Mediterranean Diet and Cardiovascular Prevention: Why Analytical Observational Designs Do Support Causality and Not Only Associations
Abstract
1. Introduction
2. Materials and Methods
2.1. The Seguimiento Universidad de Navarra (SUN) Cohort
2.2. Exposure Assessment
2.3. Outcome Assessment
2.4. Potential Confounder Assessment
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2018 Diet Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972, Erratum in Lancet 2021, 397, 2466. [Google Scholar]
- Mozaffarian, D.; Appel, L.J.; Van Horn, L. Components of a cardioprotective diet: New insights. Circulation 2011, 123, 2870–2891. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100, Erratum in Circulation 2009, 119, e379. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Changes in Diet Quality Scores and Risk of Cardiovascular Disease among US Men and Women. Circulation 2015, 132, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Association of Changes in Diet Quality with Total and Cause-Specific Mortality. N. Engl. J. Med. 2017, 377, 143–153. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef]
- Nordmann, A.J.; Suter-Zimmermann, K.; Bucher, H.C.; Shai, I.; Tuttle, K.; Estruch, R.; Briel, M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am. J. Med. 2011, 124, 841–851.e2. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Becerra-Tomás, N.; García-Gavilán, J.F.; Bulló, M.; Barrubés, L. Mediterranean Diet and Cardiovascular Disease Prevention: What Do We Know? Prog. Cardiovasc. Dis. 2018, 61, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Galbete, C.; Schwingshackl, L.; Schwedhelm, C.; Boeing, H.; Schulze, M.B. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta-analyses. Eur. J. Epidemiol. 2018, 33, 909–931. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Román-Viñas, B.; Sanchez-Villegas, A.; Guasch-Ferré, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol. Asp. Med. 2019, 67, 1–55. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef]
- Trichopoulou, A.; A Martínez-González, M.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; Predimed Investigators. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Salas-Salvadó, J.; Ros, E.; Estruch, R.; Corella, D.; Fitó, M.; Martínez-González, M.A.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. The PREDIMED trial, Mediterranean diet and health outcomes: How strong is the evidence? Nutr. Metab. Cardiovasc. Dis. 2017, 27, 624–632. [Google Scholar] [CrossRef]
- Fernández-Lázaro, C.I.; Ruiz-Canela, M.; Martínez-González, M.Á. Deep dive to the secrets of the PREDIMED trial. Curr. Opin. Lipidol. 2021, 32, 62–69. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Petersen, K.S.; Svendsen, K.; Ros, E.; Sloan, C.B.; Steffen, L.M.; Tapsell, L.C.; Kris-Etherton, P.M. Considerations to facilitate a US study that replicates PREDIMED. Metabolism 2018, 85, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. The Challenge of Reforming Nutritional Epidemiologic Research. JAMA 2018, 320, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Smith, R. Are some diets “mass murder”? BMJ 2014, 349, g7654, Erratum in BMJ 2015, 350, h408; Erratum in BMJ 2015, 351, h4884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez-Gonzalez, M.A. Are some diets “mass murder”? Evidence in support of the Mediterranean diet is strong. BMJ 2015, 350, h610. [Google Scholar] [CrossRef]
- Hu, F.B.; Willett, W.C. Current and Future Landscape of Nutritional Epidemiologic Research. JAMA 2018, 320, 2073–2074. [Google Scholar] [CrossRef]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding nutritional epidemiology and its role in policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Balduzzi, S.; Beyerbach, J.; Bröckelmann, N.; Werner, S.S.; Zähringer, J.; Nagavci, B.; Meerpohl, J.J. Evaluating agreement between bodies of evidence from randomised controlled trials and cohort studies in nutrition research: Meta-epidemiological study. BMJ 2021, 374, n1864. [Google Scholar] [CrossRef]
- Hernán, M.A. The C-Word: Scientific Euphemisms Do Not Improve Causal Inference from Observational Data. Am. J. Public Health 2018, 108, 616–619. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Renaud, S.; Salen, P.; Monjaud, I.; Mamelle, N.; Martin, J.L.; Guidollet, J.; Touboul, P.; Delaye, J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994, 343, 1454–1459. [Google Scholar] [CrossRef]
- Vincent-Baudry, S.; Defoort, C.; Gerber, M.; Bernard, M.-C.; Verger, P.; Helal, O.; Portugal, H.; Planells, R.; Grolier, P.; Amiot-Carlin, M.-J.; et al. The Medi-RIVAGE study: Reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am. J. Clin. Nutr. 2005, 82, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Marfella, R.; Ciotola, M. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Sayón-Orea, C.; Razquin, C.; Bulló, M.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; Wärnberg, J.; Martínez, J.A.; et al. Effect of a Nutritional and Behavioral Intervention on Energy-Reduced Mediterranean Diet Adherence among Patients with Metabolic Syndrome: Interim Analysis of the PREDIMED-Plus Randomized Clinical Trial. JAMA 2019, 322, 1486–1499. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Sanchez-Villegas, A.; De Irala, J.; Marti, A.; Martínez, J.A. Mediterranean diet and stroke: Objectives and design of the SUN project. Seguimiento Universidad de Navarra. Nutr. Neurosci. 2002, 5, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Carlos, S.; De La Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Razquin, C.; Rico-Campà, A.; Martínez-González, M.A.; Ruiz-Canela, M. Mediterranean Diet and Health Outcomes in the SUN Cohort. Nutrients 2018, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef]
- De la Fuente-Arrillaga, C.; Ruiz, Z.V.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef]
- Buckland, G.; González, C.A.; Agudo, A.; Vilardell, M.; Berenguer, A.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Basterretxea, M.; et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am. J. Epidemiol. 2009, 170, 1518–1529. [Google Scholar] [CrossRef]
- Schröder, H.; Marrugat, J.; Vila, J.; Covas, M.I.; Elosua, R. Adherence to the traditional Mediterranean diet is inversely associated with body mass index and obesity in a Spanish population. J. Nutr. 2004, 134, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Arciniega, A.A.; Mendez, M.A.; Baena-Díez, J.M.; Martori, M.A.; Soler, C.; Marrugat, J.; Covas, M.I.; Sanz, H.; Llopis, A.; Schröder, H. Concurrent and construct validity of Mediterranean diet scores as assessed by an FFQ. Public Health Nutr. 2011, 14, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Vadiveloo, M.; Lichtenstein, A.H.; Anderson, C.; Aspry, K.; Foraker, R.; Griggs, S.; Hayman, L.L.; Johnston, E.; Stone, N.J.; Thorndike, A.N.; et al. Rapid Diet Assessment Screening Tools for Cardiovascular Disease Risk Reduction across Healthcare Settings: A Scientific Statement from the American Heart Association. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e000094. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guasch-Ferré, M.; Chung, W.; Ruiz-Canela, M.; Toledo, E.; Corella, D.; Bhupathiraju, S.N.; Tobias, D.K.; Tabung, F.K.; Hu, J.; et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur. Heart J. 2020, 41, 2645–2656. [Google Scholar] [CrossRef] [PubMed]
- Bes-Rastrollo, M.; Sánchez-Villegas, A.; Alonso, A.; Martínez-González, M.A.; Pérez Valdivieso, J.R. Validation of self-reported weight and body mass index of the participants of a cohort of university graduates. Rev. Esp. Obes. 2005, 3, 352–358. [Google Scholar]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Zaragoza-Martí, A.; Cabañero-Martínez, M.J.; Hurtado-Sánchez, J.A.; Laguna-Pérez, A.; Ferrer-Cascales, R. Evaluation of Mediterranean diet adherence scores: A systematic review. BMJ Open 2018, 8, e019033. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Jennings, A.; Hayhoe, R.P.G.; Awuzudike, V.E.; Welch, A.A. High variability of food and nutrient intake exists across the Mediterranean Dietary Pattern-A systematic review. Food Sci. Nutr. 2020, 8, 4907–4918. [Google Scholar] [CrossRef]
- Mansournia, M.A.; Altman, D.G. Inverse probability weighting. BMJ 2016, 352, i189. [Google Scholar] [CrossRef]
- Toh, S.; Hernán, M.A. Causal inference from longitudinal studies with baseline randomization. Int. J. Biostat. 2008, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Bamia, C.; Trichopoulos, D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 2009, 338, b2337. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J.; Mathur, M.B.; Ding, P. Correcting Misinterpretations of the E-Value. Ann. Intern. Med. 2019, 170, 131–132. [Google Scholar] [CrossRef]
- Hernán, M.A.; Robins, J.M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef]
- García-Albéniz, X.; Hsu, J.; Hernán, M.A. The value of explicitly emulating a target trial when using real world evidence: An application to colorectal cancer screening. Eur. J. Epidemiol. 2017, 32, 495–500. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chavarro, J.E.; Dickerman, B.A.; Manson, J.E.; Mukamal, K.J.; Rexrode, K.M.; Rimm, E.B.; Hernán, M.A. Estimating the effect of nutritional interventions using observational data: The American Heart Association’s 2020 Dietary Goals and mortality. Am. J. Clin. Nutr. 2021, 114, 690–703. [Google Scholar] [CrossRef]
- Hernán, M.A.; Robins, J.M. Per-Protocol Analyses of Pragmatic Trials. N. Engl. J. Med. 2017, 377, 1391–1398. [Google Scholar] [CrossRef]
- Arnold, B.F.; Ercumen, A.; Benjamin-Chung, J.; Colford, J.M., Jr. Brief Report: Negative Controls to Detect Selection Bias and Measurement Bias in Epidemiologic Studies. Epidemiology 2016, 27, 637–641. [Google Scholar] [CrossRef]
- Arnold, B.F.; Ercumen, A. Negative Control Outcomes: A Tool to Detect Bias in Randomized Trials. JAMA 2016, 316, 2597–2598. [Google Scholar] [CrossRef]
- Liese, A.D.; Hense, H.W.; Brenner, H.; Löwel, H.; Keil, U. Assessing the impact of classical risk factors on myocardial infarction by rate advancement periods. Am. J. Epidemiol. 2000, 152, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Gefeller, O.; Greenland, S. Risk and rate advancement periods as measures of exposure impact on the occurrence of chronic diseases. Epidemiology 1993, 4, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Zazpe, I.; Santiago, S.; De la Fuente-Arrillaga, C.; Nuñez-Córdoba, J.M.; Bes-Rastrollo, M.; Martínez-González, M.A. Paper-Based Versus Web-Based Versions of Self-Administered Questionnaires, Including Food-Frequency Questionnaires: Prospective Cohort Study. JMIR Public Health Surveill. 2019, 5, e11997. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Seguí-Gómez, M.; de Irala, J.; Sánchez-Villegas, A.; Beunza, J.J.; Martínez-Gonzalez, M.A. Predictors of follow-up and assessment of selection bias from dropouts using inverse probability weighting in a cohort of university graduates. Eur. J. Epidemiol. 2006, 21, 351–358. [Google Scholar] [CrossRef]
- Alonso, A.; de Irala, J.; Martínez-González, M.A. Representativeness, losses to follow-up and validity in cohort studies. Eur. J. Epidemiol. 2007, 22, 481–482. [Google Scholar] [CrossRef]
- Bröckelmann, N.; Balduzzi, S.; Harms, L.; Beyerbach, J.; Petropoulou, M.; Kubiak, C.; Wolkewitz, M.; Meerpohl, J.J.; Schwingshackl, L. Evaluating agreement between bodies of evidence from randomized controlled trials and cohort studies in medical research: A meta-epidemiological study. BMC Med. 2022, 20, 174. [Google Scholar] [CrossRef]

| All Participants | Low MDS (0 to 2) | Moderate MDS (3 to 6) | High MDS (7 to 9) |
|---|---|---|---|
| N | 3133 | 13,038 | 2248 |
| Female sex (%) | 62.1 | 60.8 | 56.8 |
| Age | 33.9 (10.3) | 38.2 (12.1) | 43.3 (12.7) |
| Baseline energy intake (kcal/day) | 2192 (594) | 2352 (625) | 2517 (548) |
| Body mass index (kg/m2) | 23.0 (3.5) | 23.6 (3.5) | 24.0 (3.6) |
| Physical activity (METs-h/wk) | 17.6 (20.4) | 21.8 (22.7) | 27.3 (26.0) |
| Television watching (h/d) | 1.64 (1.3) | 1.61 (1.2) | 1.57 (1.1) |
| Years of university education | 5.02 (1.5) | 5.05 (1.5) | 5.11 (1.5) |
| Health consciousness (scale 0–10) | 3.6 (1.8) | 4.0 (1.8) | 4.6 (1.9) |
| Dyslipidemia (%) | 11.0 | 16.9 | 25.6 |
| Hypertension (%) | 7.2 | 10.5 | 14.3 |
| Diabetes Mellitus (%) | 0.7 | 1.8 | 2.8 |
| Family history of CHD (%) | 6.5 | 7.7 | 9.4 |
| Unemployment (%) | 4.6 | 4.3 | 2.7 |
| Smoking | |||
| Never smokers (%) | 54.9 | 47.8 | 41.0 |
| Current smokers (%) | 22.6 | 22.3 | 19.6 |
| Former smokers (%) | 21.7 | 29.2 | 38.8 |
| Missing (%) | 0.8 | 0.8 | 0.7 |
| Pack-years of smoking | 3.4 (7.7) | 4.8 (9.5) | 6.1 (10.6) |
| Passive smokers (%) | 14.4 | 19.3 | 22.2 |
| Marital status | |||
| Unmarried (%) | 54.8 | 43.4 | 33.5 |
| Married (%) | 42.4 | 51.7 | 59.9 |
| Divorced (%) | 1.6 | 2.5 | 3.3 |
| Widowed (%) | 0.4 | 0.9 | 1.6 |
| Others (%) | 0.8 | 1.5 | 1.7 |
| Year of entering the cohort | |||
| 1999–2001 (%) | 40.6 | 33.6 | 25.1 |
| 2002–2003 (%) | 22.9 | 19.0 | 15.9 |
| 2004–2006 (%) | 17.0 | 22.8 | 28.8 |
| 2007–2008 (%) | 11.9 | 15.3 | 19.6 |
| 2009–2016 (%) | 7.7 | 9.2 | 10.6 |
| Components of the MDS | |||
| MUFA:SFA Ratio | 1.1 (0.2) | 1.3 (0.3) | 1.6 (0.4) |
| Fruits and nuts (g/day) | 185 (142) | 357 (287) | 551 (367) |
| Vegetables (g/day) | 322 (185) | 538 (334) | 764 (368) |
| Cereals (g/day) | 74 (59) | 103 (73) | 134 (73) |
| Fish (g/day) | 64 (39) | 100 (60) | 135 (59) |
| Legumes (g/day) | 17 (14) | 23 (19) | 29 (18) |
| Dairy products (g/day) | 298 (222) | 186 (189) | 84 (100) |
| Meat (g/day) | 197 (80) | 175 (79) | 145 (67) |
| Alcohol intake (g/day) | 4.0 (8.3) | 6.7 (10.3) | 9.7 (10.3) |
| Baseline MDS (range 0 to 9) | <3 | 3 to 6 | >6 | Per SD | |
| N | 3133 | 13,037 | 2248 | ||
| Cases | 22 | 127 | 22 | ||
| Person-Years | 37,390 | 149,936 | 24,604 | ||
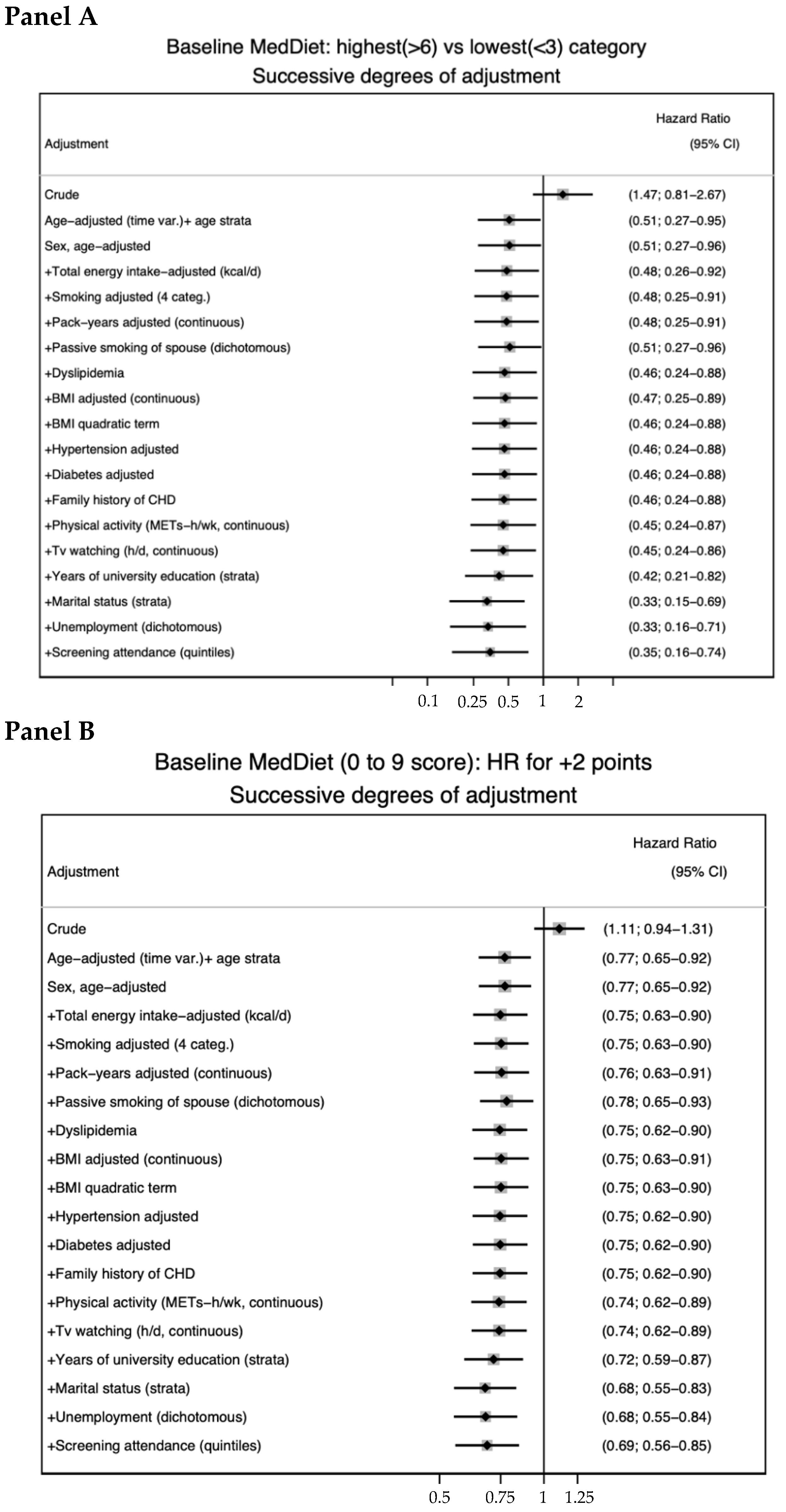
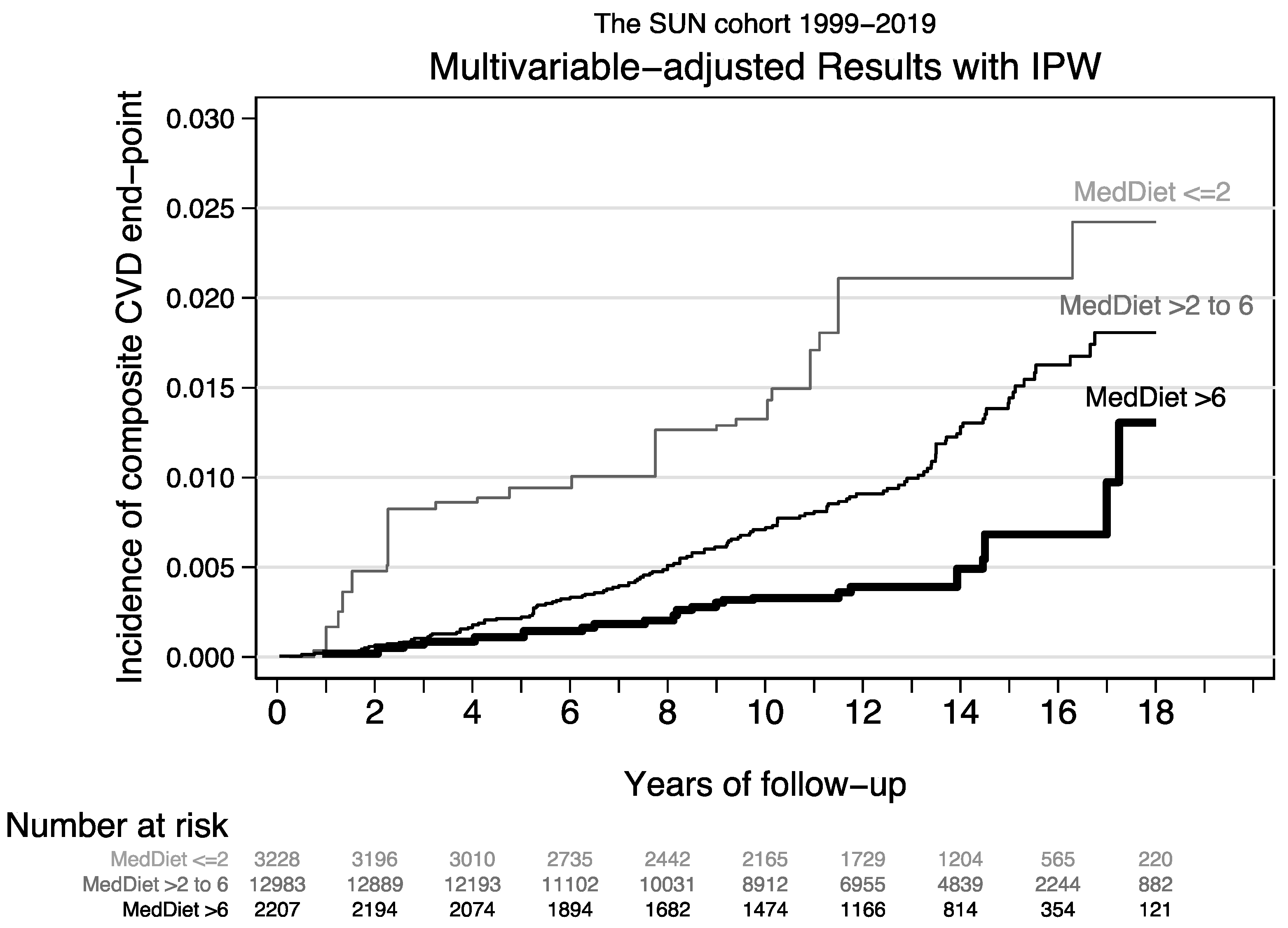
| Age-, sex-adjusted HR (95% CI) | 1 (ref.) | 0.92 (0.58–1.45) | 0.57 (0.31–1.05) | 0.82 (0.70–0.95) | |
| MV-adjusted HR (95% CI) | 1 (ref.) | 0.83 (0.49–1.41) | 0.35 (0.16–0.74) | 0.71 (0.59–0.86) | |
| Modified Mediterranean diet score (10 to 30) | <17 | 17 to 19 | 20 to 22 | 23 to 20 | Per SD |
| N | 4240 | 6227 | 5348 | 2603 | |
| Cases | 39 | 57 | 44 | 31 | |
| Person-Years | 50,480 | 72,274 | 60,819 | 28,358 | |
| Age-, sex-adjusted HR (95% CI) | 1 (ref.) | 0.88 (0.58–1.32) | 0.72 (0.46–1.12) | 0.77 (0.47–1.24) | 0.90 (0.78–1.05) |
| MV-adjusted HR (95% CI) | 1 (ref.) | 0.72 (0.45–1.16) | 0.58 (0.34–0.97) | 0.50 (0.27–0.93) | 0.80 (0.66–0.97) |
| Mediterranean diet adherence screener (MEDAS, 0 to 14) | <5 | 5 to 6 | 7 | 8 to 14 | Per SD |
| N | 4807 | 6969 | 2877 | 3765 | |
| Cases | 36 | 70 | 29 | 36 | |
| Person-Years | 57,277 | 80,741 | 32,707 | 41,206 | |
| Age-, sex-adjusted HR (95% CI) | 1 (ref.) | 1.00 (0.67–1.50) | 0.88 (0.54–1.44) | 0.68 (0.43–1.09) | 0.90 (0.77–1.05) |
| MV-adjusted HR (95% CI) | 1 (ref.) | 0.97 (0.63–1.48) | 0.85 (0.51–1.43) | 0.63 (0.38–1.05) | 0.88 (0.75–1.04) |
| Mediterranean diet adherence screener (MEDAS, 0 to 14) (restricted to >40 years) | <5 | 5 to 6 | 7 | 8 to 14 | Per SD |
| N | 1354 | 2634 | 1283 | 2032 | |
| Cases | 31 | 65 | 28 | 31 | |
| Person-Years | 16,005 | 30,573 | 14,617 | 22,636 | |
| Age-, sex-adjusted HR (95% CI) | 1 (ref.) | 1.00 (0.65–1.54) | 0.89 (0.53–1.48) | 0.60 (0.36–0.99) | 0.86 (0.74–1.01) |
| MV-adjusted HR (95% CI) | 1 (ref.) | 0.96 (0.61–1.51) | 0.82 (0.47–1.41) | 0.53 (0.30–0.91) | 0.83 (0.69–0.99) |
| MDS cumulative average, time-dependent Cox model with IPW | <3 | 3 to 6 | >6 | Per SD | |
| N | 3047 | 13,136 | 2235 | ||
| Person-Years | 35,701 | 151,622 | 24,855 | ||
| Age-, sex-adjusted HR (95% CI) | 1 (ref.) | 0.93 (0.54–1.61) | 0.61 (0.31–1.20) | 0.89 (0.74–1.07) | |
| MV-adjusted HR (95% CI) | 1 (ref.) | 0.65 (0.35–1.21) | 0.30 (0.14–0.62) | 0.63 (0.43–0.94) |
| Baseline Exposure | Cases When Item = 0 | Cases When Item = 1 | Adjusted HR (for Each Item without Adjustment for the Other Items) | p Value | Additionally Adjusted for the Other 8 Items, HR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Ratio MUFA:SFA | 84 | 87 | 0.856 (0.606–1.211) | 0.380 | 0.980 (0.682–1.409) | 0.913 |
| Fruit and nuts | 76 | 95 | 0.714 (0.494–1.031) | 0.072 | 0.797 (0.545–1.164) | 0.240 |
| Vegetables | 90 | 81 | 0.756 (0.528–1.082) | 0.126 | 0.820 (0.566–1.189) | 0.296 |
| Cereals | 102 | 69 | 0.745 (0.510–1.090) | 0.130 | 0.750 (0.507–1.109) | 0.149 |
| Fish and seafood | 83 | 88 | 0.774 (0.542–1.104) | 0.157 | 0.797 (0.555–1.146) | 0.221 |
| Legumes | 85 | 86 | 0.720 (0.501–1.035) | 0.076 | 0.717 (0.497–1.034) | 0.075 |
| Low dairy | 77 | 94 | 0.720 (0.499–1.040) | 0.080 | 0.780 (0.531–1.145) | 0.205 |
| Low meat | 74 | 97 | 0.856 (0.596–1.231) | 0.403 | 0.885 (0.609–1.286) | 0.522 |
| Mod. alcohol | 113 | 58 | 0.955 (0.660–1.381) | 0.806 | 0.949 (0.655–1.376) | 0.783 |
| Repeated measures, cumulative averages | ||||||
| Ratio MUFA:SFA | 76 | 95 | 0.894 (0.632–1.266) | 0.528 | 1.021 (0.709–1.471) | 0.910 |
| Fruit and nuts | 73 | 98 | 0.672 (0.465–0.972) | 0.035 | 0.743 (0.508–1.086) | 0.125 |
| Vegetables | 84 | 87 | 0.849 (0.593–1.217) | 0.373 | 0.917 (0.632–1.330) | 0.647 |
| Cereals | 99 | 72 | 0.762 (0.523–1.108) | 0.155 | 0.764 (0.518–1.127) | 0.175 |
| Fish and seafood | 73 | 98 | 0.770 (0.540–1.100) | 0.151 | 0.796 (0.553–1.145) | 0.219 |
| Legumes | 87 | 84 | 0.725 (0.503–1.044) | 0.084 | 0.716 (0.495–1.038) | 0.078 |
| Low dairy | 78 | 93 | 0.758 (0.525–1.094) | 0.139 | 0.803 (0.547–1.179) | 0.263 |
| Low meat | 72 | 99 | 0.779 (0.542–1.121) | 0.179 | 0.808 (0.557–1.174) | 0.263 |
| Mod. alcohol | 113 | 58 | 1.053 (0.731–1.517) | 0.780 | 1.039 (0.721–1.499) | 0.836 |
| TOTAL MDS (For +2 points) | 0.712 (0.575–0.882) | 0.002 | ||||
| Effect of removing one item at a time (baseline exposures) | % of change | |||||
| All items (+2 points) | 0.686 (0.555–0.848) | 0.001 | - | |||
| Removing MUFA:SFA ratio | 0.772 (0.643–0.928) | 0.006 | 12.56 | |||
| Removing fruits and nuts | 0.806 (0.673–0.965) | 0.019 | 17.38 | |||
| Removing vegetables | 0.829 (0.694–0.991) | 0.040 | 20.83 | |||
| Removing cereals | 0.843 (0.717–0.992) | 0.040 | 22.87 | |||
| Removing fish and seafood | 0.852 (0.717–1.013) | 0.069 | 24.17 | |||
| Removing legumes | 0.805 (0.684–0.947) | 0.009 | 17.33 | |||
| Removing low dairy | 0.781 (0.651–0.936) | 0.008 | 13.77 | |||
| Removing low meat | 0.797 (0.674–0.942) | 0.008 | 16.16 | |||
| Removing alcohol | 0.796 (0.677–0.937) | 0.006 | 16.05 | |||
| Effect of removing one item at a time (repeated measures, cumulative averages) | % of change | |||||
| All items (+2 points) | 0.712 (0.575–0.882) | 0.002 | ||||
| Removing MUFA:SFA ratio | 0.835 (0.750–0.930) | 0.001 | 17.22 | |||
| Removing fruit and nuts | 0.862 (0.777–0.958) | 0.006 | 21.04 | |||
| Removing vegetables | 0.849 (0.766–0.942) | 0.002 | 19.18 | |||
| Removing cereals | 0.865 (0.783–0.956) | 0.004 | 21.41 | |||
| Removing fish and seafood | 0.862 (0.779–0.954) | 0.004 | 21.00 | |||
| Removing legumes | 0.872 (0.790–0.962) | 0.007 | 22.35 | |||
| Removing dairy | 0.853 (0.769–0.947) | 0.003 | 19.78 | |||
| Removing meats | 0.861 (0.778–0.952) | 0.004 | 20.79 | |||
| Removing alcohol | 0.846 (0.766–0.934) | 0.001 | 18.68 | |||
| Median HR | P 1 | P 2.5 | P 25 | P 75 | P 97.5 | P 99 | |
|---|---|---|---|---|---|---|---|
| All (samples: 50%) | 0.29 | 0.11 | 0.14 | 0.22 | 0.39 | 0.82 | 0.94 |
| Age strata (samples: 75%) | |||||||
| ≤45 years | 0.44 | 0.07 | 0.14 | 0.32 | 0.58 | 1.13 | 1.41 |
| >45 & <55 years | 0.25 | 0.06 | 0.08 | 0.18 | 0.42 | 2.41 | -- |
| ≥55 years | 0.31 | 0.14 | 0.16 | 0.25 | 0.39 | 0.67 | 0.73 |
| Multivar-adj. HR (95% CI) | p Value | |
|---|---|---|
| Main outcome | ||
| Cardiovascular disease | 0.686 (0.555–0.848) | 0.001 |
| Negative controls | ||
| Mammography (women) or PSA (men) | 0.988 (0.954–1.024) | 0.510 |
| Visit to doctor | 0.970 (0.931–1.011) | 0.149 |
| Sport injury | 0.998 (0.959–1.039) | 0.937 |
| Road injury with hospitalization | 0.980 (0.818–1.174) | 0.829 |
| Road injury without hospitalization | 1.027 (0.965–1.092) | 0.404 |
| Cataract surgery | 1.091 (1.003–1.188) | 0.043 |
| Glaucoma | 1.005 (0.870–1.162) | 0.944 |
| Bronchitis | 0.946 (0.820–1.091) | 0.442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-González, M.Á.; Martín-Calvo, N.; Bretos-Azcona, T.; Carlos, S.; Delgado-Rodríguez, M. Mediterranean Diet and Cardiovascular Prevention: Why Analytical Observational Designs Do Support Causality and Not Only Associations. Int. J. Environ. Res. Public Health 2022, 19, 13653. https://doi.org/10.3390/ijerph192013653
Martínez-González MÁ, Martín-Calvo N, Bretos-Azcona T, Carlos S, Delgado-Rodríguez M. Mediterranean Diet and Cardiovascular Prevention: Why Analytical Observational Designs Do Support Causality and Not Only Associations. International Journal of Environmental Research and Public Health. 2022; 19(20):13653. https://doi.org/10.3390/ijerph192013653
Chicago/Turabian StyleMartínez-González, Miguel Ángel, Nerea Martín-Calvo, Telmo Bretos-Azcona, Silvia Carlos, and Miguel Delgado-Rodríguez. 2022. "Mediterranean Diet and Cardiovascular Prevention: Why Analytical Observational Designs Do Support Causality and Not Only Associations" International Journal of Environmental Research and Public Health 19, no. 20: 13653. https://doi.org/10.3390/ijerph192013653
APA StyleMartínez-González, M. Á., Martín-Calvo, N., Bretos-Azcona, T., Carlos, S., & Delgado-Rodríguez, M. (2022). Mediterranean Diet and Cardiovascular Prevention: Why Analytical Observational Designs Do Support Causality and Not Only Associations. International Journal of Environmental Research and Public Health, 19(20), 13653. https://doi.org/10.3390/ijerph192013653









