Abstract
Humans have always been exposed to tiny particles via dust storms, volcanic ash, and other natural processes, and our bodily systems are well adapted to protect us from these potentially harmful external agents. However, technological advancement has dramatically increased the production of nanometer-sized particles or nanoparticles (NPs), and many epidemiological studies have confirmed a correlation between NP exposure and the onset of cardiovascular diseases and various cancers. Among the adverse effects on human health, in recent years, potential hazards of nanomaterials on female reproductive organs have received increasing concern. Several animal and human studies have shown that NPs can translocate to the ovary, uterus, and placenta, thus negatively impacting female reproductive potential and fetal health. However, NPs are increasingly being used for therapeutic purposes as tools capable of modifying the natural history of degenerative diseases. Here we briefly summarize the toxic effects of few but widely diffused NPs on female fertility and also the use of nanotechnologies as a new molecular approach for either specific pathological conditions, such as ovarian cancer and infertility, or the cryopreservation of gametes and embryos.
1. Introduction to Nanoparticles
In European legislation, a nanomaterial is defined as “an insoluble or bio-persistent and intentionally manufactured material with one or more external dimensions, or an internal structure, on the scale from 1 to ≤100 nm” [1,2]. The most important parameters for defining a nanomaterial are its size and physicochemical properties. The mode of formation of nanomaterials can be of two types: “top-down” or “bottom-up.” In the first case, they are intentionally synthesized for specific purposes or result from the random degradation of materials dispersed in the environment resulting in the formation of pollutants. In both, they are obtained by the degradation of a starting material. In the second case, they result from chemical processes and not from bulk starting materials [3]. Understanding the size and chemical characteristics of the nanomaterial is crucial, because kinetic properties, which is a set of parameters designed to estimate how much material is absorbed and its path within a living organism [4], also depend on these. Considering the dimensions as the main characteristics, we can define nanoparticles (NPs) as “nano-objects with three external nanoscale dimensions”. The terms nanorod or nanoplate are employed, instead of NPs, when the longest and the shortest axis lengths of a nano-object are different [5].
NP spread in the environment is very relevant. Indeed, we can distinguish natural nanoparticles from those man-made and then dispersed [6,7]. Atmospheric NPs can originate from forest fires and volcanic eruptions, and populations living near areas with a high frequency of these phenomena have higher hospitalization rates and more frequent respiratory problems [7,8,9]. NPs released from anthropogenic activities are concentrated mainly near urban areas with a prevalence of those smaller than 50 nm [10], and major pollutants include vehicle emissions and tire-wear particles [11,12,13]. Also, occupational hazards contribute to their formation, as the most common chemically induced occupational disease is silicosis [14]. Cigarette smoke contains many NPs interacting with the gas phase and together giving a synergistic effect that could amplify the adverse effects of each individual component [15,16,17].
Other NPs gaining significance as a pollutant are nanoplastics [18]. The toxicity of plastic wastes was understood as early as 1969, but it was not until the development of new technologies that their degradation products were also identified [19,20]. They are debris of different shapes and sizes that tend to remain in suspension given their small size [21]. Being lipophilic, they accumulate mainly in fatty tissues, and by biomagnification, their concentrations increase as one moves up the trophic chain of the ecological niche considered [22].
Depending on its composition, an NP can be lipophilic or hydrophilic: the former is able to cross cell membranes by diffusion, while the latter needs active transport [23]. NPs can be chemically modified on their surface by varying their charge to increase properties either in a targeted manner (e.g., to aid adsorption when they act as carriers for drugs), or randomly (e.g., when other pollutant molecules dispersed in the environment are adsorbed on them) [24]. Uncharged NPs tend to bind with serum proteins and, when incubated with cells, their uptake is mediated by specific receptors [25,26]. In general, positively charged NPs are taken up by endocytosis much more often than negatively charged ones because of the presence of phospholipidic molecules [27,28,29]. In addition, NPs can be bound to bioconjugates, specific biological molecules recognized by cells receptors [30]. The main mechanisms of NP endocytosis are clathrin-mediated, macropinocytosis and phagocytosis [31], which can occur independently of cargo size [32,33].

Table 1.
Nanoparticles (NPs) widely used in cosmetics and food.
Table 1.
Nanoparticles (NPs) widely used in cosmetics and food.
| NPs | Where | Dimensions | References | |
|---|---|---|---|---|
| Cosmetics | ||||
| TiO2 | Sunscreens, toothpastes | 200–400 nm | [34,35,36] | |
| ZnO | Sunscreens, toothpastes | 10–200 nm | [37,38,39] | |
| Sb, As, Cd, Pb, Cr | Eye shadow, lipstick, soap, eye shadow, body creams | / | [40] | |
| Liposomes | Skin creams and drugs, soap | Variable | [41] | |
| Nanoemulsions | Hair care products | 50–200 nm | [42] | |
| Micro- and Nanoplastics | Facial scrubs, toothpastes, nail products, shower gel | 10–100 nm/0.1–1 μm | [43] | |
| Food | ||||
| Nanocellulose | Milk products, salad dressing, ice cream | 5–100 nm | [44] | |
| Sufactantcoated NPs | Beverages, ice cream, margarine | 1–100 nm | [45] | |
| SiO2 | 1–100 nm | [46] | ||
| Cu/CuO | food packaging | [47] | ||
| Nanopolyethylene | Food packaging | 1–100 nm | [48] |
After entering in the circulatory system via various items (food/oral ingestion, inhalation, skin penetration), they can exert their negative toxic effects on cells by increasing the production of reactive oxygen species (ROS), damaging DNA and mitochondria and even inducing cell death [49]. Table 1 shows the most common and everyday products in which NPs can be found. In cosmetic products they are used primarily to contribute to the desired effect, especially antiaging, and secondarily as preservatives, and for these reasons have a larger size. In food, they are added for antibacterial purpose and to increase shelf life. Also, the possibility that NPs can result from the degradation of transport coatings and subsequent passage into food cannot be excluded. A link seems to exist between NPs and cancer: for example, iron-rich NPs from traffic pollution are likely a plausible cause of brain cancer, while a significant association between wildfires and the incidence of specific cancer outcomes (as lung, brain and some haematological cancers) has been recently proposed [50,51].
With regard to metallic NPs, their effects depend mainly on their composition, morphology, size and crystal structure. They are characterized by a metal core composed of inorganic metal or metal oxide usually covered with a shell formed by organic, inorganic material or metal oxide. The most widely used metallic NPs in medicine, food, agriculture and industry include Ag, Au, ZnO, TiO2, CuO, CeO2 and FeO. Among these, TiO2 NPs are particularly popular in biomedical applications, being employed for the fabrication of biomedical devices and for bioimaging [52]. Considering the breadth of applications to date, little is known about their toxicity except for a few metal NPs used in biomedical fields [53].
Since 2015, the European Commission has launched a series of proposals with the aim of reducing their use both to safeguard human health and the environment [54]. In 2021, the European Food Safety Authority (EFSA) produced a summary document on the main procedures for determining NP counts in food [55]. For example, the use of TiO2NPs as a food coloring and flavoring agent is no longer considered safe because of its effects on DNA. Despite its low bioavailability, it can accumulate in the body reaching high chronic concentrations especially in long-living animals and breeding animals [56]. To date, it remains usable in medicinal products at depletion [57,58]. The presence of TiO2NPs in cosmetics as a preservative and colorant has been reduced to 25% in masks and hair products, a value considered safe without prejudice to its carcinogenic capacity by inhalation [59]. Other NPs, including those listed in Table 1, are still under observation.
The reproductive toxicity of these NPs is becoming a public health problem due to the use of NP-containing consumer products and occupational exposure. Both male and female reproductive organs are vulnerable, but in females the presence of NPs may compromise fertility and eventual fetal development more dramatically due to the fixed and not renewable number of oocytes present at birth in the mammalian ovaries. Furthermore, NP accumulation seems to be strongly conditioned by menstrual cycle, as demonstrated by the 2- fold increase in ovarian and uterine NP content occurring during mouse ovulation in comparison with the other stages of estrus cycle [60]. In this review we will discuss not only of the toxic effects of selected NPs on female reproductive organs but also of their importance for fertility preservation.
2. Path of NPS Access into Cells of Female Reproductive System
Since NP intake via skin is considered to be below detection level, ingestion and inhalation represent the major route by which NPs are transferred to organs and cells, as confirmed by their presence in the circulatory and lymphatic systems as well as urine [61]. Since NPs are able to cross biological barriers in a way strongly conditioned by physical characteristics (size, shape, etc.), they can be deposited in the target organs reaching cytoplasm and mitochondria. In testis, NPs can cross the blood–testis barrier which can be completely harmed due to increased ROS production [62], while they can accumulate in the cells of female reproductive organs by mechanisms not completely understood yet, but capable of inducing significant increase of ROS levels. Even if a key point is to define if and how NPs can be internalized into oocytes, their storage in the somatic compartment of the ovary results in a dramatic impairment of oocyte developmental competence, which is the ability to be successfully fertilized and to form a blastocyst. This property is acquired in a stepwise manner during folliculogenesis, and is strongly conditioned by the maintenance of bidirectional communications between the somatic and germinal compartment [63]. Below, we will report recent data focusing on the reproductive toxic effects and utilization for therapeutic purposes of some of the most-used NPs.
3. NP-Induced Toxicity in Ovary and Oocytes
3.1. Zinc Oxide Nanoparticles (ZnONPs or nZnO) and Titanium Dioxide Nanoparticle (TiO2NPs or nTiO2)
Both NPs are commonly used in a wide range of products, from paints to cosmetics. nTiO2 is produced in three commercial forms named rutile, brookite and anatase, which is the most utilized [64]. In recent years, several studies have investigated their toxic effects in vivo and in vitro.
3.1.1. In Vivo Exposure
The mechanisms by which TiO2NPs can damage ovarian cells have mainly been studied in laboratory animals. Experimental data demonstrated that a long-term administration (5–8 weeks) of TiO2NPs (1–10 mg/kg/d) to female mice caused alterations of gonadotropin and sex steroid release, cysts formation and follicular atresia together with significant reduction of pregnancy rate due to extensive ovarian inflammation caused by elevated ROS production [65,66,67]. Interestingly, these NPs can negatively interfere with the TGF beta pathway, which plays important roles in regulating several ovarian functions, including follicle development and maturation [68]. Ovarian mitochondrial damages and the expression level of apoptotic genes increased concomitantly with the accumulation of TiO2NPs (1.25–2.5–5 mg/kg for 90 consecutive days) in both the cytoplasm and nuclei of follicle cells [69]. The rise of oxidative stress level and of Cyp17a1 gene expression, together with altered regulation of many (about 288) ovarian genes, account for the decline of fertility and pregnancy rate. In vivo, increased estrogen release and decreased testosterone/progesterone levels have been detected in rodents of various strains treated with increasing concentrations of TiO2NPs [70]. Contrasting effects on steroid hormone release have been obtained by Hong and Wang [71] and Zhao et al. [72] following mice exposure to similar concentrations of anatase-nTiO2 (2.5–5–10 mg/kg for 30 days), but differences can be ascribed to different treatment times and different mouse strains used in the experiments.
The rapidly expanding production and use of ZnONPs in consumer products (e.g., tooth paste and food packaging) also stimulated studies on this nanomaterial and its ability to alter/disrupt cell functions. nZnO toxicity seems to mainly be due to Zn ion release, although an intrinsic particle toxicity should be considered especially after long-term exposure [73]. In females, reprotoxic effects induced by nZnO (5–10 μg/mL for 24 h) consisted of the alteration of ovarian gene expression, stimulation of apoptosis, interference with Sonic Hedgehog pathway, which has a key role in the regulation of tissue homeostasis, and with steroid hormone synthesis [74,75]. Exposure to nZnO of mouse fetal ovaries (0.2–0.8 mg/kg for two days) resulted in the accumulation of double-strand breaks (DSBs) in pachytene oocytes, with a consequent decrease of ovarian follicle reservoir. Interestingly, ZnO-dependent oocyte damage relies on different mechanisms depending on in vitro or in vivo exposure: in the former, excessive accumulation of DSBs lowered the number of germ cells via apoptosis; in the latter, oocyte selection mechanisms triggered the elimination of ovarian follicle reserve [76].
3.1.2. In Vitro Exposure
The presence during culture of TiO2NPs inhibited rat follicle development and oocyte maturation in a size- and concentration-dependent manner [77]. In fact, when rat preantral follicles were cultured for 10 days in the presence of 1 μm or 25 nmTiO2 at increasing concentrations (12.5–25–50 μg/mL), follicle development and oocyte maturation were dramatically inhibited only in the presence of 25 nm TiO2 at 25 μg/mL or above. The presence of TiO2NPs did not hamper the process of cumulus expansion in mice, while a dramatic negative effect was caused by ZnO NM-110 and NM-111 at relatively low concentrations (1 mg/mL). Indeed, experimental data showed that ZnONPs, but not SiO2-coated ZnONPs, affected the expression of genes fundamental for the regulation of this process. The negative effect seems to be based either onion release or NP presence per se [78].
Another recent study performed on mouse antral follicles [79] showed that when antral follicles were exposed in vitro to ZnONPs or TiO2NPs (5–50 μg/mL for 96 h), the negative impacts on somatic and germ cells were significantly different. First of all, TEM analysis showed the absence of ZnONPs in antral follicular cells, while TiO2 NPs were easily detectable in theca, granulosa and cumulus cell cytoplasm as agglomerates enclosed in small vesicles or near mitochondria. Moreover, the presence of invaginations on cell surface confirms that TiO2NPs were internalized by micropinocytosis or clathrin-mediated endocytosis. Both NPs had a great impact on cytoskeleton arrangement and trans-zonal projections (TZP) between somatic cells and oocytes, which play a central role in the fine regulation of normal oocyte and follicle development [80]. Surprisingly, the two types of NPs exerted opposite effects on follicle diameters (TiO2: increase; ZnO: decrease), but none of them have been found in cultured oocytes that were indirectly damaged because of TZP disruption. Moreover, nZnO toxicity, evidenced by ROS increase, was higher than that caused by nTiO2, probably as a result of Zn ion release in culture media. A similar mechanism has been described as occurring also in other cells [80]. The cytotoxic and genotoxic effects of nTiO2 and nAl2O3 were evaluated on Chinese hamster ovarian (CHO-K1) cells. Changes in lysosomal and mitochondrial dehydrogenase activity as well as the formation of perinuclear vesicles in CHO-K1 cells were demonstrated after treatment with both NPs, but none was detectable in the nuclei [81].
3.2. Gold and Silver NPs In Vivo and In Vitro
Other metallic NPs can impair follicle survival and ovarian steroidogenesis. Silver nanoparticles (AgNPs) are used for their antimicrobial activity in food, home appliances, water treatment and for disinfecting medical devices [82]. Gold nanoparticles (AuNPs), instead, are a new application in nanomedicine because of the absence of toxic effects at the doses used for drug delivery [83]. However, AuNPs were internalized by cultured buffalo granulosa cells and operated as ovarian endocrine disruptors by perturbing steroidogenesis. In particular, progesterone production was significantly increased, probably as a consequence of increased mitochondria permeability due to particle movement across the cells and other membranes. Such a disturbance of the mitochondrial membranes and homeostasis generates ROS that, in turn, causes oxidative stress and even cell death by stimulating the expression of pro-apoptotic genes [84,85]. Different results were obtained by Tiedemann et al., because in their experiments AuNPs showed (after 46 h) no detrimental effects on porcine cumulus–oocyte complexes regardless of size, surface ligands or concentration [86]. Despite a selective uptake of AuNPs by oocytes, they did not have toxic effects and the authors proposed these particles as a top candidate for biomedical applications.
Other studies evaluated the reproductive risks associated with exposure to widely used AgNPs. Cytotoxic responses include increased levels of ROS production, apoptosis, DNA damage and inflammation, although the molecular and cellular mechanisms by which they can exert adverse effects at the organismal level remain elusive [87]. Porcine cumulus cells can accumulate AgNPs, which caused the alteration of cell proliferation with indirect consequences to oocyte maturation, probably due to ion release [88,89]. In addition, the expression of proliferation-related and apoptosis-related peptides such as cyclin B1 and caspase-3 had been changed in porcine ovarian granulosa cells after AgNPs addition [90]. Similar results were obtained in mice intravenously injected with increasing doses (0–50 mg/mL) of these particles, since they accumulated in a dose-dependent manner in ovaries and induced inflammatory response as well as granulosa cell and theca cell apoptosis. As expected, a significant decrease in the expression levels of genes involved in steroidogenesis and in the maintenance of follicle survival from primordial to antral stage was found [90]. Oocyte damage was also recorded after in vitro exposure to AgNPs. Again, cytoskeletal modifications culminated with retraction of TZPs, which caused the impairment of cumulus expansion and of meiotic resumption in pig cumulus–oocyte complexes exposed during IVM [91].
4. Uterus and Placenta Are Targeted by NPs
The presence of NPs in the uterus can induce developmental toxicity and has deleterious consequences to reproductive performance. In in vivo-exposed animals, TiO2NPs and AgNPs (0.2, 0.4, or 0.8 µM for 2 h) have been detected in the uterus where increased oxidative stress and apoptosis of connective tissues with consequent alteration of contractile activity were observed [92,93,94].
It is generally accepted that fetuses are more sensitive to environmental toxins than adults [95]. Recent evidence showed that NPs can easily cross the placenta to reach the fetal compartment in a size-dependent manner. In fact, experiments conducted with animal models showed that NPs can negatively impact a fetus in a way strongly conditioned by chemical structure (size, composition, charge, etc.) and by the stage of pregnancy. For example, small silica nSP70 and 35 nm TiO2 NPs, administered once every three days from day three of gestation until delivery at 0.02 and 2 mg/Kg, might impair normal fetus development in comparison with higher diameters by causing DNA damage in offspring [96]. These NPs triggered fetal inflammation, apoptosis, and genotoxic and neuronal damage, which resulted in abnormal embryonic development and fetal death [97]. Most studies on placental toxicity have been carried out using laboratory animals, but the existence of several structural differences in placenta organization between rodents and humans has stimulated the use of human cellular models. In pregnant women, NPs may cross the placenta by molecular transport mechanisms that are still poorly understood, although endocytosis and passive transport are the most accredited. Exposure to AgNPs increases the risk of damage to the nervous system of fetuses and also other nano-oxides (as Zn, Fe and Cd oxide) can induce cytotoxic effects and cell death [97].
It is evident that the indirect toxicity of NPs on placental/fetal functions and long-term health requires further exploration, especially because the key steroidogenic role of placenta during pregnancy could be affected by the suspected endocrine disrupting effects of specific nanomaterials used in NP generation [98].
5. NP-Induced Genotoxicity
One of the main problems related to nanomaterials is their ability to induce genotoxic, and thus mutagenic and clastogenic, damage on DNA [99]. The damage can be direct, if there is interaction with the genetic material, or indirect, if it is mediated by the formation of chemical species such as ROS [99]. This second mechanism is very important, since NPs are unlikely to reach the nucleus [100]. A recent study on AgNP genotoxicity evidenced positive results based on several in vitro and in vivo tests (in vivo: micronucleus tests, chromosome aberration tests, comet assays; in vitro: mouse lymphoma assays, micronucleus tests, comet assays). Even if AgNPs impaired the DNA repair system and depleted cells of antioxidant molecules, results did not confirm with certainty that the genotoxic effect was produced exclusively by oxidative damage. Therefore, the authors suggested performing a complete genotoxicological assessment of this, and obviously of other NPs, before drawing definitive conclusions on human exposure [101,102]. The use of nanotechnologies for diagnostic and therapeutic purposes based on injections of TiO2 and AuNPs in laboratory animals have increased the appearance of new metastatic sites by increasing the gap between blood vessel cells and allowing cancer cells to migrate more easily to new sites, pointing to the need of accurate studies before using NPS to treat cancers [103].
6. The Benefits of Nanotechnologies
6.1. Preservation of Fertility
The correct application of cryopreservation technologies is fundamental to improve organ/cell shortage for transplantation. Fertility in young women with cancer but also in healthy young women who electively wish to delay childbearing to avoid the detrimental effects of aging on reproductive capacity. Cryopreservation of germ cells requires the use of specific freezing/thawing cocktails, in which different cryoprotectant agents (CPA) and other chemicals are added depending on freezing procedures (slow freezing or vitrification). Due to its rapid procedure, vitrification is now the most-used method to preserve germ cells and embryos.
Unfortunately, the survival rate of vitrified oocytes is still low (about 4%) [104], but recent data showed that the incorporation of specific NPs into CPA solutions could improve outcomes by avoiding recrystallization of vitrification solution during rewarming [105]. Experiments conducted on porcine oocytes arrested at GV stage and vitrified with or without different concentrations (0.01%, 0.02%, 0.05%, 0.1%, 0.5% w/w) of silicon dioxide (SiO2), aluminum oxide (Al2O3), hydroxy apatite (HA) and TiO2NPs (diameter: 20 nm) showed that HA and SiO2 are the most biocompatible nanomaterials having a reduced toxicity. In fact, when used at 0.05%, oocyte survival rate and capacity to complete meiosis up to MII stage were higher in comparison with the other NPs tested and control conditions [106]. Similar protective effects were also obtained for vitrified spermatozoa following ZnONP supplementation, and analysis by confocal and high-resolution transmission electron microscopy clearly demonstrated that these NPs did not enter spermatozoa cytoplasm [107]. More recently, Abbasi et al. also showed that Fe3O4NPs (0.004%, 0.008% and 0.016% w/v) could protect mouse GV oocytes from cryodamage during vitrification procedures. After IVM and IVF, the quality of frozen/thawed embryos has been found to be positively modulated by presence of these NPs [108].
Another interesting aspect of female fertility preservation in which NPs are starting to use is contrasting ovarian aging. Despite the existence of various strategies, no clinical treatments are currently available to delay ovarian aging. Drug delivery via NPs should be effective in postponing ovarian aging. In fact, studies are currently being pursued to use them in both 3D cultures and tissue engineering, with the purpose of increasing cell proliferation and viability [109].
6.2. Ovarian Cancer Therapy
A significant improvement in the safety of NP-delivered drugs in cancer patients has been achieved in recent years to bypass the limitations of chemotherapy. NPs are utilizable for biomedical applications to facilitate the absorption of drugs that can be bound on their surface [110,111]. To date, the most widely used in diagnostics and bioimaging as well as in cancer therapies are AuNPs because they are stable metals with low cytotoxicity, but also gold and carbon nanotubes could be used to treat some forms of cancer, for bioimaging and gene therapy [112].
Recently, the application of RNAi-based therapies has been sharply accelerated because a significant decrease in nonspecific toxicity can be obtained by encapsulating multiple payloads of drugs within nanocarriers and by monitoring si-RNA delivery by noninvasive imaging techniques. Nano-siRNA drugs can be of great help in overcoming the limitations of chemotherapy. Recently, NP-mediated siRNA delivery strategies such as polymeric- and lipid-based systems, rigid nanoparticles and NP coupled with specific ligand systems have been tested. NP-based codelivery of anticancer drugs and siRNA targeting various mechanisms of multi-drug resistance is a cutting-edge strategy for ovarian cancer therapy. Since siRNAs that are negatively charged are unable to cross cell membranes, the use of nanocarriers allows for their increased cellular uptake [113]. One example is the use of chitosan NPs, which, being positively charged, fulfill the role well [114]. The use of dendrimers has already been tested for the therapy of this deadly cancer, and reduced proliferation in vitro and reduced metastasis in vivo were described [115]. As above mentioned, AuNPs do not appear to show allergic or adverse effects and have an excellent ability to be manipulated. Indeed, their use can be associated with siRNAs that induce apoptosis in ovarian cancer cells [116]. The two molecules have been tested together to exploit their synergistic effect and to inhibit the causes of resistance to therapies. For example, it has been shown that the apoptosis of cancer cells can be increased by exploiting the effect of pro-apoptotic siRNAs with doxorubicin [117].
7. Conclusions
The fact that NPs can harm biological systems but can be used for innovative therapies capable of conferring health benefits highlights the two sides of a coin (Figure 1). To lessen their toxic side effects, it’s necessary to reduce environmental pollution and to increase our knowledge of the pathways by which NPs enter human body to modulate cell metabolism. At the same time, the production of NPs able to select specific organs or cells will be a non-invasive option for successful molecular therapy of many human pathologies as well as of genetic anomalies, especially of embryos or oocytes. In this context, the development of new treatments of pathological reproductive conditions and the most frequent complications of pregnancy are needed and are of great importance for women’s health. Different coatings able to reduce toxic effects (e.g., albumin, polyethylene glycol, aspartic acid) are under investigation to facilitate their penetration into cells and to increase NP stability and targeted distribution in human body. From inorganic and organic particles, technologies are also moving toward new chemical forms such as lipid NPs. One of the most recent applications is in vaccines. With the COVID-19 outbreak and the licensing of mRNA-based vaccines, nanostructural technologies have been used to transport mRNA. These correspond to liposomes, nanostructures characterized by an inhomogeneous mixture of lipid components: neutral or charged lipids that promote the entry of nucleic material by electrostatic interaction, cholesterol that decreases permeability and increases stability, PEG-lipids that hinder binding to nonspecific proteins and helper lipids that facilitate their fusion with target membranes [118,119,120]. Thus, distinguishing the applications of nanomedicine from nanotoxicology becomes critical, and understanding the cut-off point between biomedical application and adverse effects will allow us to improve possible therapeutic applications: only by combining diagnostic testing and prevention will it be possible to improve both sides of this coin.
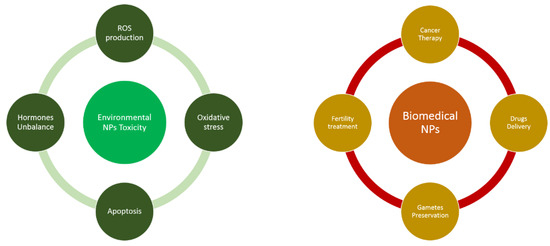
Figure 1.
Toxicity and biomedical applications of environmental and artificial NPs.
Author Contributions
M.A., G.R., S.C. (Sabrina Colafarina), M.G., S.C. (Sandra Cecconi) and A.M.G.P. jointly conceptualized the review; M.A., S.C. (Sandra Cecconi) and A.M.G.P. designed and wrote the review; G.R., S.C. (Sabrina Colafarina) and M.G. critically revised the review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). Scientific Basis for the Definition of the Term “Nanomaterial”; Pre-consultation opinion; European Union: Maastricht, The Netherlands, 2010. [Google Scholar]
- Regulation (EC) No. 1223/2009, Art. 2(1). Available online: https://ec.europa.eu/growth/sectors/cosmetics/cosmetic-products-specific-topics/nanomaterials_en (accessed on 23 June 2022).
- Alves, O.L.; De Moraes, A.C.M.; Simões, M.B.; Fonseca, L.C.; Nascimento, R.O.D.; Holtz, R.D.; De Faria, A.F. Nanomaterials. In Nanotoxicology. Nanomedicine and Nanotoxicology; Durán, N., Guterres, S., Alves, O., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Hochhaus, G.; Barrett, J.S.; Derendorf, H. Evolution of Pharmacokinetics and Pharmacokinetic/Dynamic Correlations during the 20th Century. J. Clin. Pharmacol. 2000, 40, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Shao, L.; Jones, T.P.; Lu, S. Microscopy and mineralogy of airborne particles collected during severe dust storm episodes in Beijing, China. J. Geophys. Res. 2005, 110, D01303. [Google Scholar] [CrossRef]
- Mott, J.A.; Meyer, P.; Mannino, D.; Redd, S.C.; Smith, E.M.; Gotway-Crawford, C.; Chase, E. Wildland forest fire smoke: Health effects and intervention evaluation, Hoopa, California, 1999. West. J. Med. 2002, 176, 157–162. [Google Scholar] [CrossRef]
- Carlsen, H.K.; Hauksdottir, A.; Valdimarsdottir, U.A.; Gíslason, T.; Einarsdottir, G.; Runolfsson, H.; Briem, H.; Finnbjornsdottir, R.G.; Gudmundsson, S.; Kolbeinsson, T.B.; et al. Health effects following the Eyjafjallajökull volcanic eruption: A cohort study. BMJ Open 2012, 2, e001851. [Google Scholar] [CrossRef]
- Gudmundsson, G. Respiratory health effects of volcanic ash with special reference to Iceland. A review. Clin. Respir. J. 2010, 5, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Rönkkö, T.; Timonen, H. Overview of Sources and Characteristics of Nanoparticles in Urban Traffic-Influenced Areas. J. Alzheimer’s Dis. 2019, 72, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Vecchiotti, G.; Colafarina, S.; Zarivi, O.; Arrizza, L.; Di Carlo, P.; Di Cola, A. Exposure to particle debris generated from passenger and truck tires induces different genotoxicity and inflammatory responses in the RAW 264.7 cell line. PLoS ONE 2019, 14, e0222044. [Google Scholar] [CrossRef] [PubMed]
- Lindbom, J.; Gustafsson, M.; Blomqvist, G.; Dahl, A.; Gudmundsson, A.; Swietlicki, E.; Ljungman, A.G. Wear Particles Generated from Studded Tires and Pavement Induces Inflammatory Reactions in Mouse Macrophage Cells. Chem. Res. Toxicol. 2007, 20, 937–946. [Google Scholar] [CrossRef]
- Krefft, S.; Wolff, J.; Rose, C. Silicosis: An Update and Guide for Clinicians. Clin. Chest Med. 2020, 41, 709–722. [Google Scholar] [CrossRef]
- Salvi, S. Tobacco Smoking and Environmental Risk Factors for Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2014, 35, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control 2003, 12, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Thorne, D.; Adamson, J. A review of in vitro cigarette smoke exposure systems. Exp. Toxicol. Pathol. 2013, 65, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Mofijur, M.; Ahmed, S.; Rahman, S.A.; Siddiki, S.Y.A.; Islam, A.S.; Shahabuddin, M.; Ong, H.C.; Mahlia, T.; Djavanroodi, F.; Show, P.L. Source, distribution and emerging threat of micro- and nanoplastics to marine organism and human health: Socio-economic impact and management strategies. Environ. Res. 2021, 195, 110857. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, K.W.; Kridler, E. Laysan Albatrosses Swallow Indigestible Matter. Auk 1969, 86, 339–343. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef]
- Lee, H.; Shim, W.J.; Kwon, J.-H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014, 470–471, 1545–1552. [Google Scholar] [CrossRef]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; Pillai, B.; Das, T.K.; Singh, Y.; Maiti, S. Molecular Effects of Uptake of Gold Nanoparticles in HeLa Cells. ChemBioChem 2007, 8, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Thomas, T.P.; Myc, L.A.; Kotlyar, A.; Baker, J.R., Jr. Synthesis, characterization, and intracellular uptake of carboxyl-terminated poly(amidoamine) dendrimer-stabilized iron oxide nanoparticles. Phys. Chem. Chem. Phys. 2007, 9, 5712–5720. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.L.; Bernas, L.M.; Rutt, B.K.; Foster, P.; Gillies, E.R. Enhanced Cell Uptake of Superparamagnetic Iron Oxide Nanoparticles Functionalized with Dendritic Guanidines. Bioconjug. Chem. 2008, 19, 2375–2384. [Google Scholar] [CrossRef]
- Castagna, A.; Zander, A.J.; Sautkin, I.; Schneider, M.; Shegokar, R.; Königsrainer, A.; Reymond, M.A. Enhanced intraperitoneal delivery of charged, aerosolized curcumin nanoparticles by electrostatic precipitation. Nanomedicine 2021, 16, 109–120. [Google Scholar] [CrossRef]
- Braeken, Y.; Cheruku, S.; Ethirajan, A.; Maes, W. Conjugated Polymer Nanoparticles for Bioimaging. Materials 2017, 10, 1420. [Google Scholar] [CrossRef]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef]
- Park, J.; Lim, D.-H.; Lim, H.-J.; Kwon, T.; Choi, J.-S.; Jeong, S.; Choi, I.-H.; Cheon, J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem. Commun. 2011, 47, 4382–4384. [Google Scholar] [CrossRef]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailänder, V.; Landfester, K.; et al. Differential Uptake of Functionalized Polystyrene Nanoparticles by Human Macrophages and a Monocytic Cell Line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. 7), 34–46. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2018, 35, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef]
- Ng, C.T.; Yong, L.Q.; Hande, M.P.; Ong, C.N.; Yu, L.E.; Bay, B.H.; Baeg, G.H. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int. J. Nanomed. 2017, 12, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52, Erratum in Adv. Colloid Interface Sci. 2018, 254, 100. [Google Scholar] [CrossRef]
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic metals contained in cosmetics: A status report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef]
- Rahimpour, Y.; Hamishehkar, H. Liposomes in cosmeceutics. Expert Opin. Drug Deliv. 2012, 9, 443–455. [Google Scholar] [CrossRef]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Bin Johan, M.R.; Fen, L.B. Comprehensive review on nanocellulose: Recent developments, challenges and future prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahajan, P.; Kaur, R.; Gautam, S. Nanotechnology and its challenges in the food sector: A review. Mater. Today Chem. 2020, 17, 100332. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Metal-based nanoparticles, sensors, and their multifaceted application in food packaging. J. Nanobiotechnol. 2021, 19, 256. [Google Scholar] [CrossRef] [PubMed]
- Brito, S.D.C.; Bresolin, J.D.; Sivieri, K.; Ferreira, M.D. Low-density polyethylene films incorporated with silver nanoparticles to promote antimicrobial efficiency in food packaging. Food Sci. Technol. Int. 2020, 26, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Chraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.-H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. Chest 2018, 155, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Korsiak, J.; Pinault, L.; Christidis, T.; Burnett, R.T.; Abrahamowicz, M.; Weichenthal, S. Long-term exposure to wildfires and cancer incidence in Canada: A population-based observational cohort study. Lancet Planet. Health 2022, 6, e400–e409. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. Nanostructured titanium oxide hybrids-based electrochemical biosensors for healthcare applications. Colloids Surfaces B Biointerfaces 2019, 178, 385–394. [Google Scholar] [CrossRef]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef]
- European Commission. European Green Deal: Commission Consults on Reducing the Release of Microplastics into the Envi-ronment. Available online: https://environment.ec.europa.eu/news/microplastics-public-consultation-2022-02-22_en (accessed on 8 October 2022).
- EFSA. Outcome of the public consultation on the draft Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA Support. Publ. 2021, 18, 6804E. [Google Scholar] [CrossRef]
- EFSA. Safety and efficacy of a feed additive consisting of titaniumdioxide for all animal species (Kronos International, Inc.). EFSA J. 2021, 19, e06630. [Google Scholar] [CrossRef]
- European Commission. Amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as Regards the Food Additive Titanium Dioxide (E 171). 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R0063 (accessed on 8 October 2022).
- FDA-2011-D-0489; Guidance for Industry: Safety of Nanomaterials in Cosmetic Products. Food and Drugs Administration: Silver Spring, MD, USA, 2014.
- SCCS/1617/20; OPINION on Titanium Dioxide (TiO2) Used in Cosmetic Products that Lead to Exposure by Inhalation. European Commission: Brussels, Belgium, 2020.
- Poley, M.; Mora-Raimundo, P.; Shammai, Y.; Kaduri, M.; Koren, L.; Adir, O.; Shklover, J.; Shainsky-Roitman, J.; Ramishetti, S.; Man, F.; et al. Nanoparticles Accumulate in the Female Reproductive System during Ovulation Affecting Cancer Treatment and Fertility. ACS Nano 2022, 16, 5246–5257. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, C.; Ji, G.; Liu, H.; Mo, Y.; Tollerud, D.J.; Gu, A.; Zhang, Q. Sublethal effects of zinc oxide nanoparticles on male reproductive cells. Toxicol. Vitr. 2016, 35, 131–138. [Google Scholar] [CrossRef]
- Zuccotti, M.; Merico, V.; Cecconi, S.; Redi, C.A.; Garagna, S. What does it take to make a developmentally competent mammalian egg? Hum. Reprod. Updat. 2011, 17, 525–540. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- You, D.G.; Deepagan, V.G.; Um, W.; Jeon, S.; Son, S.; Chang, H.; Yoon, H.I.; Cho, Y.W.; Swierczewska, M.; Lee, S.; et al. ROS-generating TiO2 nanoparticles for non-invasive sonodynamic therapy of cancer. Sci. Rep. 2016, 6, 23200. [Google Scholar] [CrossRef]
- Tassinari, R.; Cubadda, F.; Moracci, G.; Aureli, F.; D’Amato, M.; Valeri, M.; De Berardis, B.; Raggi, A.; Mantovani, A.; Passeri, D.; et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology 2014, 8, 654–662. [Google Scholar] [CrossRef]
- Karimipour, M.; Javanmard, M.Z.; Ahmadi, A.; Jafari, A. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. Int. J. Reprod. Biomed. (IJRM) 2018, 16, 397–404. [Google Scholar] [CrossRef]
- Zhou, Y.; Hong, F.; Wu, N.; Ji, J.; Cui, Y.; Li, J.; Zhuang, J.; Wang, L. Suppression of ovarian follicle development by nano TiO2 is associated with TGF-β-mediated signaling pathways. J. Biomed. Mater. Res. Part A 2019, 107, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Ze, Y.; Li, B.; Zhao, X.; Zhang, T.; Sheng, L.; Hu, R.; Gui, S.; Sang, X.; Sun, Q.; et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J. Hazard. Mater. 2012, 243, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Santacruz-Márquez, R.; Santos, M.G.-D.L.; Hernández-Ochoa, I. Ovarian toxicity of nanoparticles. Reprod. Toxicol. 2021, 103, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Wang, L. Nanosized titanium dioxide-induced premature ovarian failure is associated with abnormalities in serum parameters in female mice. Int. J. Nanomed. 2018, 13, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ze, Y.; Gao, G.; Sang, X.; Li, B.; Gui, S.; Sheng, L.; Sun, Q.; Cheng, J.; Cheng, Z.; et al. Nanosized TiO2-Induced Reproductive System Dysfunction and Its Mechanism in Female Mice. PLoS ONE 2013, 8, e59378. [Google Scholar] [CrossRef]
- Youn, S.-M.; Choi, S.-J. Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity. Int. J. Mol. Sci. 2022, 23, 6074. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, L.; Min, L.-J.; Zhu, L.-Q.; Sun, Q.-Y.; Zhang, H.-F.; Liu, X.-Q.; Zhang, W.-D.; Ge, W.; Wang, J.-J.; et al. Regulation of MicroRNAs, and the Correlations of MicroRNAs and Their Targeted Genes by Zinc Oxide Nanoparticles in Ovarian Granulosa Cells. PLoS ONE 2016, 11, e0155865. [Google Scholar] [CrossRef]
- Kuang, H.; Zhang, W.; Yang, L.; Aguilar, Z.P.; Xu, H. Reproductive organ dysfunction and gene expression after orally administration of ZnO nanoparticles in murine. Environ. Toxicol. 2021, 36, 550–561. [Google Scholar] [CrossRef]
- Zhai, Q.-Y.; Ge, W.; Wang, J.-J.; Sun, X.-F.; Ma, J.-M.; Liu, J.-C.; Zhao, Y.; Feng, Y.-Z.; Dyce, P.W.; De Felici, M.; et al. Exposure to Zinc oxide nanoparticles during pregnancy induces oocyte DNA damage and affects ovarian reserve of mouse offspring. Aging 2018, 10, 2170–2189. [Google Scholar] [CrossRef]
- Hou, J.; Wan, X.-Y.; Wang, F.; Xu, G.-F.; Liu, Z.; Zhang, T.-B. Effects of titanium dioxide nanoparticles on development and maturation of rat preantral follicle in vitro. Acad. J. Second Mil. Med. Univ. 2009, 29, 869–873. [Google Scholar] [CrossRef]
- Camaioni, A.; Massimiani, M.; Lacconi, V.; Magrini, A.; Salustri, A.; Sotiriou, G.A.; Singh, D.; Bitounis, D.; Bocca, B.; Pino, A.; et al. Silica encapsulation of ZnO nanoparticles reduces their toxicity for cumulus cell-oocyte-complex expansion. Part. Fibre Toxicol. 2021, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Santacruz-Márquez, R.; Solorio-Rodríguez, A.; González-Posos, S.; García-Zepeda, S.P.; Santoyo-Salazar, J.; De Vizcaya-Ruiz, A.; Hernández-Ochoa, I. Comparative effects of TiO2 and ZnO nanoparticles on growth and ultrastructure of ovarian antral follicles. Reprod. Toxicol. 2020, 96, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Canipari, R.; Cellini, V.; Cecconi, S. The Ovary Feels Fine when Paracrine and Autocrine Networks Cooperate with Gonadotropins in the Regulation of Folliculogenesis. Curr. Pharm. Des. 2012, 18, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, A.; Reigosa, M.; Arnal, P.; de Mele, M.F.L. Comparative study of the cytotoxic and genotoxic effects of titanium oxide and aluminium oxide nanoparticles in Chinese hamster ovary (CHO-K1) cells. J. Hazard. Mater. 2010, 177, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Stelzer, R.; Hutz, R.J. Gold Nanoparticles Enter Rat Ovarian Granulosa Cells and Subcellular Organelles, and Alter In-Vitro Estrogen Accumulation. J. Reprod. Dev. 2009, 55, 685–690. [Google Scholar] [CrossRef]
- Lyngdoh, E.L.; Nayan, V.; Vashisht, M.; Kumari, S.; Bhardwaj, A.; Bhatia, T.; Dalal, J.; Pawaria, S.; Onteru, S.K.; Sikka, P.; et al. Gold nanoparticles modulate the steroidogenic and apoptotic pathway in a buffalo granulosa cell model. Biotechnol. Lett. 2020, 42, 1383–1395. [Google Scholar] [CrossRef]
- Tiedemann, D.; Taylor, U.; Rehbock, C.; Jakobi, J.; Klein, S.; Kues, W.A.; Barcikowski, S.; Rath, D. Reprotoxicity of gold, silver, and gold–silver alloy nanoparticles on mammalian gametes. Analyst 2014, 139, 931–942. [Google Scholar] [CrossRef]
- Mao, B.-H.; Chen, Z.-Y.; Wang, Y.-J.; Yan, S.-J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018, 8, 2445. [Google Scholar] [CrossRef]
- Kolesarova, A.; Capcarova, M.; Sirotkin, A.V.; Medvedova, M.; Kovacik, J. In vitro assessment of silver effect on porcine ovarian granulosa cells. J. Trace Elements Med. Biol. 2011, 25, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Jeong, J.-K.; Gurunathan, S.; Choi, Y.-J.; Das, J.; Kwon, D.-N.; Cho, S.-G.; Park, C.; Seo, H.G.; Park, J.-K.; et al. Male- and female-derived somatic and germ cell-specific toxicity of silver nanoparticles in mouse. Nanotoxicology 2016, 10, 361–373. [Google Scholar] [CrossRef]
- Dianová, L.; Tirpák, F.; Halo, M.; Slanina, T.; Massányi, M.; Stawarz, R.; Formicki, G.; Madeddu, R.; Massányi, P. Effects of Selected Metal Nanoparticles (Ag, ZnO, TiO2) on the Structure and Function of Reproductive Organs. Toxics 2022, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Appeltant, R.; Somfai, T.; Nakai, M.; Bodó, S.; Maes, D.; Kikuchi, K.; Van Soom, A. Interactions between oocytes and cumulus cells during in vitro maturation of porcine cumulus-oocyte complexes in a chemically defined medium: Effect of denuded oocytes on cumulus expansion and oocyte maturation. Theriogenology 2015, 83, 567–576. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K.; et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-S.; Kim, S.; Lee, S.H.; Jo, E.; Lee, B.; Yoon, J.; Eom, I.-C.; Kim, H.-M.; Kim, P.; Choi, K.; et al. Combined repeated-dose toxicity study of silver nanoparticles with the reproduction/developmental toxicity screening test. Nanotoxicology 2014, 8, 349–362. [Google Scholar] [CrossRef]
- Wigle, D.T.; Arbuckle, T.E.; Turner, M.C.; Bérubé, A.; Yang, Q.; Liu, S.; Krewski, D. Epidemiologic Evidence of Relationships Between Reproductive and Child Health Outcomes and Environmental Chemical Contaminants. J. Toxicol. Environ. Health Part B Crit. Rev. 2008, 11, 373–517. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-C.; Zhu, J.-Q. Nanoparticles and female reproductive system: How do nanoparticles affect oogenesis and embryonic development. Oncotarget 2017, 8, 109799–109817. [Google Scholar] [CrossRef] [PubMed]
- Yaman, S.; Çömelekoğlu, Ü.; Değirmenci, E.; Karagül, M.İ.; Yalın, S.; Ballı, E.; Yıldırımcan, S.; Yıldırım, M.; Doğaner, A.; Ocakoğlu, K. Effects of silica nanoparticles on isolated rat uterine smooth muscle. Drug Chem. Toxicol. 2017, 41, 465–475. [Google Scholar] [CrossRef]
- Ghaderi, S.; Tabatabaei, S.R.F.; Varzi, H.N.; Rashno, M. Induced adverse effects of prenatal exposure to silver nanoparticles on neurobehavioral development of offspring of mice. J. Toxicol. Sci. 2015, 40, 263–275. [Google Scholar] [CrossRef]
- Dugershaw, B.B.; Aengenheister, L.; Hansen, S.S.K.; Hougaard, K.S.; Buerki-Thurnherr, T. Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part. Fibre Toxicol. 2020, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Vales, G.; Rubio, L.; Marcos, R. Genotoxic and cell-transformation effects of multi-walled carbon nanotubes (MWCNT) following in vitro sub-chronic exposures. J. Hazard. Mater. 2016, 306, 193–202. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, M.B.; Kapila, Y.L. Cellular Mechanisms in Nanomaterial Internalization, Intracellular Trafficking, and Toxicity. In Nanotoxicology. Nanomedicine and Nanotoxicology; Durán, N., Guterres, S., Alves, O., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Courbiere, B.; Auffan, M.; Rollais, R.; Tassistro, V.; Bonnefoy, A.; Botta, A.; Rose, J.; Orsière, T.; Perrin, J. Ultrastructural Interactions and Genotoxicity Assay of Cerium Dioxide Nanoparticles on Mouse Oocytes. Int. J. Mol. Sci. 2013, 14, 21613–21628. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garraus, A.; Azqueta, A.; Vettorazzi, A.; de Cerain, A.L. Genotoxicity of Silver Nanoparticles. Nanomaterials 2020, 10, 251. [Google Scholar] [CrossRef]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef]
- Seshadri, S.; Saab, W.; Exeter, H.; Drew, E.; Petrie, A.; Davies, M.; Serhal, P. Clinical outcomes of a vitrified donor oocyte programme: A single UK centre experience. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 136–140. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Zhang, D.; Dai, J. Hydroxyapatite nanoparticles improved survival rate of vitrified porcine oocytes and its mechanism. CryoLetters 2015, 36, 45–50. [Google Scholar]
- Li, W.J.; Zhou, X.L.; Liu, B.L.; Dai, J.J.; Song, P.; Teng, Y. Effect of Nanoparticles on the Survival and Development of Vitrified Porcine GV Oocytes. CryoLetters 2017, 37, 401–405. [Google Scholar]
- Isaac, A.V.; Kumari, S.; Nair, R.; Urs, D.R.; Salian, S.R.; Kalthur, G.; Adiga, S.K.; Manikkath, J.; Mutalik, S.; Sachdev, D.; et al. Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem. Biophys. Res. Commun. 2017, 494, 656–662. [Google Scholar] [CrossRef]
- Abbasi, Y.; Hajiaghalou, S.; Baniasadi, F.; Mahabadi, V.P.; Ghalamboran, M.R.; Fathi, R. Fe3O4 magnetic nanoparticles improve the vitrification of mouse immature oocytes and modulate the pluripotent genes expression in derived pronuclear-stage embryos. Cryobiology 2021, 100, 81–89. [Google Scholar] [CrossRef]
- Wu, M.; Guo, Y.; Wei, S.; Xue, L.; Tang, W.; Chen, D.; Xiong, J.; Huang, Y.; Fu, F.; Wu, C.; et al. Biomaterials and advanced technologies for the evaluation and treatment of ovarian aging. J. Nanobiotechnol. 2022, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Kaniowski, D.; Ebenryter-Olbińska, K.; Kulik, K.; Suwara, J.; Cypryk, W.; Jakóbik-Kolon, A.; Leśnikowski, Z.; Nawrot, B. Composites of Nucleic Acids and Boron Clusters (C2B10H12) as Functional Nanoparticles for Downregulation of EGFR Oncogene in Cancer Cells. Int. J. Mol. Sci. 2021, 22, 4863. [Google Scholar] [CrossRef] [PubMed]
- Kaniowski, D.; Ebenryter-Olbinska, K.; Kulik, K.; Janczak, S.; Maciaszek, A.; Bednarska-Szczepaniak, K.; Nawrot, B.; Lesnikowski, Z. Boron clusters as a platform for new materials: Composites of nucleic acids and oligofunctionalized carboranes (C2B10H12) and their assembly into functional nanoparticles. Nanoscale 2020, 12, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef] [PubMed]
- Aghamiri, S.; Mehrjardi, K.F.; Shabani, S.; Keshavarz-Fathi, M.; Kargar, S.; Rezaei, N. Nanoparticle-siRNA: A potential strategy for ovarian cancer therapy? Nanomedicine 2019, 14, 2083–2100. [Google Scholar] [CrossRef]
- Veilleux, D.; Panicker, R.K.G.; Chevrier, A.; Biniecki, K.; Lavertu, M.; Buschmann, M.D. Lyophilisation and concentration of chitosan/siRNA polyplexes: Influence of buffer composition, oligonucleotide sequence, and hyaluronic acid coating. J. Colloid Interface Sci. 2018, 512, 335–345. [Google Scholar] [CrossRef]
- Ma, J.; Kala, S.; Yung, S.; Chan, T.M.; Cao, Y.; Jiang, Y.; Liu, X.; Giorgio, S.; Peng, L.; Wong, A.S. Blocking Stemness and Metastatic Properties of Ovarian Cancer Cells by Targeting p70S6K with Dendrimer Nanovector-Based siRNA Delivery. Mol. Ther. 2018, 26, 70–83. [Google Scholar] [CrossRef]
- Agasti, S.S.; Chompoosor, A.; You, C.-C.; Ghosh, P.; Kim, C.K.; Rotello, V.M. Photoregulated Release of Caged Anticancer Drugs from Gold Nanoparticles. J. Am. Chem. Soc. 2009, 131, 5728–5729. [Google Scholar] [CrossRef]
- Shuai, X.-T.; Zou, S.; Cao, N.; Cheng, D.; Zheng, R.; Wang, J.; Zhu, K. Enhanced apoptosis of ovarian cancer cells via nanocarrier-mediated codelivery of siRNA and doxorubicin. Int. J. Nanomed. 2012, 7, 3823–3835. [Google Scholar] [CrossRef][Green Version]
- Eygeris, Y.; Patel, S.; Jozic, A.; Sahay, G. Deconvoluting Lipid Nanoparticle Structure for Messenger RNA Delivery. Nano Lett. 2020, 20, 4543–4549. [Google Scholar] [CrossRef]
- Pilkington, E.H.; Suys, E.J.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery Strategies for mRNA Vaccines. Pharm. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).