Perspective: Might Maternal Dietary Monosodium Glutamate (MSG) Consumption Impact Pre- and Peri-Implantation Embryos and Their Subsequent Development?
Abstract
1. Introduction
2. MSG Ingestion Appears to Alter Brain Glutamate Signaling and Whole-Body Metabolism: A Delicate Balance?
3. MSG Apparently Reaches Embryos/Fetuses in the Uterus after Ingestion by Their Mothers
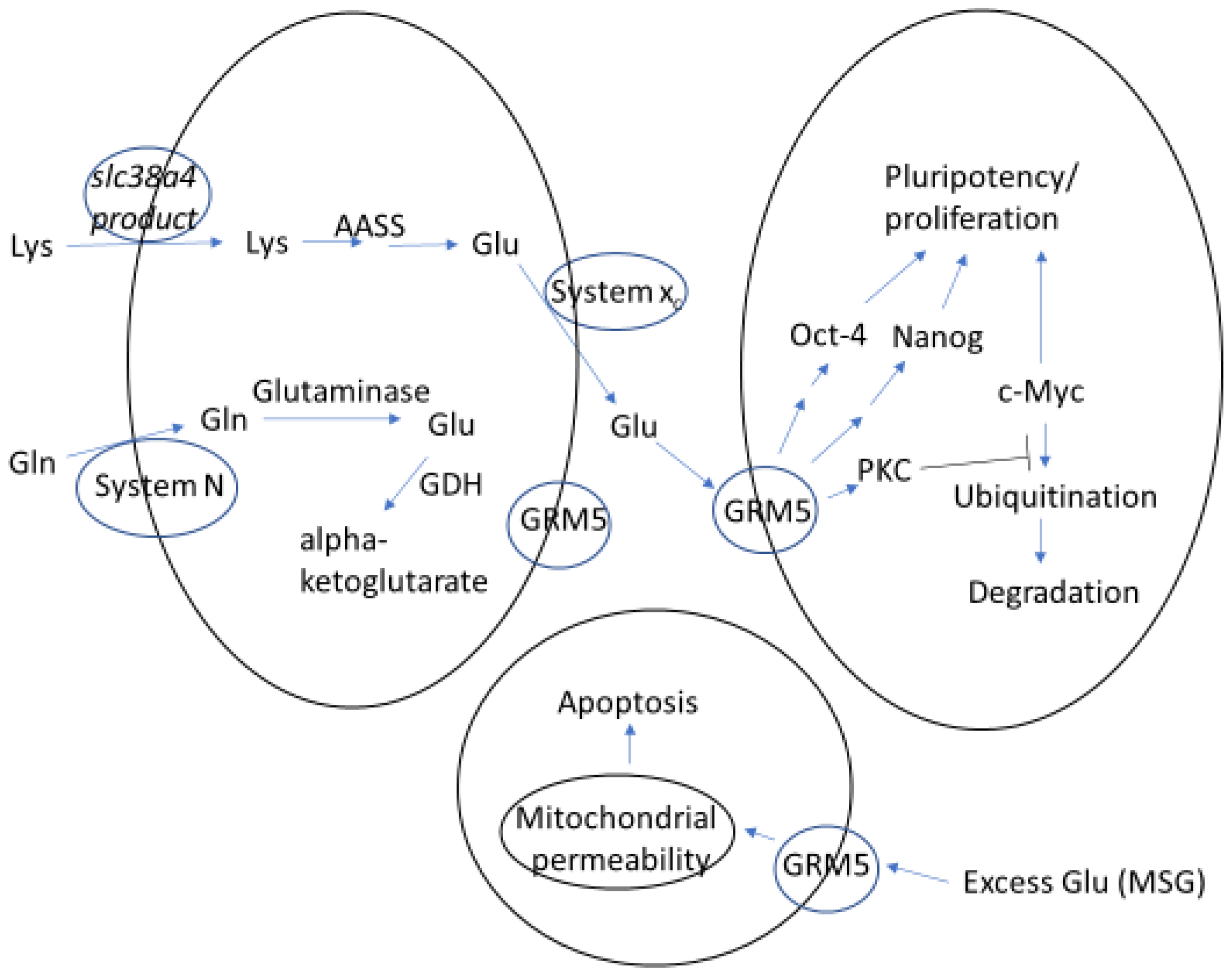
4. Unwanted Effects of Glutamate on Preimplantation Development
5. Possible Importance of Glutamate Receptors to Normal Pre- and Peri-Implantation Embryo Development
6. Compartmentalized Conversion of Lysine to Glutamate Likely Supports Embryonic Stem Cell Pluripotency and Proliferation
7. How Might Exogenous Glutamate Overwhelm This Regulated Way of Providing Glutamate Signaling for ICM Maintenance?
8. Need for Further Studies
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdul-Hamid, M.; Galaly, S.R.; Ahmed, R.R.; Hamdalla, H.M. Monosodium glutamate as a food additive: Toxic implications and the protective role of quercetin. Merit Res. J. Med. Med. Sci. 2017, 5, 384–402. [Google Scholar]
- Onaolapo, A.Y.; Odetunde, I.; Akintola, A.S.; Ogundeji, M.O.; Ajao, A.; Obelawo, A.Y.; Onaolapo, O.J. Dietary composition modulates impact of food-added monosodium glutamate on behaviour, metabolic status and cerebral cortical morphology in mice. Biomed. Pharmacother. 2019, 109, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Holton, K.F.; Hargrave, S.L.; Davidson, T.L. Differential effects of dietary MSG on hippocampal dependent memory are mediated by diet. Front. Neurosci. 2019, 13, 968. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, A.E.; Baron, M.; VanMeter, J.W.; Baraniuk, J.N.; Holton, K.F. The low glutamate diet improves cognitive functioning in veterans with Gulf War Illness and resting-state EEG potentially predicts response. Nutr. Neurosci. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Biney, R.P.; Djankpa, F.T.; Osei, S.A.; Egbenya, D.L.; Aboagye, B.; Karikari, A.A.; Ussif, A.; Wiafe, G.A.; Nuertey, D. Effects of in utero exposure to monosodium glutamate on locomotion, anxiety, depression, memory and KCC2 expression in offspring. Int. J. Dev. Neurosci. 2022, 82, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Fishell, G.; Kriegstein, A. Cortical development: New concepts. Neuron 2005, 46, 361–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Špirková, A.; Kovaříková, V.; Šefčíková, Z.; Pisko, J.; Kšiňanová, M.; Koppel, J.; Fabian, D.; Čikoš, Š. Glutamate can act as a signaling molecule in mouse preimplantation embryos. Biol. Reprod. 2022, 107, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, I.; Spinsanti, P.; Porcellini, A.; Desiderati, F.; De Vita, T.; Storto, M.; Capobianco, L.; Battaglia, G.; Nicoletti, F.; Melchiorri, D. Endogenous activation of mGlu5 metabotropic glutamate receptors supports self-renewal of cultured mouse embryonic stem cells. Neuropharmacology 2005, 49, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J. Amino Acid Transport and Metabolism Regulate Early Embryo Development: Species Differences, Clinical Significance, and Evolutionary Implications. Cells 2021, 10, 3154. [Google Scholar] [CrossRef]
- Spinsanti, P.; De Vita, T.; Di Castro, S.; Storto, M.; Formisano, P.; Nicoletti, F.; Melchiorri, D. Endogenously activated mGlu5 metabotropic glutamate receptors sustain the increase in c-Myc expression induced by leukaemia inhibitory factor in cultured mouse embryonic stem cells. J. Neurochem. 2006, 99, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J.; Galat, V.; Iannaccone, P.M. Lysine Deprivation during Maternal Consumption of Low-Protein Diets Could Adversely Affect Early Embryo Development and Health in Adulthood. Int. J. Environ. Res. Public Health 2020, 17, 5462. [Google Scholar] [CrossRef]
- Van Winkle, L.J.; Tesch, J.K.; Shah, A.; Campione, A.L. System B0,+ amino acid transport regulates the penetration stage of blastocyst implantation with possible long-term developmental consequences through adulthood. Hum. Reprod. Update 2006, 12, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Papes, F.; Surpili, M.J.; Langone, F.; Trigo, J.R.; Arruda, P. The essential amino acid lysine acts as precursor of glutamate in the mammalian central nervous system. FEBS Lett. 2001, 488, 34–38. [Google Scholar] [CrossRef]
- Sacksteder, K.A.; Biery, B.J.; Morrell, J.C.; Goodman, B.K.; Geisbrecht, B.V.; Cox, R.P.; Gould, S.J.; Geraghty, M.T. Identification of the α-aminoadipic semialdehyde synthase gene, which is defective in familial hyperlysinemia. Am. J. Hum. Genet. 2000, 66, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Crowther, L.M.; Mathis, D.; Poms, M.; Plecko, B. New insights into human lysine degradation pathways with relevance to pyridoxine-dependent epilepsy due to antiquitin deficiency. J. Inherit. Metab. Dis. 2019, 42, 620–628. [Google Scholar]
- Bell, S.; Maussion, G.; Jefri, M.; Peng, H.; Theroux, J.F.; Silveira, H.; Soubannier, V.; Wu, H.; Hu, P.; Galat, E.; et al. Disruption of GRIN2B impairs differentiation in human neurons. Stem Cell Rep. 2018, 11, 183–196. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.C. The glutamate-glutamine cycle is not stoichiometric: Fates of glutamate in brain. J. Neurosci. Res. 2007, 85, 3347–3358. [Google Scholar] [CrossRef]
- Hussein, A.M.; Wang, Y.; Mathieu, J.; Margaretha, L.; Song, C.; Jones, D.C.; Cavanaugh, C.; Miklas, J.W.; Mahen, E.; Showalter, M.R.; et al. Metabolic control over mTOR-dependent diapause-like state. Dev. Cell 2020, 52, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, V.A.; Bulut-Karslioglu, A. Molecular Regulation of Paused Pluripotency in Early Mammalian Embryos and Stem Cells. Front. Cell Dev. Biol. 2021, 9, 708318. [Google Scholar] [CrossRef]
- Carey, B.W.; Finley, L.W.; Cross, J.R.; Allis, C.D.; Thompson, C.B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 2015, 518, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Comes, S.; Gagliardi, M.; Laprano, N.; Fico, A.; Cimmino, A.; Palamidessi, A.; De Cesare, D.; De Falco, S.; Angelini, C.; Scita, G.; et al. L-Proline induces a mesenchymal-like invasive program in embryonic stem cells by remodeling H3K9 and H3K36 methylation. Stem Cell Rep. 2013, 1, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J.; Ryznar, R. Amino acid transporters: Roles for nutrition, signaling and epigenetic modifications in embryonic stem cells and their progenitors. eLS 2019, 1–13. [Google Scholar] [CrossRef]
- Van Winkle, L.J.; Dickinson, H.R. Differences in amino acid content of preimplantation mouse embryos that develop in vitro versus in vivo: In vitro effects of five amino acids that are abundant in oviductal secretions. Biol. Reprod. 1995, 52, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Master, J.S.; Thouas, G.A.; Harvey, A.J.; Sheedy, J.R.; Hannan, N.J.; Gardner, D.K.; Wlodek, M. Low female birth weight and advanced maternal age programme alterations in next-generation blastocyst development. Reproduction 2015, 149, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Alexander, P.; Wu, L.; Hammer, R.; Cleaver, O.; McKnight, S.L. Dependence of mouse embryonic stem cells on threonine catabolism. Science 2009, 325, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, N.; Shiraki, Y.; Tsuyama, T.; Obata, F.; Miura, M.; Nagae, G.; Aburatani, H.; Kume, K.; Endo, F.; Kume, S. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 2014, 19, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Gopichandran, N.; Leese, H.J. Metabolic characterization of the bovine blastocyst, inner cell mass, trophectoderm and blastocoel fluid. Reproduction 2003, 126, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J.; Ryznar, R. One-carbon metabolism regulates embryonic stem cell fate through epigenetic DNA and histone modifications: Implications for transgenerational metabolic disorders in adults. Front. Cell Dev. Biol. 2019, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J.; Mann, D.F.; Weimer, B.D.; Campione, A.L. Na+-dependent transport of aniomic amino acids by preimplantation mouse blastocysts. Biochim. Biophys. Acta (BBA)-Biomembr. 1991, 1068, 231–236. [Google Scholar] [CrossRef]
- Elhassan, Y.M.; Wu, G.; Leanez, A.C.; Tasca, R.J.; Watson, A.J.; Westhusin, M.E. Amino acid concentrations in fluids from the bovine oviduct and uterus and in KSOM-based culture media. Theriogenology 2001, 55, 1907–1918. [Google Scholar] [CrossRef]
- Eckert, J.J.; Porter, R.; Watkins, A.J.; Burt, E.; Brooks, S.; Leese, H.J.; Humpherson, P.G.; Cameron, I.T.; Fleming, T.P. Metabolic induction and early responses of mouse blastocyst developmental programming following maternal low protein diet affecting life-long health. PLoS ONE 2012, 7, e52791. [Google Scholar] [CrossRef] [PubMed]
- Kassab, R.B.; Theyab, A.; Al-Ghamdy, A.O.; Algahtani, M.; Mufti, A.H.; Alsharif, K.F.; Abdella, E.M.; Habotta, O.A.; Omran, M.M.; Lokman, M.S.; et al. Protocatechuic acid abrogates oxidative insults, inflammation, and apoptosis in liver and kidney associated with monosodium glutamate intoxication in rats. Environ. Sci. Pollut. Res. 2022, 29, 12208–12221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.; Wang, M.; Chang, Y.; Zhang, F.; Ban, Z.; Tang, R.; Gan, Q.; Wu, S.; Guo, Y.; et al. The lysine catabolite saccharopine impairs development by disrupting mitochondrial homeostasis. J. Cell Biol. 2019, 218, 580–597. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, J.; Wang, M.; Wang, X.; Jian, Y.; Yang, C.; Guo, W. The metabolite saccharopine impairs neuronal development by inhibiting the neurotrophic function of glucose-6-phosphate isomerase. J. Neurosci. 2022, 42, 2631–2646. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Winkle, L.J. Perspective: Might Maternal Dietary Monosodium Glutamate (MSG) Consumption Impact Pre- and Peri-Implantation Embryos and Their Subsequent Development? Int. J. Environ. Res. Public Health 2022, 19, 13611. https://doi.org/10.3390/ijerph192013611
Van Winkle LJ. Perspective: Might Maternal Dietary Monosodium Glutamate (MSG) Consumption Impact Pre- and Peri-Implantation Embryos and Their Subsequent Development? International Journal of Environmental Research and Public Health. 2022; 19(20):13611. https://doi.org/10.3390/ijerph192013611
Chicago/Turabian StyleVan Winkle, Lon J. 2022. "Perspective: Might Maternal Dietary Monosodium Glutamate (MSG) Consumption Impact Pre- and Peri-Implantation Embryos and Their Subsequent Development?" International Journal of Environmental Research and Public Health 19, no. 20: 13611. https://doi.org/10.3390/ijerph192013611
APA StyleVan Winkle, L. J. (2022). Perspective: Might Maternal Dietary Monosodium Glutamate (MSG) Consumption Impact Pre- and Peri-Implantation Embryos and Their Subsequent Development? International Journal of Environmental Research and Public Health, 19(20), 13611. https://doi.org/10.3390/ijerph192013611





