Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.-C.; Werorilangi, S.; Teh, S.J. Anthropogenic Debris in Seafood: Plastic Debris and Fibers from Textiles in Fish and Bivalves Sold for Human Consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N.; Thomson, K.T. Microplastics in the Edible Tissues of Shellfishes Sold for Human Consumption. Chemosphere 2021, 264, 128554. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.J.R.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and Retention of Microplastics by the Shore Crab Carcinus Maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wen, K.; Ding, D.; Liu, J.; Lei, Z.; Chen, X.; Ye, G.; Zhang, J.; Shen, H.; Yan, C.; et al. Size-Dependent Adverse Effects of Microplastics on Intestinal Microbiota and Metabolic Homeostasis in the Marine Medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Mussels along the Coastal Waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]
- Imasha, H.U.E.; Babel, S. Microplastics Contamination in Commercial Green Mussels from Selected Wet Markets in Thailand. Arch. Environ. Contam. Toxicol. 2021, 81, 449–459. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and Nano-Plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Bai, C.L.; Liu, L.Y.; Hu, Y.B.; Zeng, E.Y.; Guo, Y. Microplastics: A Review of Analytical Methods, Occurrence and Characteristics in Food, and Potential Toxicities to Biota. Sci. Total Environ. 2022, 806, 150263. [Google Scholar] [CrossRef]
- Vendel, A.L.; Bessa, F.; Alves, V.E.N.; Amorim, A.L.A.; Patrício, J.; Palma, A.R.T. Widespread Microplastic Ingestion by Fish Assemblages in Tropical Estuaries Subjected to Anthropogenic Pressures. Mar. Pollut. Bull. 2017, 117, 448–455. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of Microplastics by Commercial Fish off the Portuguese Coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Tang, K.H.D. Effects of Microplastics on Agriculture: A Mini-Review. Asian J. Environ. Ecol. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in Fish and Fishmeal: An Emerging Environmental Challenge? Sci. Rep. 2021, 11, 2045. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and Mesoplastic Contamination in Canned Sardines and Sprats. Sci. Total Environ. 2018, 612, 1380–1386. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Galhano, V.; Hartmann, S.; Monteiro, M.S.; Zeumer, R.; Mozhayeva, D.; Steinhoff, B.; Müller, K.; Prenzel, K.; Kunze, J.; Kuhnert, K.-D.; et al. Impact of Wastewater-Borne Nanoparticles of Silver and Titanium Dioxide on the Swimming Behaviour and Biochemical Markers of Daphnia Magna: An Integrated Approach. Aquat. Toxicol. 2020, 220, 105404. [Google Scholar] [CrossRef] [PubMed]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. First Study of Its Kind on the Microplastic Contamination of Soft Drinks, Cold Tea and Energy Drinks—Future Research and Environmental Considerations. Sci. Total Environ. 2020, 726, 138580. [Google Scholar] [CrossRef]
- Hernandez-Milian, G.; Lusher, A.; MacGabban, S.; Rogan, E. Microplastics in Grey Seal (Halichoerus grypus) Intestines: Are They Associated with Parasite Aggregations? Mar. Pollut. Bull. 2019, 146, 349–354. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic Contamination of Packaged Meat: Occurrence and Associated Risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Fadare, O.O.; Wan, B.; Guo, L.-H.; Zhao, L. Microplastics from Consumer Plastic Food Containers: Are We Consuming It? Chemosphere 2020, 253, 126787. [Google Scholar] [CrossRef]
- Dessì, C.; Okoffo, E.D.; O’Brien, J.W.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Rauert, C.; Wang, X.; Thomas, K.V. Plastics Contamination of Store-Bought Rice. J. Hazard. Mater. 2021, 416, 125778. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Ren, J. Analysis of Microplastics in Takeaway Food Containers in China Using FPA-FTIR Whole Filter Analysis. Molecules 2022, 27, 2646. [Google Scholar] [CrossRef] [PubMed]
- Hee, Y.Y.; Weston, K.; Suratman, S. The Effect of Storage Conditions and Washing on Microplastic Release from Food and Drink Containers. Food Packag. Shelf Life 2022, 32, 100826. [Google Scholar] [CrossRef]
- Cella, C.; La Spina, R.; Mehn, D.; Fumagalli, F.; Ceccone, G.; Valsesia, A.; Gilliland, D. Detecting Micro- and Nanoplastics Released from Food Packaging: Challenges and Analytical Strategies. Polymers 2022, 14, 1238. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.; Kocareva, J.; Noack, D.; Kuballa, T. Microplastic Identification in German Beer—An Artefact of Laboratory Contamination? Dtsch. Lebensm.-Rundsch. Z. Für Lebensm. Und Lebensm. 2015, 111, 437–440. [Google Scholar] [CrossRef]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in Take-out Food Containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential Human Health Risks Due to Environmental Exposure to Nano- and Microplastics and Knowledge Gaps: A Scoping Review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Luqman, A.; Nugrahapraja, H.; Wahyuono, R.A.; Islami, I.; Haekal, M.H.; Fardiansyah, Y.; Putri, B.Q.; Amalludin, F.I.; Rofiqa, E.A.; Götz, F.; et al. Microplastic Contamination in Human Stools, Foods, and Drinking Water Associated with Indonesian Coastal Population. Environments 2021, 8, 138. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Zeytin, S.; Wagner, G.; Mackay Roberts, N.; Gerdts, G.; Schuirmann, E.; Klockmann, S.; Slater, M. Quantifying microplastic translocation from feed to the fillet in European sea bass Dicentrarchus labrax. Mar. Pollut. Bull. 2000, 156, 111210. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yang, K.; Cheng, X.; Guo, C.; Xing, X.; Sun, H.; Liu, D.; Liu, X.; Wang, D. Accumulation of Polystyrene Microplastics Induces Liver Fibrosis by Activating CGAS/STING Pathway. Environ. Pollut. 2022, 300, 118986. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, M.; Salmistraro, N.; Porro, D.; Pinsino, A.; Colangelo, A.M.; Gaglio, D. Polystyrene Micro and Nano-Particles Induce Metabolic Rewiring in Normal Human Colon Cells: A Risk Factor for Human Health. Chemosphere 2022, 303, 134947. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.Z.; Poulose, V.; Alsaidi, R.; al Kendi, R.; Iftikhar, S.H.; Mourad, A.-H.I.; Kittaneh, W.F.; Thiemann, T. Plastic Cutting Boards as a Source of Microplastics in Meat. Food Addit. Contam. Part A 2022, 39, 609–619. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning Microplastics into Nanoplastics through Digestive Fragmentation by Antarctic Krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Luís, L.G.; Ferreira, P.; Fonte, E.; Oliveira, M.; Guilhermino, L. Does the Presence of Microplastics Influence the Acute Toxicity of Chromium(VI) to Early Juveniles of the Common Goby (Pomatoschistus microps)? A Study with Juveniles from Two Wild Estuarine Populations. Aquat. Toxicol. 2015, 164, 163–174. [Google Scholar] [CrossRef]
- Wong, J.X.; Gan, S.N.; Aishah, M.J. Chromium (III) based Ziegler Natta catalysts for olefin polymerization. Sains Malays. 2011, 40, 771–779. [Google Scholar]
- Mourad, A.-H.I. Thermo-Mechanical Characteristics of Thermally Aged Polyethylene/Polypropylene Blends. Mater. Des. 2010, 31, 918–929. [Google Scholar] [CrossRef]
- Djakhdane, K.; Dehbi, A.; Mourad, A.-H.I.; Zaoui, A.; Picuno, P. The Effect of Sand Wind, Temperature and Exposure Time on Tri-Layer Polyethylene Film Used as Greenhouse Roof. Plast. Rubber Compos. 2016, 45, 346–351. [Google Scholar] [CrossRef]
- Babaghayou, M.I.; Mourad, A.-H.I.; Cherunurakal, N. Anisotropy Evaluation of LDPE/LLDPE/PIB Trilayer Films. In Proceedings of the 2020 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 4 February–9 April 2020; pp. 1–3. [Google Scholar]
- Khanam, P.N.; AlMaadeed, M.A. Processing and characterization of polyethylene-based composites. Adv. Manufact. Polym. Compos. Sci. 2015, 1, 63–79. [Google Scholar] [CrossRef]
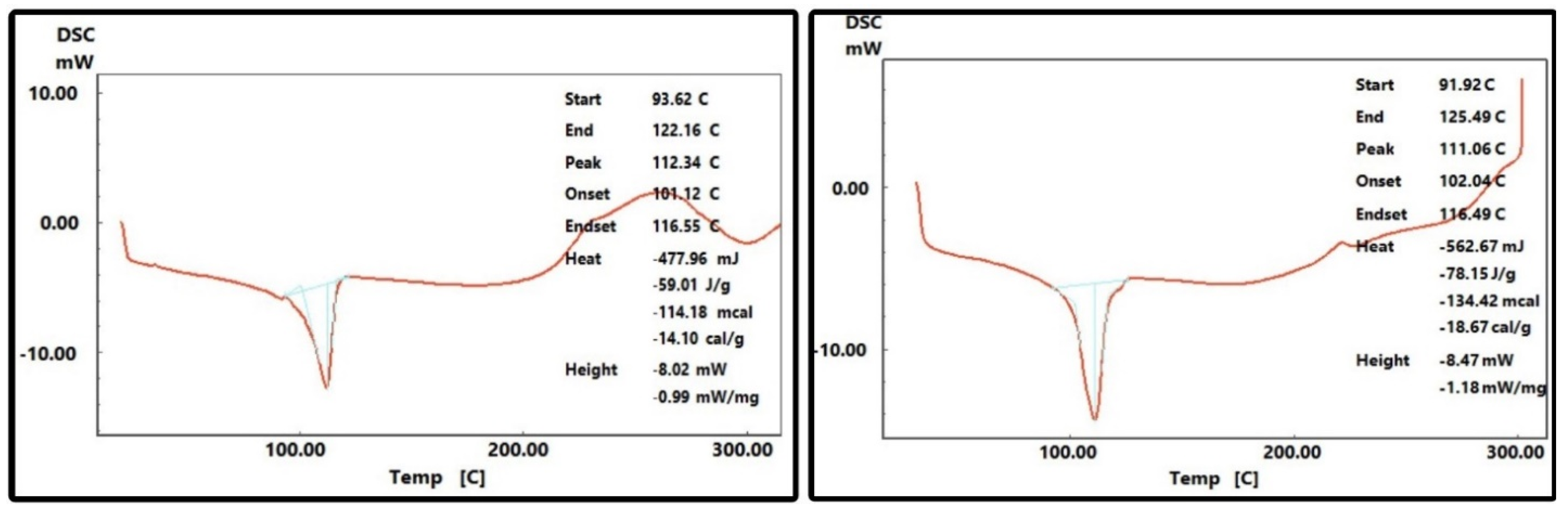
- Majewksy, M.; Bitter, H.; Eiche, E.; Horn, H. Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci. Total Environ. 2016, 568, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, L.; Wang, X.; Yang, X. Effect of Crystallinity of Polyethylene with DifferentDensities on Breakdown Strength and Conductance Property. Materials 2019, 12, 1746. [Google Scholar] [CrossRef]
- Boursillon, D.; Riethmüller, V. The Safety of Wooden Cutting Boards. Br. Food J. 2007, 109, 315–322. [Google Scholar] [CrossRef]
- Ak, N.O.; Cliver, D.O.; Kaspar, C.W. Cutting Boards of Plastic and Wood Contaminated Experimentally with Bacteria. J. Food Prot. 1994, 57, 16–22. [Google Scholar] [CrossRef]
- Food Safety and Inspection Service, USDA. Available online: https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/cutting-boards (accessed on 8 August 2022).
- Garcia-Garin, O.; Vighi, M.; Aguilar, A.; Tsangaris, C.; Digka, N.; Kaberi, H.; Borrell, A. Boops boops as a Bioindicator of Microplastic Pollution along the Spanish Catalan Coast. Mar. Pollut. Bull. 2019, 149, 110648. [Google Scholar] [CrossRef]
- Cheung, P.K.; Fok, L. Characterization of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef]
- Habib, R.Z.; Abdoon, M.; Al Meqbaali, R.; Ghebremedhin, F.; Elkashlan, M.; Kittaneh, W.F.; Cherupurakal, N.; Mourad, A.H.I.; Thiemann, T.; Al Kindi, R. Analysis of microbeads in cosmetic products in the United Arab Emirates. Environ. Pollut. 2020, 258, 113831. [Google Scholar] [CrossRef]
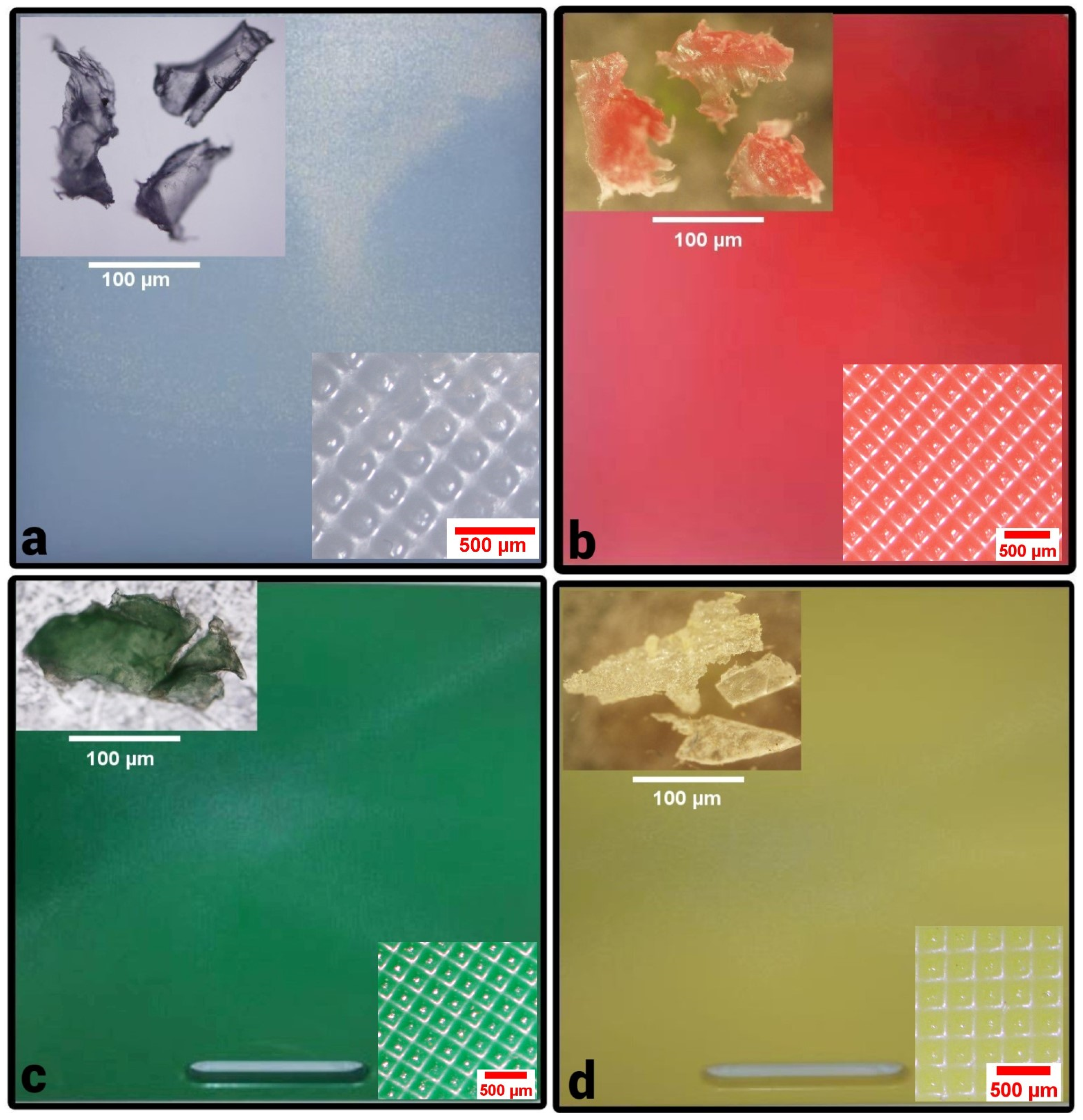
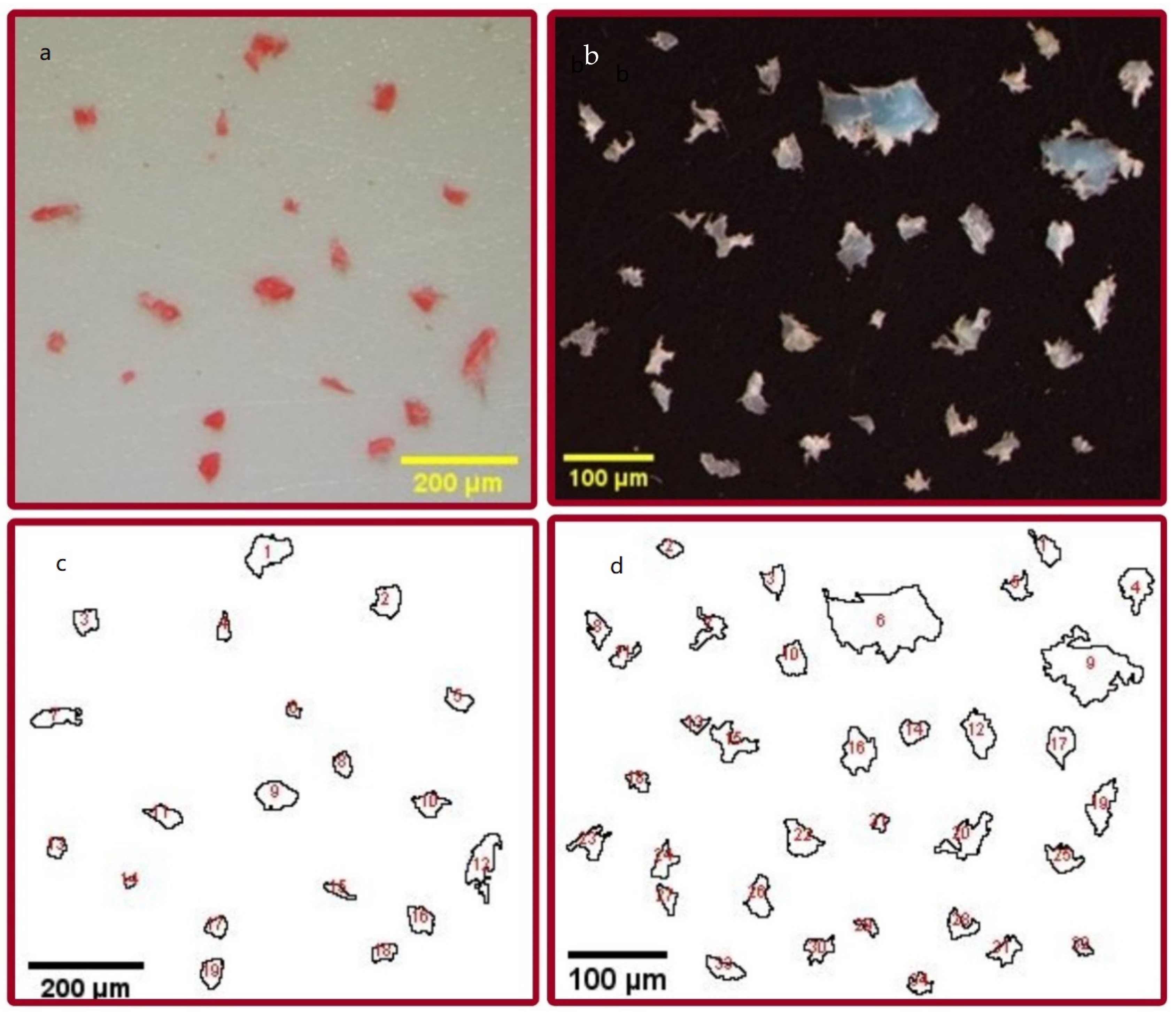
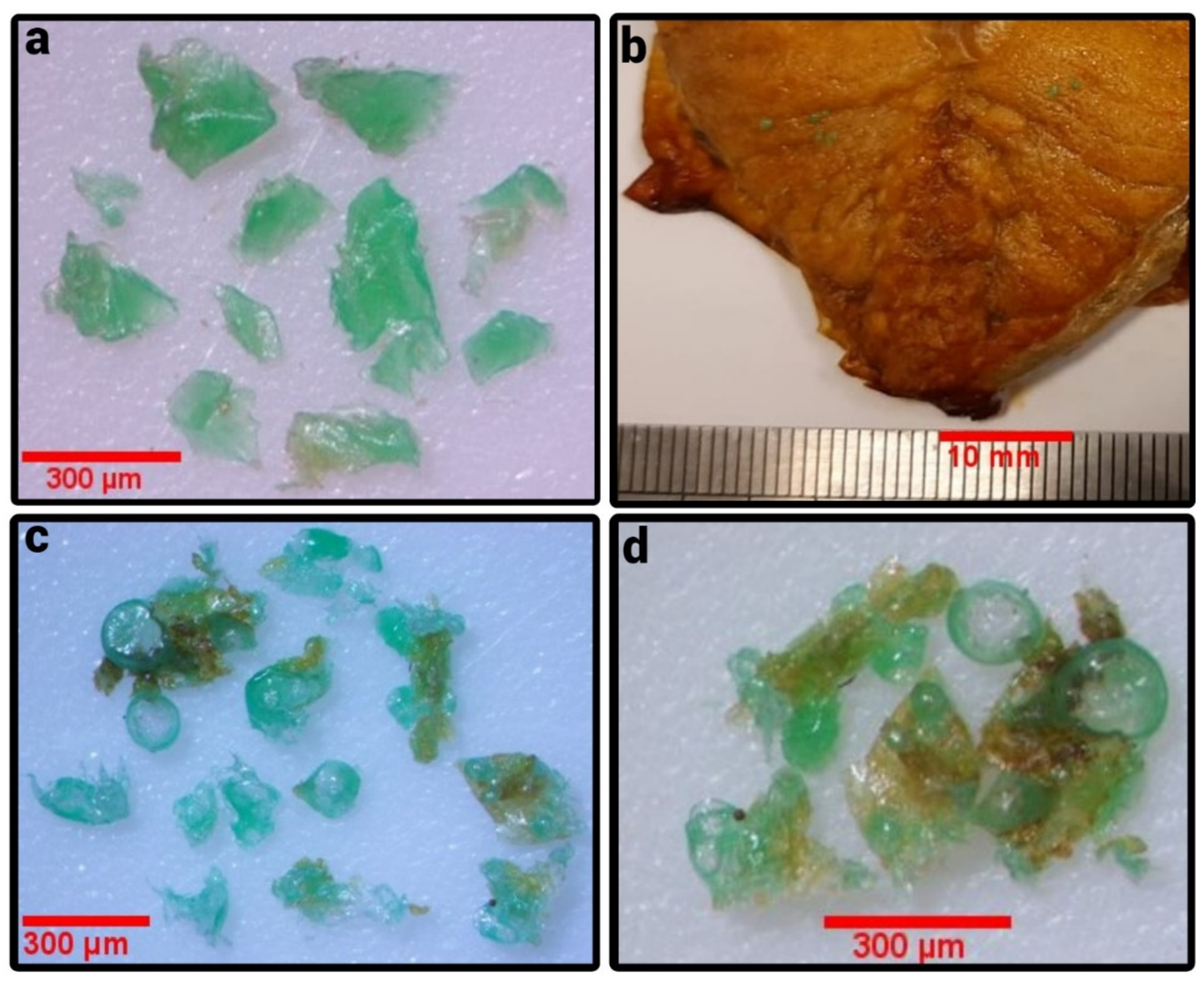

| Sample Name | Wt. (mg) of MPs/g of Food ± SD | No. of MPs Pieces/g of Food ± SD | MPs Size Range (μm) | Mean Size (µm) of MPs ± SD | Color of MPs | Color of CB Used |
|---|---|---|---|---|---|---|
| C1 (UAE) | 0.22 ± 0.11 | 1.1 ± 0.76 | 59.1–249.7 | 117.2 ± 42.5 | white/colorless | white |
| C2 (UAE) | 0.29 ± 0.25 | 1.19 ± 0.72 | 23.7–1454.5 | 148.6 ± 97 | white/colorless | white |
| C3 (UAE) | 0.147 ± 0.06 | 0.9 ± 0.36 | 8.24–984.2 | 95.1 ± 215.1 | red | red |
| C4 (UAE) | 0.11 ± 0.031 | 0.63 ± 0.4 | 33.9–462.3 | 178.3 ± 143.6 | yellow | yellow |
| C5 (UAE) | 0.066 ± 0.025 | 0.52 ± 0.38 | 14.3–152.7 | 74.6 ± 7.8 | yellow | yellow |
| C6 (Kuwait) | 0.01 ± 0.02 | 0.03 ± 0.04 | 34.1 | 11.35 ± 19.7 | green | green |
| Sample Name | Fish | Wt. (mg) of MPs/g of Food ± SD | No. of MPs Pieces/g of Food ± SD | MPs Size Range (μm) | Mean Size (µm) of MPs ± SD | Color of MPs | Color of CB Used |
|---|---|---|---|---|---|---|---|
| F1 (UAE) | Giant trevally (Caranx ignobilis) | 0.074 ± 0.013 | 0.024 ± 0.42 | 62.1–199.5 | 136.4 ± 69.4 | red | red |
| F2 (UAE) | Nile tilapia (Tilapia nilotica) | 0.0041 ± 0.0071 | 0.014 ± 0.024 | 144.47 | 48.2 ± 83.4 | green | green |
| F3 (UAE) | Threadfin bream (Nemipterus bipunctatus) | 0.31 ± 0.47 | 0.52 ± 0.22 | 15.6–94.6 | 52.69 ± 20.6 | green | green |
| F4 (UAE) | Mackarel scad (Decapterus macarellus) | 0 | 0 | 0 | 0 | yellow | yellow |
| F5 (UAE) | Orange-spotted trevally (Carangoides bajad) | 0.097 ± 0.09 | 2.60 ± 2.80 | 16.6–146.3 | 49.4 ± 8.72 | blue | blue |
| F6 (UAE) | King mackerel (Scomberomorus cavalla) | 2.47 ± 0.4 | 1.17 ± 0.57 | 51.8–503.5 | 192.0 ± 89.1 | green | green |
| F6A (UAE) | King mackerel (Scomberomorus cavalla) washed | 0.0022 ± 0.0021 | 0.03 ± 0.03 | 89.7–737 | 309.7 ± 335.3 | green | green |
| F7 (Kuwait) | Atlantic salmon (Salmo salar) | 0.08 ± 0.01 | 0.16 ± 0.06 | 67.7–1151.1 | 544.3 ± 459.8 | white | white |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, R.Z.; Kindi, R.A.; Salem, F.A.; Kittaneh, W.F.; Poulose, V.; Iftikhar, S.H.; Mourad, A.-H.I.; Thiemann, T. Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards. Int. J. Environ. Res. Public Health 2022, 19, 13442. https://doi.org/10.3390/ijerph192013442
Habib RZ, Kindi RA, Salem FA, Kittaneh WF, Poulose V, Iftikhar SH, Mourad A-HI, Thiemann T. Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards. International Journal of Environmental Research and Public Health. 2022; 19(20):13442. https://doi.org/10.3390/ijerph192013442
Chicago/Turabian StyleHabib, Rana Zeeshan, Ruwaya Al Kindi, Feras Al Salem, Wajeeh Faris Kittaneh, Vijo Poulose, Syed Haris Iftikhar, Abdel-Hamid Ismail Mourad, and Thies Thiemann. 2022. "Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards" International Journal of Environmental Research and Public Health 19, no. 20: 13442. https://doi.org/10.3390/ijerph192013442
APA StyleHabib, R. Z., Kindi, R. A., Salem, F. A., Kittaneh, W. F., Poulose, V., Iftikhar, S. H., Mourad, A.-H. I., & Thiemann, T. (2022). Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards. International Journal of Environmental Research and Public Health, 19(20), 13442. https://doi.org/10.3390/ijerph192013442







