholzer, U.; Normann, C. Learning as a model of neural plasticity in major depression. Biol. Psychiatry 2010, 68, 544–552. [Google Scholar] [CrossRef]
- Sigmon, S.; Withcomb-Smith, S.; Boulard, N.; Pells, J.J.; Endelfield, T.M.; LaMattina, S.M.; Schartel, J.G. Sensoal reactivity: Attentional bias and psychophysiological arousal in seasonal and nonseasonal depression. Cogn. Ther. Res. 2007, 31, 619–638. [Google Scholar] [CrossRef]
- Falkenberg, I.; Kohn, N.; Schoepker, R.; Rabel, U. Mood induction in depressive patients: A comparative multimensional approach. PLoS ONE 2012, 7, e30016. [Google Scholar] [CrossRef] [Green Version]
- Albus, M.; Muller-Spahn, F.; Ackenheil, M.; Engel, R.R. Psychiatric patients, and their response to experimental stress. Stress Med. 1987, 3, 233–238. [Google Scholar] [CrossRef]
- Brankovic, S.B. System identification of skin conductance response in depression—An attempt to probe the neurochemistry of limbic system. Psychiatr. Danub. 2008, 20, 310–322. [Google Scholar] [PubMed]
- Donat, D.C.; McCullough, J.P. Psychophysiological discriminants of depression at rest and in response to stress. J. Clin. Psychol. 1983, 39, 315–320. [Google Scholar] [CrossRef]
- Jayanthi, A.; Nivedha, R.; Vani, C. Galvanic skin response measurement and analysis. Int. J. Appl. Eng. Res. 2015, 10, 12447–12452. [Google Scholar]
- Phitayakorn, R.; Minehart, R.D.; Pian-Smith, M.C.M.; Hemingway, M.W.; Petrusa, E.R. Practicality of using galvanic skin response to measure intraoperative physiologic autonomic activation in operating room team members. Surgery 2015, 158, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.G.; Doerr, H.O.; Storrie, M.C. Skin conductance: A potentially sensitive test for depression. Psychiatry Res. 1983, 10, 295–302. [Google Scholar] [CrossRef]
- Rosebrock, L.E.; Hoxha, D.; Norris, C.; Cacioppo, J.T.; Gollan, J.K. Skin conductance and subjective arousal in anxiety, depression, and comorbidity: Implications for affective reactivity. J. Psychophysiol. 2017, 31, 145–157. [Google Scholar] [CrossRef]
- Myslobodsky, M.S.; Horesh, N. Bilateral electrodermal activity in depressive patients. Biol. Psychol. 1978, 6, 111–120. [Google Scholar] [CrossRef]
- Topoglu, Y.; Watson, J.; Suri, R.; Ayaz, H. Electrodermal activity in ambulatory settings: A narrative review of literature. In Advances in Neuroergonomics and Cignitive Engineering. AHFE 2019. Advances in Intelligent Systems and Computing; Ayaz, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 953. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Pazderka-Robinson, H.; Morrison, J.W.; Flor-Henry, P. Electrodermal dissociation of chronic fatigue and depression: Evidence for distinct physiological mechanisms. Int. J. Psychophysiol. 2004, 53, 171–182. [Google Scholar] [CrossRef]
- Sławiński, A. GSR Biofeedback Verim 3.0. 2017. Available online: https://verim.pl/wp-content/uploads/2017/05/VERIM-Polski-2017-11-02.pdf (accessed on 4 September 2022).
- Nagai, Y.; Goldstein, L.H.; Fenwick, P.B.C.; Trimble, M.R. Clinical efficacy of galvanic skin response biofeedback training in reducing seizures in adult epilepsy: A preliminary randomized controlled study. Epilepsy Behav. 2004, 5, 216–223. [Google Scholar] [CrossRef]
- Lim, C.L.; Gordon, E.; Rennie, C.; Wright, J.J.; Bahramali, H.; Li, W.M.; Clouston, P.; Morris, G.L. Dynamics of SCR, EEG, and ERP activity in an oddball paradigm with short interstimulus intervals. Psychophysiology 1999, 36, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Jones, C.I.; Sen, A. Galvanic skin response (GSR)/Electrodermal/Skin Conductance Biofeedback on epilepsy: A systematic review and meta-analysis. Front. Neurol. 2019, 10, 377. [Google Scholar] [CrossRef] [Green Version]
- Maren, S. Synaptic mechanisms of associative memory in the amygdala. Neuron 2005, 47, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.L.; Ressler, K.J.; Lu, K.-T.; Davis, M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J. Neurosci. 2002, 22, 2343–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrson, E.; Nanobashvili, A.; Kokaia, Z.; Lindvall, O. Evidence of neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J. Cereb. Blood Flow Metab. 1999, 19, 1220–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Xiong, L.-J.; Tong, Y.; Mao, M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed. Rep. 2013, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Naghara, A.H.; Tuszynski, M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011, 10, 209–219. [Google Scholar] [CrossRef]
- Kossut, M. Mechanizmy Plastyczności Mózgu [Mechanisms of Brain Plasticity]; PZWL: Warszawa, Poland, 1993. [Google Scholar]
- Oh, H.; Lewis, D.A.; Sibille, E. The role of BDNF in age-dependent changes of excitatory and inhibitory synaptic markers in the human prefrontal cortex. Neuropsychopharmacology 2016, 41, 3080–3091. [Google Scholar] [CrossRef] [Green Version]
- Birbaumer, N.; Ruiz, S.; Sitaram, R. Learned regulation of brain metabolism. Trends Cogn. Sci. 2013, 17, 295–302. [Google Scholar] [CrossRef]
- Mathiak, K.A.; koush, Y.; Dyck, M.; Gaber, T.J.; Alawi, E.; Zepf, F.D.; Zvyagubtsev, M.; Mathiak, K. Social reinforcement can regulate localized brain activity. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, S132–S136. [Google Scholar] [CrossRef]
- Blasé, K.; Vermetten, E.; Lehrer, P.; Gevitz, R. Neurophysiological approach by self-control of your stress-related autonomic nervous system with depression, stress, and anxiety patients. Int. J. Environ. Res. Public Health 2021, 18, 3329. [Google Scholar] [CrossRef] [PubMed]
- Zaferi, E.; Dedes, V.; Tzirogianniś, K.; Kandylaki, A.; Polikandrioiti, M.; Panidis, D.; Panoutsopoulos, G. Managing anxiety disorders with the neuro-biofeedback method of Brain Boy Universal Professional. Health Psychol. Res. 2022, 10, 35644. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Aram, J.; Koepp, M.; Lemieux, L.; Mula, M.; Critchley, H.; Sisodiya, S.; Cercignami, M. Epileptic seizures are reduced by autonomic biofeedback therapy through enhancement of fronto-limbic connectivity: A controlled trial and neuroimaging study. EBioMedicine 2018, 27, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Reza Alvani, S.; Levy, P.T.; McCarron, R.; Szeth, S.; Emamirad, R. Paroxymal dyskinesia and electrodermal volatility: The role of mindfulness, self-compassion and psychophysiological interventions. Appl. Neoropsychol. Adult 2022, 1–12. [Google Scholar] [CrossRef]
- Markiewicz, R.; Dobrowolska, B. Cognitive and social rehabilitation in schizophrenia—From neurophysiology to neuromodulation. Pilot study. Int. J. Environ. Res. Public Health 2020, 17, 4034. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, R.; Dobrowolska, B. Reinforcement of self-regulated brain activity in schizophrenia patients undergoing rehabilitation. BioMed. Res. Int. 2021, 2021, 8030485. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, R.; Markiewicz-Gospodarek, A.; Dobrowolska, B.; Łoza, B. Improving clinical, cognitive, and psychosocial dysfunctions in patients with schizophrenia: A neurofeedback randomized control trial. Neural Plast. 2021, 2021, 4488664. [Google Scholar] [CrossRef]
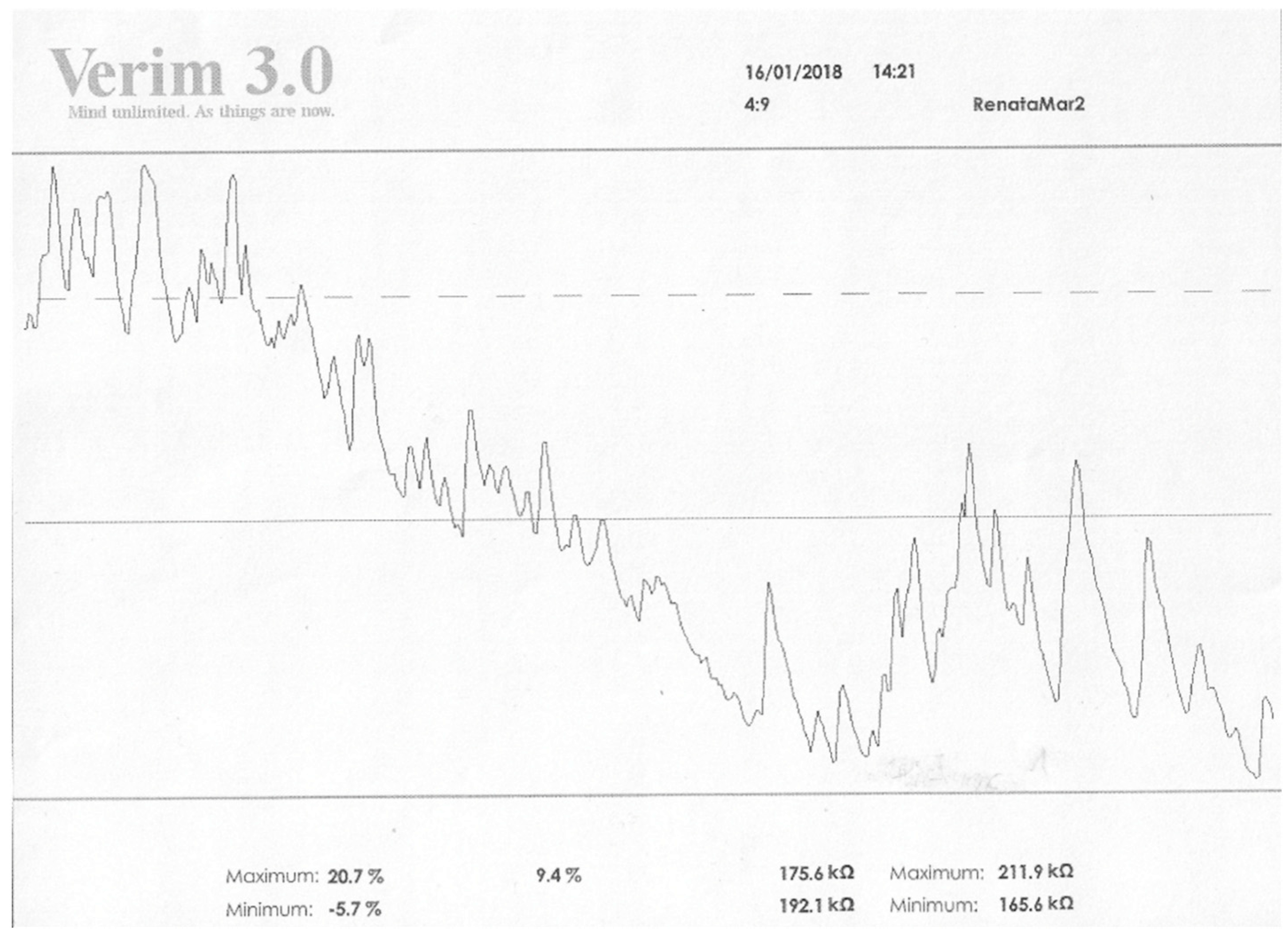

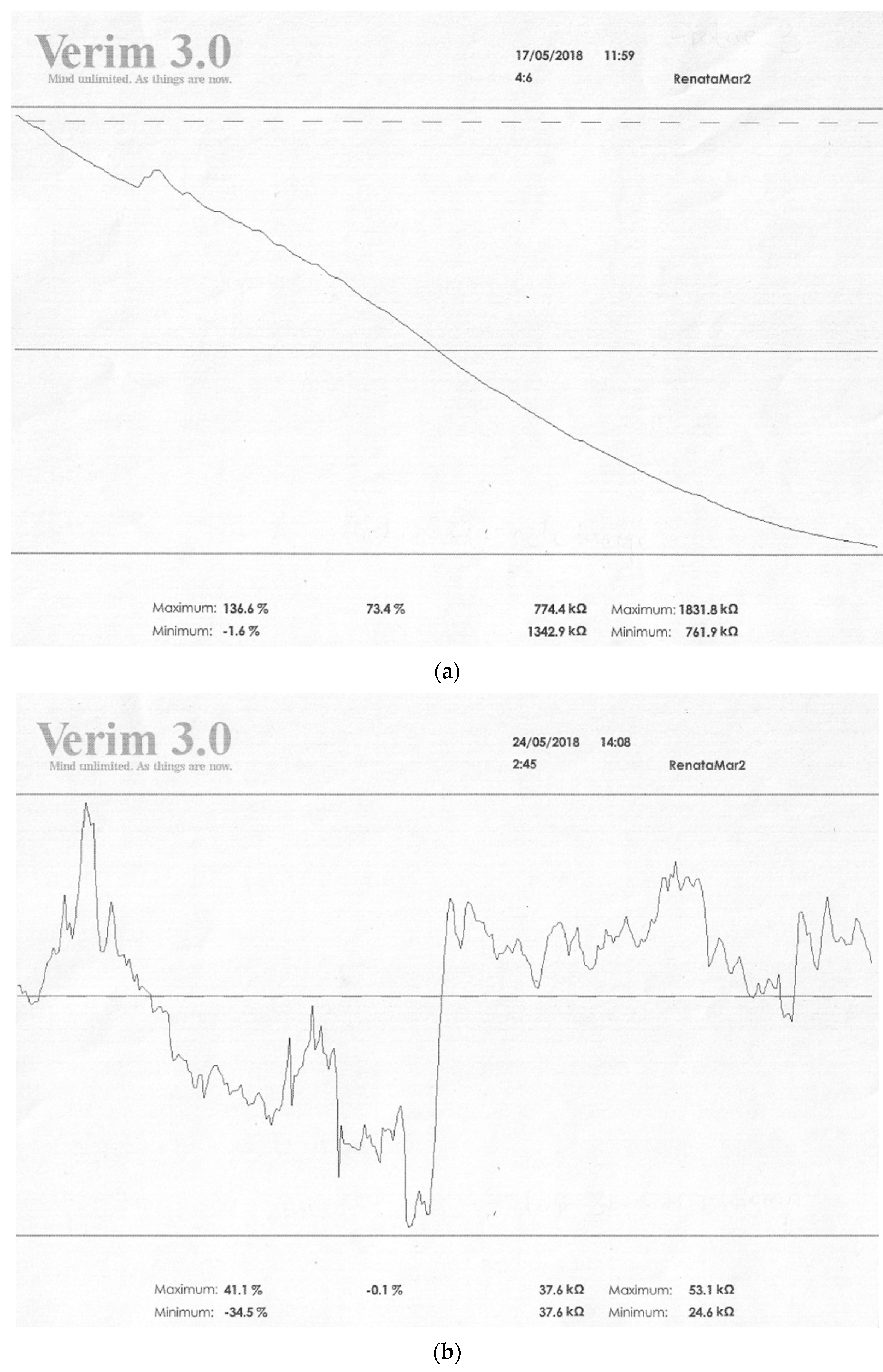
| Type of Mental Disorder | EDA Profile (Skin Conductivity) SCL | EDA Profile (Change in Conductivity) SCR | Ref. |
|---|---|---|---|
| Anxiety disorders | Reduced | SCR lability | [15,25,26] |
| Depressive disorders | Reduced, “flat profile”, deficit of psychological flexibility | Lowered or elevated (remission period) | [27,28,29,30] |
| Suicidal tendencies | Lowered | Lowered | [31,32] |
| Bipolar affective disorder | Value fluctuations, decreased in depression, increased in mania | Stabilization of values in the period of remission | [33,34,35] |
| Schizophrenia | Labile or lowered values (spontaneous, non-specific reaction) | Lowered values (dominance of negative symptoms) or increased values (dominance of positive symptoms) | [36,37,38,39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markiewicz, R.; Markiewicz-Gospodarek, A.; Dobrowolska, B. Galvanic Skin Response Features in Psychiatry and Mental Disorders: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 13428. https://doi.org/10.3390/ijerph192013428
Markiewicz R, Markiewicz-Gospodarek A, Dobrowolska B. Galvanic Skin Response Features in Psychiatry and Mental Disorders: A Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(20):13428. https://doi.org/10.3390/ijerph192013428
Chicago/Turabian StyleMarkiewicz, Renata, Agnieszka Markiewicz-Gospodarek, and Beata Dobrowolska. 2022. "Galvanic Skin Response Features in Psychiatry and Mental Disorders: A Narrative Review" International Journal of Environmental Research and Public Health 19, no. 20: 13428. https://doi.org/10.3390/ijerph192013428
APA StyleMarkiewicz, R., Markiewicz-Gospodarek, A., & Dobrowolska, B. (2022). Galvanic Skin Response Features in Psychiatry and Mental Disorders: A Narrative Review. International Journal of Environmental Research and Public Health, 19(20), 13428. https://doi.org/10.3390/ijerph192013428










