Prenatal Depressive Symptoms, Self-Rated Health, and Diabetes Self-Efficacy: A Moderated Mediation Analysis
Abstract
1. Introduction
1.1. Self-Rated Health
1.2. Diabetes Self-Efficacy and Depression
1.3. Research Objectives
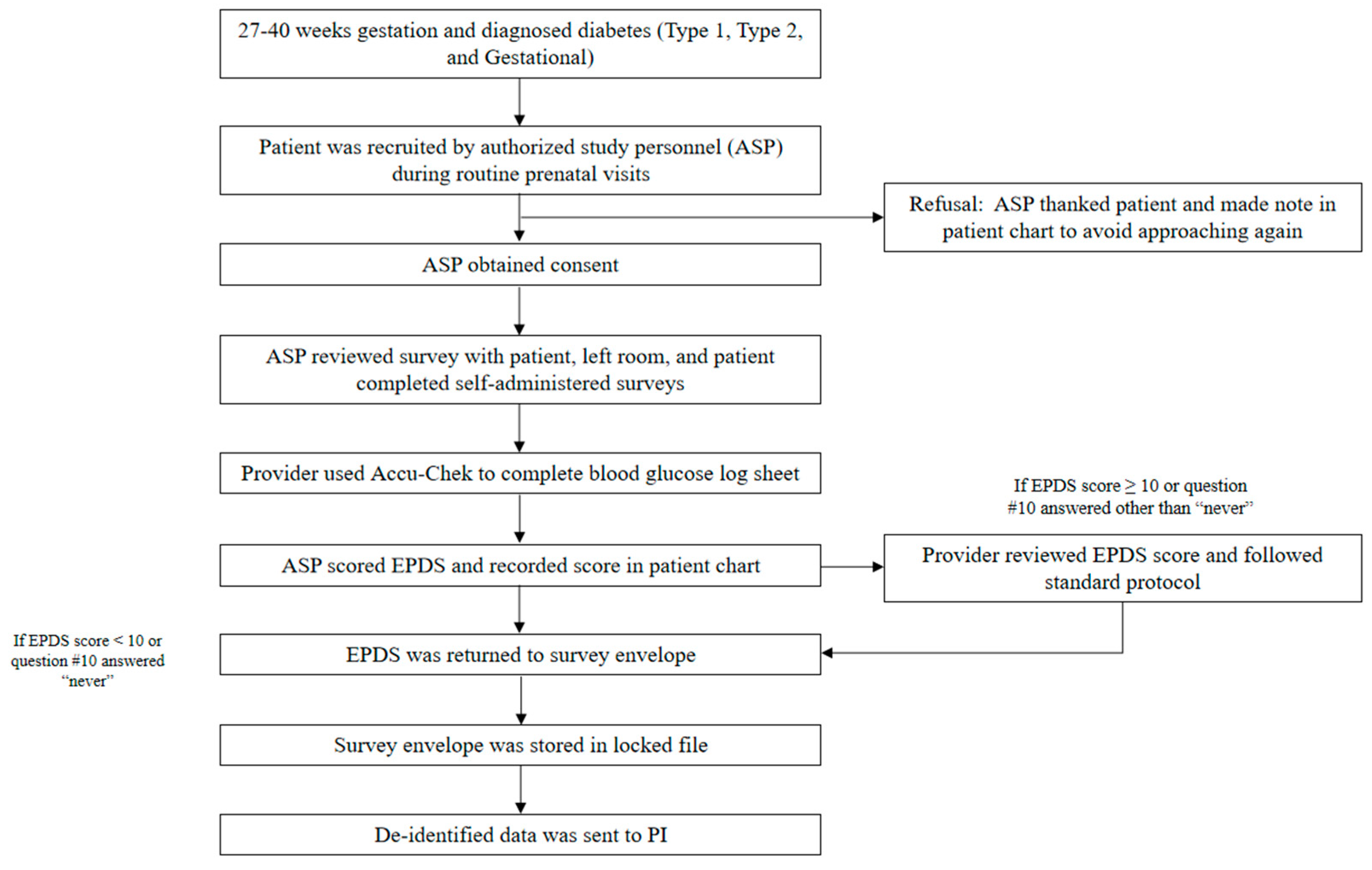
2. Materials and Methods
2.1. Measures
2.1.1. Demographic Questionnaire
2.1.2. Edinburgh Postnatal Depression Scale (EPDS)
2.1.3. Diabetes Self-Efficacy Scale (DSES)
2.1.4. Self-Rated Health (SRH)
2.2. Statistical Analysis Plan
3. Results
3.1. Depressive Symptoms, Self-Rated Health, and Diabetes Self-Efficacy
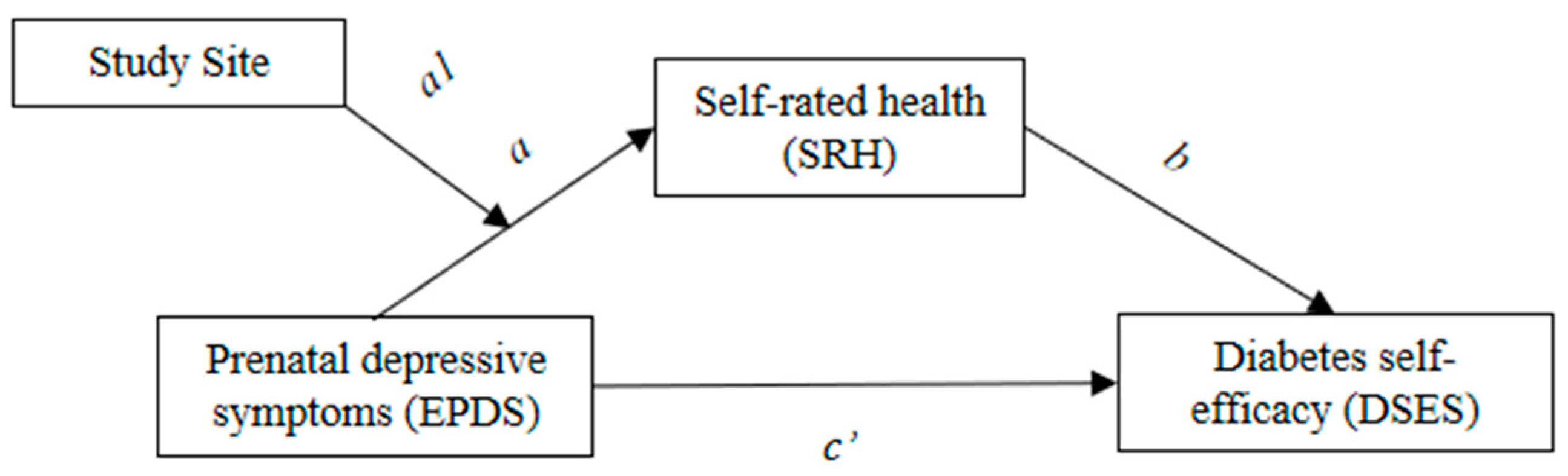
3.2. Mediation Analysis
3.3. Moderated Mediation Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control. Reproductive Health: Diabetes during Pregnancy; Centers for Disease Control: Atlanta, GA, USA, 2018; Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/diabetes-during-pregnancy.htm#:~:text=In%20the%20United%20States%2C%20about,pregnant%20women%20develop%20gestational%20diabetes (accessed on 15 July 2022).
- Acolet, D.; Fleming, K.; Macintosh, M.; Modder, J. Confidential Enquiry into Maternal and Child Health: Pregnancy in Women with Type 1 and Type 2 Diabetes in 2002–03, England, Wales and Northern Ireland; Cemach: London, UK, 2005. [Google Scholar]
- Kunasegaran, T.; Balasubramaniam, V.R.M.T.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. Gestational Diabetes Mellitus in Southeast Asia: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 1272. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Klick, J.; Stratmann, T. Diabetes treatments and moral hazard. J. Law Econ. 2007, 50, 519–538. [Google Scholar] [CrossRef][Green Version]
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Kubik, M.; Li, L. Screening for gestational diabetes: US preventive services task force recommendation statement. JAMA 2021, 326, 531–538. [Google Scholar]
- Association, A.D. 4. Lifestyle management: Standards of medical care in diabetes—2018. Diabetes Care 2018, 41 (Suppl. 1), S38–S50. [Google Scholar] [CrossRef]
- Malaza, N.; Masete, M.; Adam, S.; Dias, S.; Nyawo, T.; Pheiffer, C. A Systematic Review to Compare Adverse Pregnancy Outcomes in Women with Pregestational Diabetes and Gestational Diabetes. Int. J. Environ. Res. Public Health 2022, 19, 10846. [Google Scholar] [CrossRef]
- Carolan, M. Women’s experiences of gestational diabetes self-management: A qualitative study. Midwifery 2013, 29, 637–645. [Google Scholar] [CrossRef]
- Hui, A.L.; Sevenhuysen, G.; Harvey, D.; Salamon, E. Stress and anxiety in women with gestational diabetes during dietary management. Diabetes Educ. 2014, 40, 668–677. [Google Scholar] [CrossRef]
- Miilunpalo, S.; Vuori, I.; Oja, P.; Pasanen, M.; Urponen, H. Self-rated health status as a health measure: The predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. J. Clin. Epidemiol. 1997, 50, 517–528. [Google Scholar] [CrossRef]
- Latham, K.; Peek, C.W. Self-rated health and morbidity onset among late midlife US adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2013, 68, 107–116. [Google Scholar] [CrossRef]
- Valadares, A.L.; Machado, V.S.; Costa-Paiva, L.S.; de Sousa, M.H.; Pinto-Neto, A.M. Factors associated with the age of the onset of diabetes in women aged 50 years or more: A population-based study. BMJ Open 2014, 4, e004838. [Google Scholar] [CrossRef]
- Wennberg, P.; Rolandsson, O.; Jerdén, L.; Boeing, H.; Sluik, D.; Kaaks, R.; Teucher, B.; Spijkerman, A.; De Mesquita, B.B.; Dethlefsen, C. Self-rated health and mortality in individuals with diabetes mellitus: Prospective cohort study. BMJ Open 2012, 2, e000760. [Google Scholar] [CrossRef]
- Schytt, E.; Hildingsson, I. Physical and emotional self-rated health among Swedish women and men during pregnancy and the first year of parenthood. Sex. Reprod. Healthc. 2011, 2, 57–64. [Google Scholar] [CrossRef]
- Juarez Padilla, J.; Singleton, C.R.; Pedersen, C.A.; Lara-Cinisomo, S. Associations between Self-Rated Health and Perinatal Depressive and Anxiety Symptoms among Latina Women. Int. J. Environ. Res. Public Health 2022, 19, 11978. [Google Scholar] [CrossRef]
- Lara-Cinisomo, S.; Swinford, C.; Massey, D.; Hardt, H. Diabetes, Prenatal Depression, and Self-Rated Health in Latina Mothers. Diabetes Spectr. 2018, 31, 159–165. [Google Scholar] [CrossRef][Green Version]
- Graffigna, G.; Barello, S.; Libreri, C.; Bosio, C.A. How to engage type-2 diabetic patients in their own health management: Implications for clinical practice. BMC Public Health 2014, 14, 648. [Google Scholar] [CrossRef]
- Hailu, F.B.; Moen, A.; Hjortdahl, P. Diabetes self-management education (DSME)—Effect on knowledge, self-care behavior, and self-efficacy among type 2 diabetes patients in Ethiopia: A controlled clinical trial. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2489. [Google Scholar] [CrossRef]
- Miller, W.R.; Rollnick, S. Motivational Interviewing: Helping People Change; Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Tharek, Z.; Ramli, A.S.; Whitford, D.L.; Ismail, Z.; Mohd Zulkifli, M.; Ahmad Sharoni, S.K.; Shafie, A.A.; Jayaraman, T. Relationship between self-efficacy, self-care behaviour and glycaemic control among patients with type 2 diabetes mellitus in the Malaysian primary care setting. BMC Fam. Pract. 2018, 19, 39. [Google Scholar] [CrossRef]
- Piette, J.D.; Kerr, E.A. The impact of comorbid chronic conditions on diabetes care. Diabetes Care 2006, 29, 725–731. [Google Scholar] [CrossRef]
- Weinger, K.; Beverly, E.A.; Smaldone, A. Diabetes self-care and the older adult. West. J. Nurs. Res. 2014, 36, 1272–1298. [Google Scholar] [CrossRef]
- De Groot, M.; Golden, S.H.; Wagner, J. Psychological conditions in adults with diabetes. Am. Psychol. 2016, 71, 552. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.H.; Von Korff, M.; Consortium, W.W.S. Mental disorders among persons with diabetes—Results from the World Mental Health Surveys. J. Psychosom. Res. 2008, 65, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Dadi, A.F.; Miller, E.R.; Bisetegn, T.A.; Mwanri, L. Global burden of antenatal depression and its association with adverse birth outcomes: An umbrella review. BMC Public Health 2020, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Míguez, M.C.; Vázquez, M.B. Prevalence of Depression during Pregnancy in Spanish Women: Trajectory and Risk Factors in Each Trimester. Int. J. Environ. Res. Public Health 2021, 18, 6789. [Google Scholar] [CrossRef]
- França, U.L.; McManus, M.L. Frequency, trends, and antecedents of severe maternal depression after three million US births. PLoS ONE 2018, 13, e0192854. [Google Scholar] [CrossRef]
- Jacques, N.; de Mola, C.L.; Joseph, G.; Mesenburg, M.A.; da Silveira, M.F. Prenatal and postnatal maternal depression and infant hospitalization and mortality in the first year of life: A systematic review and meta-analysis. J. Affect. Disord. 2019, 243, 201–208. [Google Scholar] [CrossRef]
- Madigan, S.; Oatley, H.; Racine, N.; Fearon, R.P.; Schumacher, L.; Akbari, E.; Cooke, J.E.; Tarabulsy, G.M. A meta-analysis of maternal prenatal depression and anxiety on child socioemotional development. J. Am. Acad. Child Adolesc. Psychiatry 2018, 57, 645–657.e8. [Google Scholar] [CrossRef]
- Grigoriadis, S.; VonderPorten, E.H.; Mamisashvili, L.; Tomlinson, G.; Dennis, C.-L.; Koren, G.; Steiner, M.; Mousmanis, P.; Cheung, A.; Radford, K. The impact of maternal depression during pregnancy on perinatal outcomes: A systematic review and meta-analysis. J. Clin. Psychiatry 2013, 74, 8615. [Google Scholar] [CrossRef]
- Shields, L.; Tsay, G.S. California Diabetes and Pregnancy Program Sweet Success Guidelines for Care; California Department of Public Health; Maternal Child and Adolescent Health Division: California, CA, USA, 2015. [Google Scholar]
- Coustan, D.R.; Lowe, L.P.; Metzger, B.E.; Dyer, A.R. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: Paving the way for new diagnostic criteria for gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2010, 202, 654.e1–654.e6. [Google Scholar] [CrossRef]
- Diabetes, I.A.o.; Panel, P.S.G.C. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar]
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- Kozinszky, Z.; Dudas, R.B. Validation studies of the Edinburgh Postnatal Depression Scale for the antenatal period. J. Affect. Disord 2015, 176, 95–105. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Fortner, R.T.; Gollenberg, A.; Buonnaccorsi, J.; Dole, N.; Markenson, G. A prospective cohort study of modifiable risk factors for gestational diabetes among Hispanic women: Design and baseline characteristics. J. Womens Health 2010, 19, 117–124. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry J. Ment. Sci. 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Vázquez, M.B.; Míguez, M.C. Validation of the Edinburgh postnatal depression scale as a screening tool for depression in Spanish pregnant women. J. Affect. Disord. 2019, 246, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Lorig, K.; Ritter, P.L.; Villa, F.J.; Armas, J. Community-Based Peer-Led Diabetes Self-management. Diabetes Educ. 2009, 35, 641–651. [Google Scholar] [CrossRef]
- Ritter, P.L.; Lorig, K.; Laurent, D.D. Characteristics of the Spanish-and English-language self-efficacy to manage diabetes scales. Diabetes Educ. 2016, 42, 167–177. [Google Scholar] [CrossRef]
- Wuorela, M.; Lavonius, S.; Salminen, M.; Vahlberg, T.; Viitanen, M.; Viikari, L. Self-rated health and objective health status as predictors of all-cause mortality among older people: A prospective study with a 5-, 10-, and 27-year follow-up. BMC Geriatr. 2020, 20, 120. [Google Scholar] [CrossRef]
- Noh, J.-W.; Chang, Y.; Park, M.; Kwon, Y.D.; Ryu, S. Self-rated health and the risk of incident type 2 diabetes mellitus: A cohort study. Sci. Rep. 2019, 9, 3697. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide, 8th ed.; 1998–2017; Muthén & Muthén: Los Angeles, CA, USA, 2017. [Google Scholar]
- Shrout, P.E.; Bolger, N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol. Methods 2002, 7, 422. [Google Scholar] [CrossRef]
- Adam, J.; Folds, L. Depression, self-efficacy, and adherence in patients with type 2 diabetes. J. Nurse Pract. 2014, 10, 646–652. [Google Scholar] [CrossRef]
- Fritz, M.S.; MacKinnon, D.P. Required Sample Size to Detect the Mediated Effect. Psychol. Sci. 2007, 18, 233–239. [Google Scholar] [CrossRef]
- Yu, F.; Lv, L.; Liang, Z.; Wang, Y.; Wen, J.; Lin, X.; Zhou, Y.; Mai, C.; Niu, J. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: A prospective cohort study. J. Clin. Endocrinol. Metab. 2014, 99, 4674–4682. [Google Scholar] [CrossRef]
- González-Quintero, V.H.; Istwan, N.B.; Rhea, D.J.; Rodriguez, L.I.; Cotter, A.; Carter, J.; Mueller, A.; Stanziano, G.J. The impact of glycemic control on neonatal outcome in singleton pregnancies complicated by gestational diabetes. Diabetes Care 2007, 30, 467–470. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, H.; Gao, X.; Lintu, H.; Sun, W. Pregnancy outcome in gestational diabetes. Int. J. Gynecol. Obstet. 2006, 94, 12–16. [Google Scholar] [CrossRef]

| Characteristics | CA Site (n = 82) | IL Site (n = 55) | Total (n = 137) |
|---|---|---|---|
| Age M (SD) | 31.23 (6.58) | 29.35 (5.57) | 30.47 (6.24) |
| Age of diabetes diagnosis M (SD) | 29.98 (6.79) | 27.00 (7.77) | 28.60 (7.38) |
| Language; n (%) | |||
| English | 51 (62.2) | 55 (100) | 106 (77.4) |
| Spanish | 31 (37.8) | - | 31 (22.6) |
| Race/Ethnicity; n (%) | |||
| White, non-Hispanic | 2 (2.4) | 39 (70.9) | 41 (29.9) |
| Black, non-Hispanic | 5 (6.1) | 9 (16.4) | 14 (10.2) |
| Hispanic/Latina | 69 (84.1) | 3 (5.5) | 72 (52.6) |
| Asian | 4 (4.9) | 2 (3.6) | 6 (4.4) |
| Biracial | 2 (2.4) | 2 (3.6) | 4 (2.9) |
| Marital Status; n (%) | |||
| Married | 36 (45.6) | 24 (43.6%) | 60 (44.8) |
| Cohabitating | 18 (22.8) | 13 (23.6) | 31 (23.1) |
| Single/Separated | 25 (31.6) | 18 (32.7) | 42 (31.3) |
| Employment; n (%) | |||
| Full-time employed | 19 (25.7) | 26 (48.1) | 45 (35.2) |
| Part-time employed | 16 (21.6) | 13 (23.6) | 29 (22.7) |
| Not currently employed | 39 (52.7) | 15 (27.8) | 54 (42.2) |
| Education; n (%) | |||
| Less than high school | 17 (21.5) | 8 (14.5) | 25 (18.2) |
| High school/GED or equivalent | 27 (34.1) | 17 (30.9) | 44 (21.1) |
| Some College/Associate’s Degree | 31 (39.2) | 21 (38.2) | 52 (38.0) |
| Bachelor’s degree or more | 4 (5.0) | 9 (16.4) | 13 (9.5) |
| Income; n (%) | |||
| No income | 5 (6.3) | 1 (1.8%) | 6 (4.4) |
| Less than $15,000 | 14 (17.7) | 12 (21.8) | 26 (19.4) |
| $15,000 to $29,999 | 26 (32.9) | 15 (27.3) | 41 (30.5) |
| $30,000 to more | 19 (24.0) | 21 (38.2) | 40 (29.8) |
| Declined to answer | 11(13.9) | 6 (10.9) | 17 (12.6) |
| History of depression diagnosis; n (%) | |||
| No | 68 (85.0) | 25 (45.5) | 93 (68.9) |
| Yes | 9 (11.3) | 27 (49.1) | 36 (26.7) |
| Unsure | 3 (3.8) | 3 (5.5) | 6 (4.4) |
| EPDS cut-off; n (%) | |||
| Not at risk for depression | 68 (82.9) | 44 (80.0) | 112 (81.8) |
| At risk for depression | 9 (11.0) | 10 (18.2) | 19 (13.9) |
| CA | IL | Total | Independent t-Test | |
|---|---|---|---|---|
| (n = 82) | (n = 55) | (n = 137) | ||
| EPDS score; M (SD) | 4.57 (4.32) | 6.06 (4.32) | 5.18 (4.37) | t (129) = −1.935, p = 0.055 |
| DSES score; M (SD) | 8.48 (1.58) | 7.40 (1.69) | 8.05 (1.70) | t (133) = 3.789, p < 0.001 |
| SRH; M (SD) | 3.00 (1.10) | 2.80 (0.91) | 2.92 (1.03) | t (134) = 1.117, p = 0.266 |
| 95% CI (Bias Corrected) | |||||
|---|---|---|---|---|---|
| Path | Estimate | S.E. | Lower 2.5% | Upper 2.5% | |
| EPDS → SRH | a | −0.09 | 0.019 | −0.129 | −0.053 |
| SRH → DSES | b | 0.407 | 0.157 | 0.099 | 0.715 |
| EPDS → DSES | c’ | −0.075 | 0.035 | −0.148 | −0.009 |
| Total indirect effect | −0.037 | 0.016 | −0.077 | −0.011 | |
| 95% CI (Bias Corrected) | |||||
|---|---|---|---|---|---|
| Path | Estimate | S.E. | Lower 2.5% | Upper 2.5% | |
| EPDS → SRH | a | −0.236 | 0.059 | −0.359 | −0.126 |
| SRH → DSES | b | 0.409 | 0.157 | 0.101 | 0.716 |
| EPDS → DSES | c’ | −0.074 | 0.036 | −0.148 | −0.008 |
| EPDS × Site → SRH | a1 | 0.103 | 0.039 | 0.028 | 0.182 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Cinisomo, S.; Loret de Mola, J.R.; Flores-Carter, K.; Tabb, K.M.; Roloff, K. Prenatal Depressive Symptoms, Self-Rated Health, and Diabetes Self-Efficacy: A Moderated Mediation Analysis. Int. J. Environ. Res. Public Health 2022, 19, 13603. https://doi.org/10.3390/ijerph192013603
Lara-Cinisomo S, Loret de Mola JR, Flores-Carter K, Tabb KM, Roloff K. Prenatal Depressive Symptoms, Self-Rated Health, and Diabetes Self-Efficacy: A Moderated Mediation Analysis. International Journal of Environmental Research and Public Health. 2022; 19(20):13603. https://doi.org/10.3390/ijerph192013603
Chicago/Turabian StyleLara-Cinisomo, Sandraluz, Julio Ricardo Loret de Mola, Kendra Flores-Carter, Karen M. Tabb, and Kristina Roloff. 2022. "Prenatal Depressive Symptoms, Self-Rated Health, and Diabetes Self-Efficacy: A Moderated Mediation Analysis" International Journal of Environmental Research and Public Health 19, no. 20: 13603. https://doi.org/10.3390/ijerph192013603
APA StyleLara-Cinisomo, S., Loret de Mola, J. R., Flores-Carter, K., Tabb, K. M., & Roloff, K. (2022). Prenatal Depressive Symptoms, Self-Rated Health, and Diabetes Self-Efficacy: A Moderated Mediation Analysis. International Journal of Environmental Research and Public Health, 19(20), 13603. https://doi.org/10.3390/ijerph192013603






