Enhanced Adsorption of Tetracycline by Thermal Modification of Coconut Shell-Based Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Characterization
2.3. Experiment Procedures
2.4. Modeling
3. Results
3.1. Effects of the Thermal Treatment Temperature on TC Adsorption
3.2. Characterization
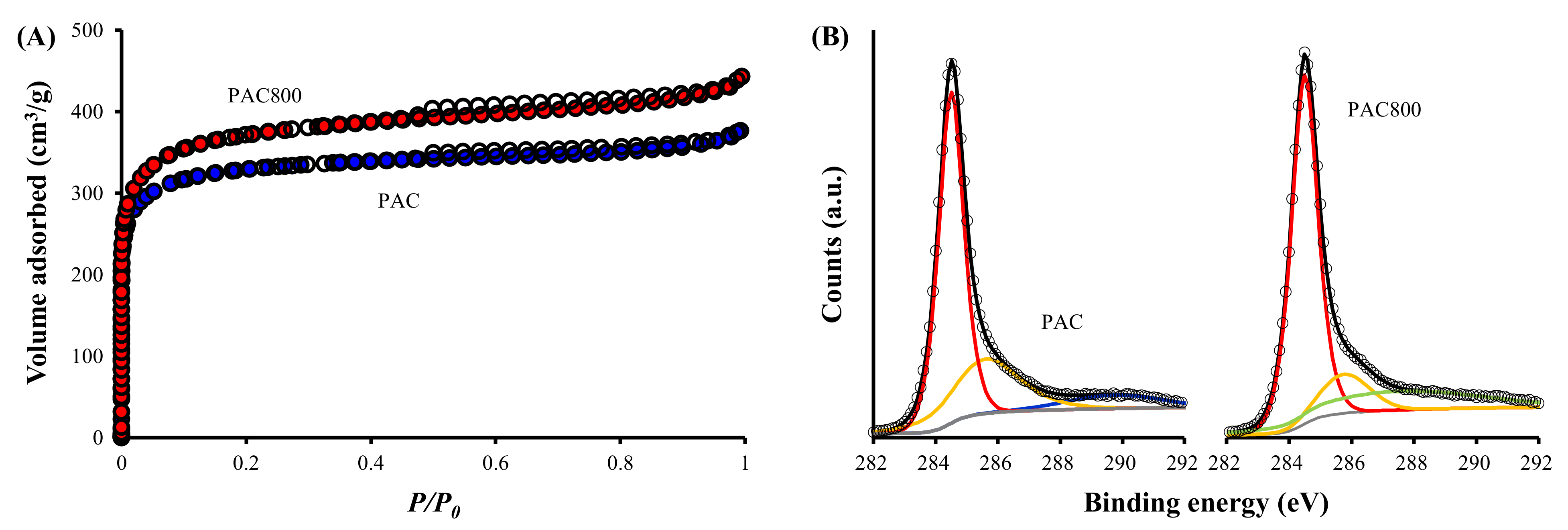
3.2.1. N2 Adsorption/Desorption
3.2.2. XPS
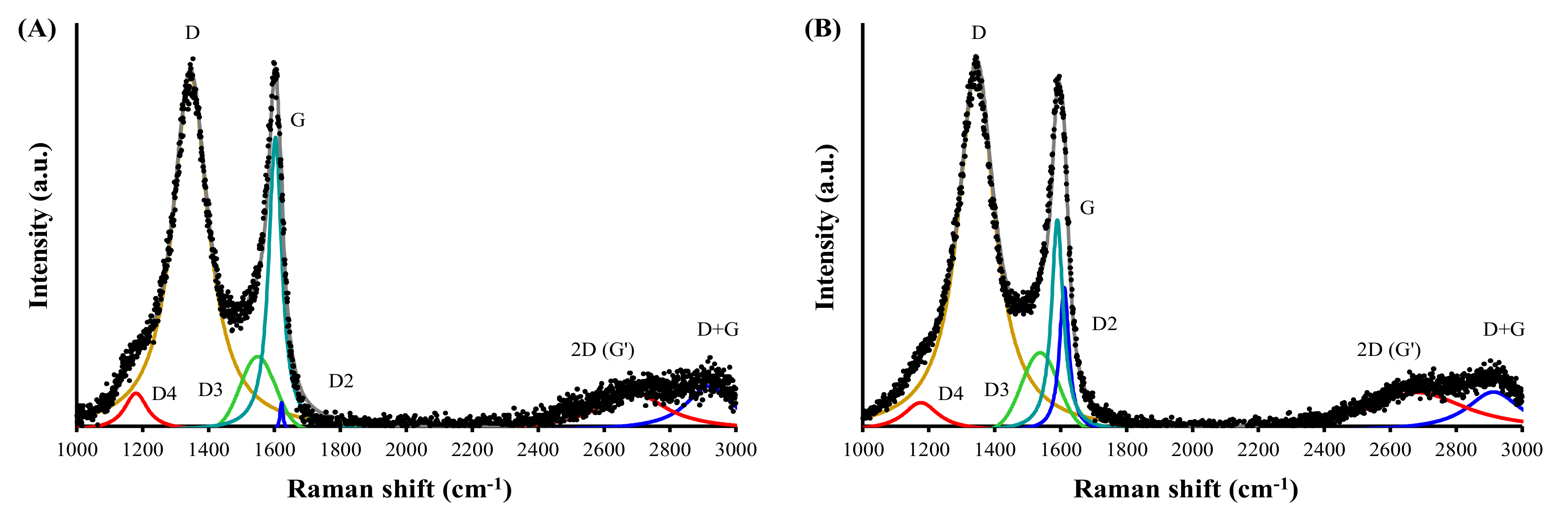
3.2.3. Raman Spectroscopy
3.2.4. XRD, Zeta Potential, and Titration
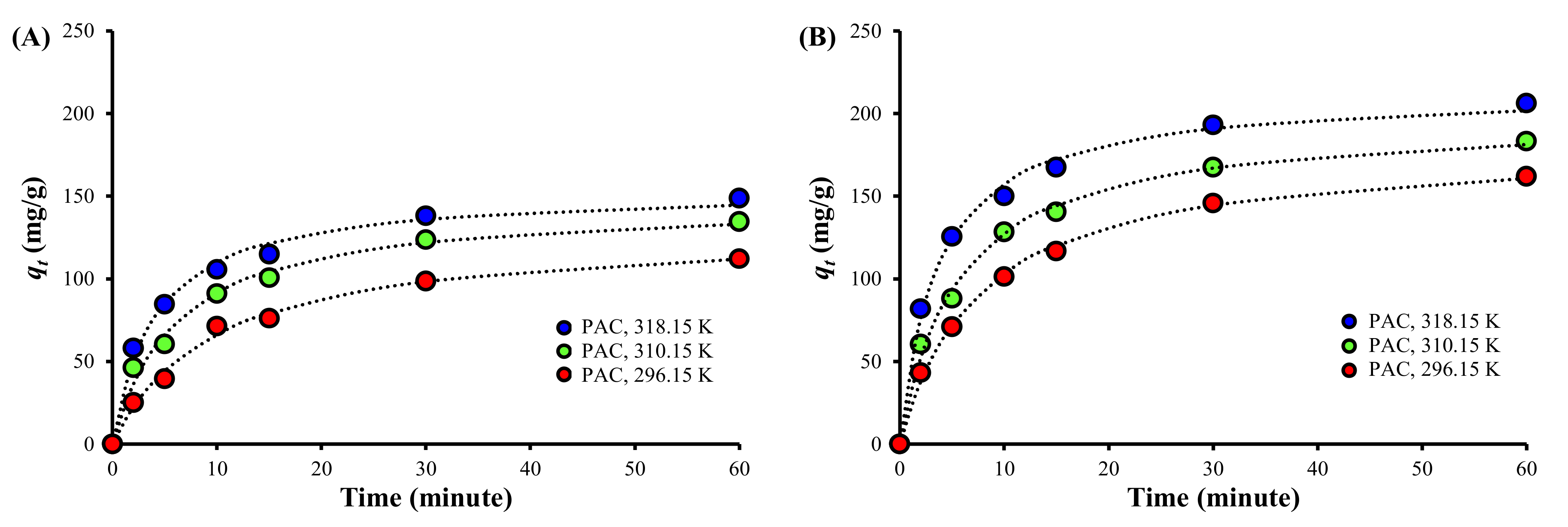
3.3. Adsorption Kinetics
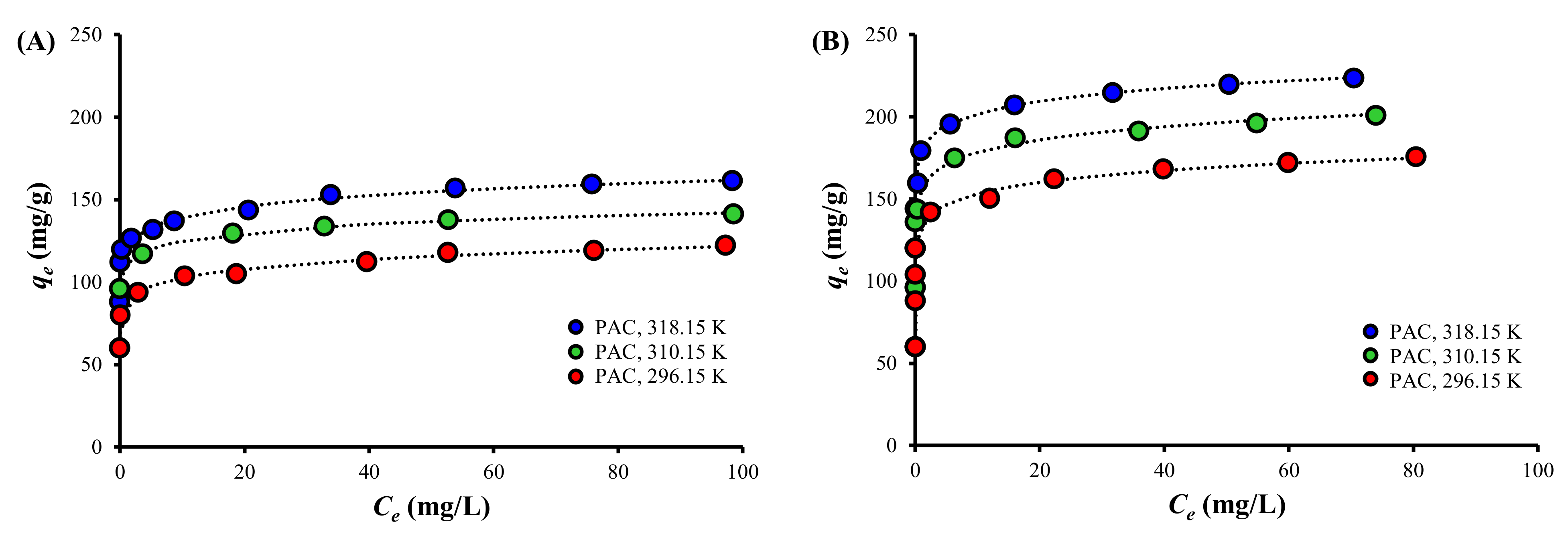
3.4. Adsorption Isotherm
3.5. Thermodynamics
3.6. Factors Affecting TC Adsorption and the Potentials in Real Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, Fate, and Risk Assessment of Typical Tetracycline Antibiotics in the Aquatic Environment: A Review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef] [PubMed]
- Jafari Ozumchelouei, E.; Hamidian, A.H.; Zhang, Y.; Yang, M. Physicochemical Properties of Antibiotics: A Review with an Emphasis on Detection in the Aquatic Environment. Water Environ. Res. 2020, 92, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Scaria, J.; Anupama, K.V.; Nidheesh, P.V. Tetracyclines in the Environment: An Overview on the Occurrence, Fate, Toxicity, Detection, Removal Methods, and Sludge Management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef] [PubMed]
- Lundström, S.V.; Östman, M.; Bengtsson-Palme, J.; Rutgersson, C.; Thoudal, M.; Sircar, T.; Blanck, H.; Eriksson, K.M.; Tysklind, M.; Flach, C.-F.; et al. Minimal Selective Concentrations of Tetracycline in Complex Aquatic Bacterial Biofilms. Sci. Total Environ. 2016, 553, 587–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menz, J.; Olsson, O.; Kümmerer, K. Antibiotic Residues in Livestock Manure: Does the EU Risk Assessment Sufficiently Protect against Microbial Toxicity and Selection of Resistant Bacteria in the Environment? J. Hazard. Mater. 2019, 379, 120807. [Google Scholar] [CrossRef]
- Gu, J.; Chen, C.; Huang, X.; Mo, J.; Xie, Q.; Zeng, Q. Occurrence and Risk Assessment of Tetracycline Antibiotics in Soils and Vegetables from Vegetable Fields in Pearl River Delta, South China. Sci. Total Environ. 2021, 776, 145959. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, Q.; Wei, D. A Critical Review on Antibiotics and Hormones in Swine Wastewater: Water Pollution Problems and Control Approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Ye, J.; Du, Y.; Wang, L.; Qian, J.; Chen, J.; Wu, Q.; Hu, X. Toxin Release of Cyanobacterium Microcystis Aeruginosa after Exposure to Typical Tetracycline Antibiotic Contaminants. Toxins 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.A.; Kareem, S.L. Adsorption of Tetracycline Fom Wastewater by Using Pistachio Shell Coated with ZnO Nanoparticles: Equilibrium, Kinetic and Isotherm Studies. Alex. Eng. J. 2019, 58, 917–928. [Google Scholar] [CrossRef]
- Qiu, G.; Chen, H.; Srinivasa Raghavan, D.S.; Ting, Y.-P. Removal Behaviors of Antibiotics in a Hybrid Microfiltration-Forward Osmotic Membrane Bioreactor for Real Municipal Wastewater Treatment. Chem. Eng. J. 2021, 417, 129146. [Google Scholar] [CrossRef]
- Khaledi, K.; Valdes Labrada, G.M.; Soltan, J.; Predicala, B.; Nemati, M. Adsorptive Removal of Tetracycline and Lincomycin from Contaminated Water Using Magnetized Activated Carbon. J. Environ. Chem. Eng. 2021, 9, 105998. [Google Scholar] [CrossRef]
- Son, H.-J.; Jung, J.-M.; Hwang, Y.-D.; Roh, J.-S.; Yu, P.-J. Effects of Activated Carbon Types and Service Life on Adsorption OfTetracycline Antibiotic Compounds in GAC Process. J. Korean Soc. Environ. Eng. 2008, 30, 925–932. (In Korean) [Google Scholar]
- Expert Market Research (EMR). E.M.R. Global Activated Carbon Market: By Type: Powdered Activated Carbon, Granular Activated Carbon, Others; By Applications: Water Treatment, Air Treatment, Gold Treatment, Respirators, Solvency Recover; By Feedstock; Regional Analysis; Historical Market; E.M.R.: Sheridan, WY, USA, 2020. [Google Scholar]
- Sarkar, J.K.; Wang, Q. Different pyrolysis process conditions of South Asian waste coconut shell and characterization of gas, bio-char, and bio-oil. Energies 2020, 13, 1970. [Google Scholar] [CrossRef]
- Siengchum, T.; Isenberg, M.; Chuang, S.S. Fast pyrolysis of coconut biomass–an FTIR study. Fuel 2013, 105, 559–565. [Google Scholar] [CrossRef]
- Coconut Production Worldwide in 2020, by Leading Country. Available online: https://www.statista.com/statistics/1040499/world-coconut-production-by-leading-producers (accessed on 13 October 2022).
- Yerima, I.; Grema, M.Z. The potential of coconut shell as biofuel. J. Middle East N. Afr. Sci. 2018, 4, 11–15. [Google Scholar]
- Ayrilmis, N.; Jarusombuti, S.; Fueangvivat, V.; Bauchongkol, P.; White, R. Coir fiber reinforced polypropylene composite panel for automotive interior applications. Fibers Polym. 2011, 11, 919–926. [Google Scholar] [CrossRef]
- Santos, D.E.; Martinez, F.C.C.; Juiz, P.J.L. A Fibra de Coco como Matéria-Prima para o Desenvolvimento de Produtos: Uma prospecção tecnológica em bancos de patentes. Cadernos. Prospecção 2019, 1, 153. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Zanariah Ngah, C.W. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Su, W.; Zhou, L.; Zhou, Y.P. Preparation of microporous activated carbon from coconut shells without activating agents. Carbon 2006, 41, 861–863. [Google Scholar] [CrossRef]
- Transparency Market Research (TMR). Coconut Activated Carbon Market-Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2019–2027; Transparency Market Research: Wilmington, DE, USA, 2019. [Google Scholar]
- Kuśmierek, K.; Świątkowski, A.; Skrzypczyńska, K.; Błażewicz, S.; Hryniewicz, J. The Effects of the Thermal Treatment of Activated Carbon on the Phenols Adsorption. Korean J. Chem. Eng. 2017, 34, 1081–1090. [Google Scholar] [CrossRef]
- Wazir, A.H.; ul Haq, I.; Manan, A.; Khan, A. Preparation and characterization of activated carbon from coal by chemical activation with KOH. Int. J. Coal Prep. Util. 2022, 42, 1477–1488. [Google Scholar] [CrossRef]
- Song, G.; Deng, R.; Yao, Z.; Chen, H.; Romero, C.; Lowe, T.; Driscoll, G.; Kreglow, B.; Schobert, H.; Baltrusaitis, J. Anthracite coal-based activated carbon for elemental Hg adsorption in simulated flue gas: Preparation and evaluation. Fuel 2020, 275, 117921. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Bie, R. Temperature rise characteristics of coal-KOH adduct under microwave heating and the properties of resultant activated carbon for catalytic methane decomposition. J. Anal. Appl. Pyrol. 2020, 145, 104739. [Google Scholar] [CrossRef]
- Kim, D.-G.; Ko, S.-O. Effects of Thermal Modification of a Biochar on Persulfate Activation and Mechanisms of Catalytic Degradation of a Pharmaceutical. Chem. Eng. J. 2020, 399, 125377. [Google Scholar] [CrossRef]
- Miyazato, T.; Nuryono, N.; Kobune, M.; Rusdiarso, B.; Otomo, R.; Kamiya, Y. Phosphate Recovery from an Aqueous Solution through Adsorption-Desorption Cycle over Thermally Treated Activated Carbon. J. Water Process Eng. 2020, 36, 101302. [Google Scholar] [CrossRef]
- Yue, X.-M.; An, Z.-Y.; Ye, M.; Liu, Z.-J.; Xiao, C.-C.; Huang, Y.; Han, Y.-J.; Zhang, S.-Q.; Zhu, J.-S. Preparation of Porous Activated Carbons for High Performance Supercapacitors from Taixi Anthracite by Multi-Stage Activation. Molecules 2019, 24, 3588. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wang, Q.; Wang, H.; Zhao, F.; Li, D. One-step chemical activation facilitates synthesis of activated carbons from Acer truncatum seed shells for premium capacitor electrodes. Ind. Crops Prod. 2022, 187, 115458. [Google Scholar] [CrossRef]
- Osman, A.I.; O’Connor, E.; McSpadden, G.; Abu-Dahrieh, J.K.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Rooney, D.W. Upcycling brewer’s spent grain waste into activated carbon and carbon nanotubes for energy and other applications via two-stage activation. J. Chem. Technol. Biotechnol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, S.-C.; Sun, G.-T.; Kang, K.; Zhu, M.-Q.; Geng, Z.-C. Comparison of activated carbons prepared by one-step and two-step chemical activation process based on cotton stalk for supercapacitors application. Energy 2021, 215, 119144. [Google Scholar] [CrossRef]
- Jiao, S.; Zhang, L.; Li, C.; Zhang, H.; Zhang, J.; Li, P.; Tao, Y.; Zhao, X.; Chen, H.; Jiang, J.C. Efficient construction of a carbon-based symmetric supercapacitor from soybean straw by coupling multi-stage carbonization and mild activation. Ind. Crop. Prod. 2022, 183, 114906. [Google Scholar] [CrossRef]
- Rong, H.; Ryu, Z.; Zheng, J.; Zhang, Y. Influence of heat treatment of rayon-based activated carbon fibers on the adsorption of formaldehyde. J. Colloid Interface Sci. 2003, 261, 207–212. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.-K.; Hussain, M. A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Shen, Y.; Hou, J.; Gao, J.; He, D.; Huang, J.; He, H.; Lei, L.; Chen, W. One-Step Synthesis of Nitrogen and Sulfur Co-Doped Mesoporous Graphite-like Carbon Nanosheets as a Bifunctional Material for Tetracycline Removal via Adsorption and Catalytic Degradation Processes: Performance and Mechanism. Chem. Eng. J. 2021, 412, 128521. [Google Scholar] [CrossRef]
- Peng, X.; Hu, F.; Huang, J.; Wang, Y.; Dai, H.; Liu, Z. Preparation of a Graphitic Ordered Mesoporous Carbon and Its Application in Sorption of Ciprofloxacin: Kinetics, Isotherm, Adsorption Mechanisms Studies. Microporous Mesoporous Mater. 2016, 228, 196–206. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Kao, C.-L.; Hung, P.-Y. Preparation of Activated Carbon from Waste Cation Exchange Resin and Its Application in Wastewater Treatment. Carbon Lett. 2022, 32, 461–474. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Elovich, S.Y.; Larinov, O.G. Theory of adsorption from solutions of non electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form,(II) verification of the equation of adsorption isotherm from solutions. Izv. Akad. Nauk. SSSR Otd. Khim. Nauk. 1962, 2, 209–216. [Google Scholar]
- Langmuir, I. The Constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Tempkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. U.S.S.R. 1940, 12, 327–356. [Google Scholar]
- Yu, Q.; Li, M.; Ji, X. Characterization and methanol adsorption of walnutshell activated carbon prepared by KOH activation. J. Wuhan. Univ. Technol. Mat. Sci. 2016, 31, 260–268. [Google Scholar] [CrossRef]
- Mojoudi, N.; Mirghafari, N.; Soleimani, M.; Shariatmadari, H.; Belver, C.; Bedia, J. Phenol adsorption on high microporous activated carbons prepared from oily sludge: Equilibrium, kinetic and thermodynamic studies. Sci. Rep. 2019, 9, 19352. [Google Scholar] [CrossRef] [Green Version]
- Roman, S.; Nabais, J.M.V.; Ledesma, B.; González, J.F.; Laginhas, C.; Titirici, M.M. Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Micropor. Mesopor. Mat. 2013, 165, 127–133. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, N.; Xing, X.; Sun, Y.; Zhang, Z.; Hao, Z. Gaseous Adsorption of Hexamethyldisiloxane on Carbons: Isotherms, Isosteric Heats and Kinetics. Chemosphere 2020, 247, 125862. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, X.; Liu, S.; Liu, Y.; Zeng, G.; Ye, S.; Yin, Z.; Hu, X.; Liu, N. Catalytic Degradation of Estrogen by Persulfate Activated with Iron-Doped Graphitic Biochar: Process Variables Effects and Matrix Effects. Chem. Eng. J. 2019, 378, 122141. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A Review on Surface Modification of Activated Carbon for Carbon Dioxide Adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Nwamba, O.C.; Echeverria, E.; McIlroy, D.N.; Austin, A.; Shreeve, J.M.; Aston, D.E. Thermal Modification of Graphite for Fast Electron Transport and Increased Capacitance. ACS Appl. Nano Mater. 2019, 2, 228–240. [Google Scholar] [CrossRef]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [Green Version]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman Microspectroscopy of Soot and Related Carbonaceous Materials: Spectral Analysis and Structural Information. Carbon N. Y. 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, J.; Wang, J. Pyrolytic Characteristics of Pine Wood in a Slowly Heating and Gas Sweeping Fixed-Bed Reactor. J. Anal. Appl. Pyrolysis 2009, 84, 179–184. [Google Scholar] [CrossRef]
- Yoo, S.; Chung, C.-C.; Kelley, S.S.; Park, S. Graphitization Behavior of Loblolly Pine Wood Investigated by in Situ High Temperature X-Ray Diffraction. ACS Sustain. Chem. Eng. 2018, 6, 9113–9119. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, B.; Zhou, Q.; He, Y.; Wang, Z.; Radacsi, N. Fe-Doped ZnO/Reduced Graphene Oxide Nanocomposite with Synergic Enhanced Gas Sensing Performance for the Effective Detection of Formaldehyde. ACS Omega 2019, 4, 10252–10262. [Google Scholar] [CrossRef] [Green Version]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Akdemir Evrendilek, G.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Intraparticle Diffusion of Cr(VI) through Biomass and Magnetite Coated Biomass: A Comparative Kinetic and Diffusion Study. S. Afr. J. Chem. Eng. 2020, 32, 39–55. [Google Scholar] [CrossRef]
- Hu, Q.; Pang, S.; Wang, D. In-Depth Insights into Mathematical Characteristics, Selection Criteria and Common Mistakes of Adsorption Kinetic Models: A Critical Review. Sep. Purif. Rev. 2022, 51, 281–299. [Google Scholar] [CrossRef]
- Schwaab, M.; Steffani, E.; Barbosa-Coutinho, E.; Severo Júnior, J.B. Critical Analysis of Adsorption/Diffusion Modelling as a Function of Time Square Root. Chem. Eng. Sci. 2017, 173, 179–186. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Razavi, M.M. Water Reuse: Brackish Water Desalination Using Prosopis Juliflora. Environ. Technol. Innov. 2020, 17, 100614. [Google Scholar] [CrossRef]
- Sivaraman, S.; Michael Anbuselvan, N.; Venkatachalam, P.; Ramiah Shanmugam, S.; Selvasembian, R. Waste Tire Particles as Efficient Materials towards Hexavalent Chromium Removal: Characterisation, Adsorption Behaviour, Equilibrium, and Kinetic Modelling. Chemosphere 2022, 295, 133797. [Google Scholar] [CrossRef]
- Yu, F.; Pan, J.; Li, Y.; Yang, Y.; Zhang, Z.; Nie, J.; Ma, J. Batch and Continuous Fixed-Bed Column Adsorption of Tetracycline by Biochar/MOFs Derivative Covered with κ-Carrageenan/Calcium Alginate Hydrogels. J. Environ. Chem. Eng. 2022, 10, 107996. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Y.; Lu, H.; Fang, Q.; Rong, H. Efficient Adsorption of Tetracycline from Aqueous Solutions by Modified Alginate Beads after the Removal of Cu(II) Ions. ACS Omega 2021, 6, 6240–6251. [Google Scholar] [CrossRef]
- Moussavi, G.; Hossaini, Z.; Pourakbar, M. High-Rate Adsorption of Acetaminophen from the Contaminated Water onto Double-Oxidized Graphene Oxide. Chem. Eng. J. 2016, 287, 665–673. [Google Scholar] [CrossRef]
- Ding, W.; Zhou, G.; Wen, S.; Yin, J.; Liu, C.; Fu, Y.; Zhang, L. Two-dimensional activated carbon nanosheets for rapid removal of tetracycline via strong π-π electron donor receptor interactions. Bioresour. Technol. 2022, 360, 127544. [Google Scholar] [CrossRef]
- Wei, M.; Marrakchi, F.; Yuan, C.; Cheng, X.; Jiang, D.; Zafar, F.F.; Fu, Y.; Wang, S. Adsorption modeling, thermodynamics, and DFT simulation of tetracycline onto mesoporous and high-surface-area NaOH-activated macroalgae carbon. J. Hazard. Mater. 2022, 425, 127887. [Google Scholar] [CrossRef] [PubMed]
- Ania, C.O.; Armstrong, P.A.; Bandosz, T.J.; Beguin, F.; Carvalho, A.P.; Celzard, A.; Frackowiak, E.; Gilarranz, M.A.; László, K.; Matos, J.; et al. Engaging nanoporous carbons in “beyond adsorption” applications: Characterization, challenges and performance. Carbon 2020, 164, 69–84. [Google Scholar] [CrossRef]
- Lin, J.; Hu, Y.; Xiao, J.; Huang, Y.; Wang, M.; Yang, H.; Zou, J.; Yuan, B.; Ma, J. Enhanced diclofenac elimination in Fe(II)/peracetic acid process by promoting Fe (III)/Fe(II) cycle with ABTS as electron shuttle. Chem. Eng. J. 2021, 420, 129692. [Google Scholar] [CrossRef]
- Que, W.; Jiang, L.H.; Wang, C.; Liu, Y.G.; Zeng, Z.W.; Wang, X.H.; Ning, Q.M.; Liu, S.H.; Zhang, P.; Liu, S.B. Influence of sodium dodecyl sulfate coating on adsorption of methylene blue by biochar from aqueous solution. J. Environ. Sci. 2018, 70, 166–174. [Google Scholar] [CrossRef]
- Viegas, R.M.C.; Mestre, A.S.; Mesquita, E.; Machuqueiro, M.; Andrade, M.A.; Carvalho, A.P.; Rosa, M.J. Key Factors for Activated Carbon Adsorption of Pharmaceutical Compounds from Wastewaters: A Multivariate Modelling Approach. Water 2022, 14, 166. [Google Scholar] [CrossRef]
- Marzbali, M.H.; Esmaieli, M.; Abolghasemi, H.; Marzbali, M.H. Tetracycline adsorption by H3PO4-activated carbon produced from apricot nut shells: A batch study. Process Saf. Environ. Prot. 2016, 102, 700–709. [Google Scholar] [CrossRef]
| SBET (m2/g) | Vp (m3/g) | dp (Average, nm) | |
|---|---|---|---|
| PAC | 1252.9 | 0.581 | 1.855 |
| PAC800 | 1410.8 | 0.681 | 1.930 |
| Basicity | Acidity | Phenolic Groups | Lactonic Groups | Carboxylic Groups | |
|---|---|---|---|---|---|
| PAC | 2.086 | 0.890 | 0.531 | 0.246 | 0.113 |
| PAC800 | 2.736 | 0.174 | 0.115 | 0.059 | ND |
| Model | Constants | PAC | PAC800 | ||||
|---|---|---|---|---|---|---|---|
| 296.15 K | 300.15 K | 318.15 K | 296.15 K | 300.15 K | 318.15 K | ||
| Pseudo first order | ka1 (min−1) | 0.094 | 0.130 | 0.182 | 0.110 | 0.141 | 0.205 |
| qe1 (mg/g) | 108.4 | 127.8 | 136.9 | 155.0 | 173.0 | 190.4 | |
| r2 | 0.986 | 0.969 | 0.957 | 0.983 | 0.977 | 0.966 | |
| Pseudo second order | ka2 (×10−3 g/mg․min) | 0.80 | 1.12 | 1.59 | 0.72 | 0.90 | 1.29 |
| qe2 (mg/g) | 129.9 | 146.5 | 154.3 | 181.3 | 198.0 | 214.0 | |
| r2 | 0.993 | 0.989 | 0.992 | 0.998 | 0.995 | 0.996 | |
| Intraparticle diffusion | kid (mg/g·min−0.5) | 16.8 | 21.3 | 24.2 | 25.0 | 29.3 | 32.5 |
| r2 | 0.916 | 0.829 | 0.727 | 0.886 | 0.807 | 0.676 | |
| Elovich equation | αEl (mg/g·min) | 31.0 | 65.2 | 120.2 | 57.6 | 93.9 | 199.5 |
| βEl (g/mg) | 0.0371 | 0.0358 | 0.0364 | 0.0274 | 0.0264 | 0.0269 | |
| r2 | 0.980 | 0.979 | 0.952 | 0.994 | 0.985 | 0.985 |
| PAC | PAC800 | ||||||
|---|---|---|---|---|---|---|---|
| 296.15 K | 310.15 K | 318.15 K | 296.15 K | 310.15 K | 318.15 K | ||
| Langmuir | qmax (mg/g) | 117.4 | 138.5 | 154.7 | 169.6 | 199.5 | 224.5 |
| KL (L/mg) | 1.151 | 1.426 | 1.852 | 1.846 | 1.073 | 1.196 | |
| r2 | 0.778 | 0.902 | 0.714 | 0.733 | 0.829 | 0.920 | |
| Freundlich | KF ((mg/g)(L/g)1/n) | 85.8 | 109.3 | 119.5 | 132.1 | 154.9 | 178.6 |
| 1/n | 0.076 | 0.057 | 0.066 | 0.064 | 0.061 | 0.053 | |
| r2 | 0.981 | 0.994 | 0.983 | 0.969 | 0.989 | 0.999 | |
| Temkin | bT (J/mol) | 1.07 × 10−3 | 1.26 × 10−3 | 1.41 × 10−3 | 1.47 × 10−3 | 2.28 × 10−3 | 2.44 × 10−3 |
| AT (L/mg) | 7.28 × 1016 | 8.52 × 1016 | 1.81 × 1016 | 6.12 × 1016 | 1.50 × 1013 | 1.78 × 1013 | |
| r2 | 0.529 | 0.663 | 0.606 | 0.601 | 0.800 | 0.813 |
| PAC | PAC800 | |||||
|---|---|---|---|---|---|---|
| 296.15 K | 310.15 K | 318.15 K | 296.15 K | 310.15 K | 318.15 K | |
| Ea (J/mol) | 23.7 | 19.6 | ||||
| ΔG0 (kJ/mol) | −34.23 | −47.89 | −51.47 | −41.17 | −48.91 | −51.28 |
| ΔH0 (kJ/mol) | 196.7 | 98.5 | ||||
| ΔS0 (kJ/mol·K) | 0.780 | 0.473 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-G.; Boldbaatar, S.; Ko, S.-O. Enhanced Adsorption of Tetracycline by Thermal Modification of Coconut Shell-Based Activated Carbon. Int. J. Environ. Res. Public Health 2022, 19, 13741. https://doi.org/10.3390/ijerph192113741
Kim D-G, Boldbaatar S, Ko S-O. Enhanced Adsorption of Tetracycline by Thermal Modification of Coconut Shell-Based Activated Carbon. International Journal of Environmental Research and Public Health. 2022; 19(21):13741. https://doi.org/10.3390/ijerph192113741
Chicago/Turabian StyleKim, Do-Gun, Shinnee Boldbaatar, and Seok-Oh Ko. 2022. "Enhanced Adsorption of Tetracycline by Thermal Modification of Coconut Shell-Based Activated Carbon" International Journal of Environmental Research and Public Health 19, no. 21: 13741. https://doi.org/10.3390/ijerph192113741







