Association between Statin Use and Diabetes Risk in Patients with Transient Ischemic Attack
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Participants and Exposure Ascertainment
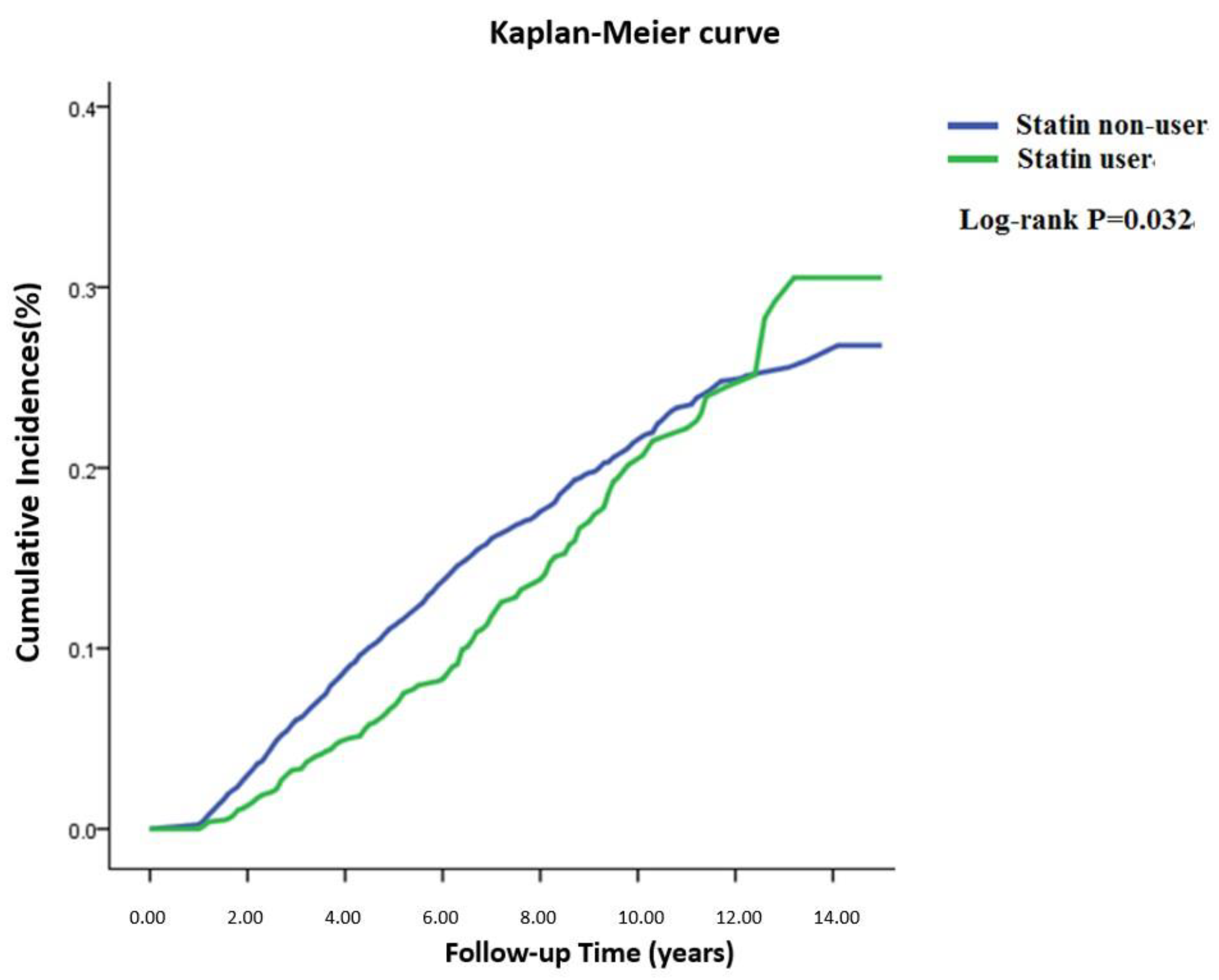
3. Results
4. Discussion
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cereda, C.W.; Olivot, J.-M. Emergency Department (ED) Triage for Transient Ischemic Attack (TIA). Curr. Atheroscler. Rep. 2018, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Tomari, S.; Magin, P.; Lasserson, D.; Quain, D.; Valderas, J.M.; Dewey, H.M.; Barber, P.A.; Spratt, N.J.; Cadilhac, D.A.; Feigin, V.L.; et al. The Characteristics of Patients with Possible Transient Ischemic Attack and Minor Stroke in the Hunter and Manning Valley Regions, Australia (the INSIST Study). Front. Neurol. 2020, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Coutts, S.B. Diagnosis and Management of Transient Ischemic Attack. Continuum 2017, 23, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Oza, R.; Rundell, K.; Garcellano, M. Recurrent Ischemic Stroke: Strategies for Prevention. Am. Fam. Physician 2017, 96, 436–440. [Google Scholar] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129 (Suppl. S2), S1–S45. [Google Scholar] [CrossRef]
- Kernan, W.N.; Ovbiagele, B.; Kittner, S.J. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-S.; Cheng, C.-L.; Yang, Y.-H.K.; Li, Y.-H. Statin Adherence After Ischemic Stroke or Transient Ischemic Attack Is Associated with Clinical Outcome. Circ. J. 2016, 80, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Kazi, D.S.; Penko, J.M.; Bibbins-Domingo, K. Statins for Primary Prevention of Cardiovascular Disease: Review of Evidence and Recommendations for Clinical Practice. Med. Clin. N. Am. 2017, 101, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Kattoor, A.J.; Pothineni, N.V.; Palagiri, R.D.R.; Romeo, F.; Mehta, J.L. Balancing Primary Prevention and Statin-Induced Diabetes Mellitus Prevention. Am. J. Cardiol. 2017, 120, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Mansi, I.A.; Chansard, M.; Lingvay, I.; Zhang, S.; Halm, E.A.; Alvarez, C.A. Association of Statin Therapy Initiation with Diabetes Progression: A Retrospective Matched-Cohort Study. JAMA Intern. Med. 2021, 181, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Cederberg, H.; Stančáková, A.; Yaluri, N.; Modi, S.; Kuusisto, J.; Laakso, M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: A 6-year follow-up study of the METSIM cohort. Diabetologia 2015, 58, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Casula, M.; Mozzanica, F.; Scotti, L.; Tragni, E.; Pirillo, A.; Corrao, G.; Catapano, A. Statin use and risk of new-onset diabetes: A meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, A.; Kulkarni, S.; Maddox, T. The Association of Statin Therapy with Incident Diabetes: Evidence, Mechanisms, and Recommendations. Curr. Cardiol. Rep. 2018, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.J.; Norrie, J.; Sattar, N.; Neely RD, G.; Cobbe, S.M.; Ford, I. Pravastatin and the development of diabetes mellitus: Evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001, 103, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Bonilla, H.; Yan, P.; Chung, R.T.; Butt, A.A. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology 2016, 64, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.M.; Xu, J.H.; Chen, W.; Srinivasan, S.R.; Berenson, G.S. Correlates of age onset of type 2 diabetes among relatively young black and white adults in a community: The Bogalusa Heart Study. Diabetes Care 2012, 35, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Prattichizzo, F. Variability of risk factors and diabetes complications. Cardiovasc. Diabetol. 2021, 20, 101. [Google Scholar]
- Almeida, S.O.; Budoff, M. Effect of statins on atherosclerotic plaque. Trends. Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]

| All TIA | Statin User | Non-Statin User | |||||
|---|---|---|---|---|---|---|---|
| (N = 8342) | (N = 1255; 15.1%) | (N= 7087; 84.9%) | |||||
| Variable | No. | % | No. | % | No. | % | p Value |
| Age of TIA onset † | <0.0001 | ||||||
| <45 | 1029 | 12.34 | 100 | 7.97 | 929 | 13.11 | |
| 45–65 | 3199 | 38.35 | 615 | 49.00 | 2584 | 36.46 | |
| >65 | 4114 | 49.31 | 540 | 43.03 | 3574 | 50.43 | |
| Med (IQR) | 14.6 (52.70–74.40) | 62.7 (53.20–70.90) | 65.2 (52.60–75.10) | ||||
| Mean age ± SD | 63.0 ± 14.80 | 61.90 ± 11.70 | 63.20 ± 15.20 | ||||
| Male sex | 4167 | 49.45 | 612 | 48.76 | 3555 | 50.16 | 0.3615 |
| Insurance premium †, NT$ | <0.0001 | ||||||
| 0 | 2547 | 30.53 | 448 | 35.70 | 2099 | 29.62 | |
| 1–15,840 | 1770 | 21.22 | 231 | 18.41 | 1539 | 21.72 | |
| 15,841–25,000 | 2817 | 33.77 | 379 | 30.20 | 2438 | 34.40 | |
| ≥25,000 | 1208 | 14.48 | 197 | 15.70 | 1011 | 14.27 | |
| Incident DM Comorbidity | 1185 | 14.21 | 188 | 14.98 | 997 | 14.07 | |
| Hypertension | 6596 | 70.62 | 1075 | 85.66 | 4855 | 68.51 | <0.0001 |
| Dyslipidemia | 3402 | 36.42 | 1097 | 87.41 | 2034 | 28.70 | <0.0001 |
| Atrial fibrillation | 1215 | 13.01 | 148 | 11.79 | 950 | 13.40 | 0.1195 |
| Heart failure | 1746 | 18.69 | 249 | 19.84 | 1334 | 18.82 | 0.3969 |
| Coronary artery disease | 4527 | 48.47 | 758 | 60.40 | 3354 | 47.33 | <0.0001 |
| Peripheral arterial disease | 750 | 8.03 | 140 | 11.16 | 557 | 7.86 | 0.0001 |
| Chronic kidney disease | 869 | 9.30 | 143 | 11.39 | 622 | 8.78 | 0.0031 |
| COPD | 2653 | 28.40 | 363 | 28.92 | 2027 | 28.60 | 0.8158 |
| ARD | 224 | 2.40 | 19 | 1.51 | 167 | 2.36 | 0.0624 |
| Statin dose † | <0.0001 | ||||||
| <28 cDDDs | 0 | 0.00 | 7087 | 100.00 | |||
| 28–90 cDDDs | 383 | 30.51 | 0 | 0.00 | |||
| 90–180 cDDDs | 263 | 20.96 | 0 | 0.00 | |||
| >180 cDDDs | 609 | 48.53 | 0 | 0.00 | |||
| Aspirin | 4180 | 50.11 | 900 | 71.71 | 3280 | 46.28 | <0.0001 |
| NSAIDs | 6146 | 73.68 | 1049 | 83.59 | 5097 | 71.92 | <0.0001 |
| Antihypertensive agent | |||||||
| ACEi | 2304 | 17.62 | 519 | 41.35 | 1785 | 25,19 | <0.0001 |
| ARB | 2438 | 29.23 | 592 | 47.17 | 1846 | 26.05 | <0.0001 |
| Beta blocker | 2973 | 35.64 | 688 | 54.82 | 2285 | 32.24 | <0.0001 |
| CCB | 4214 | 50.52 | 868 | 69.16 | 3346 | 47.21 | <0.0001 |
| Diuretic | 2850 | 34.16 | 558 | 44.46 | 2292 | 32.34 | <0.0001 |
| Antihyperlipidemic agent | |||||||
| Nonstatin lipid-lowering drug | 108 | 1.29 | 66 | 5.26 | 42 | 0.59 | <0.0001 |
| Fibrate | 557 | 6.68 | 275 | 21.91 | 282 | 3.98 | <0.0001 |
| Follow-up time (years) | |||||||
| Med (IQR) | 5.8 (3.20–9.20) | 7.60 (4.70–10.10) | 5.50 (2.90–8.90) | ||||
| Mean (SD) | 6.3 ± 3.60 | 7.50 ± 3.40 | 6.1 ± 3.60 | ||||
| Variable | Crude Diabetes HR (95% CI) | Adjust Diabetes HR (95% CI) |
|---|---|---|
| Statin nonuser (<28 cDDDs) | 1.000 | 1.000 |
Statin user (≥28 cDDDs)  | 0.843 (0.722–0.986) * | 0.545(0.457–0.650) ** |
Statins dose  | ||
| 28–90 cDDDs | 0.911 (0.694–1.195) | 0.689 (0.520–0.911) ** |
| 90–180 cDDDs | 0.973 (0.715–1.326) | 0.594 (0.431–0.818) ** |
| >180 cDDDs | 0.762 (0.615–0.945) * | 0.463 (0.367–0.584) ** |
 Statin use and categorization was defined by the cumulative prescription (cDDDs) of statins. Models adjusted for gender, age, income, urbanization, comorbidity, and drug used. * p < 0.05, ** p < 0.01.
Statin use and categorization was defined by the cumulative prescription (cDDDs) of statins. Models adjusted for gender, age, income, urbanization, comorbidity, and drug used. * p < 0.05, ** p < 0.01.| Statin Nonuser | Statin Use Levels * | p Trend | ||||||
|---|---|---|---|---|---|---|---|---|
| 28–89 cDDDs | 90–180 cDDDs | >180 cDDDs | ||||||
| HR | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| ARB | 1.0 | 0.931 | 0.689–1.257 | 0.477 | 0.289–0.786 | 0.642 | 0.382–1.078 | 0.0123 |
| Aspirin | 1.0 | 0.886 | 0.656–1.197 | 0.452 | 0.274–0.745 | 0.604 | 0.360–1.013 | 0.0043 |
| NSAIDs | 1.0 | 0.943 | 0.699–1.272 | 0.484 | 0.294–0.797 | 0.659 | 0.393–1.104 | 0.0159 |
| Subgroup analysis † | ||||||||
| Gender | ||||||||
| Female | 1.0 | 0.805 | 0.525–1.232 | 0.658 | 0.368–1.176 | 0.731 | 0.375–1.427 | 0.3223 |
| Male | 1.0 | 1.146 | 0.750–1.749 | 0.271 | 0.101–0.730 | 0.572 | 0.253–1.292 | 0.0297 |
| Age | ||||||||
| <65 | 1.0 | 1.007 | 0.693–1.463 | 0.583 | 0.326–1.043 | 0.635 | 0.324–1.243 | 0.1796 |
| ≥65 | 1.0 | 0.782 | 0.471–1.299 | 0.300 | 0.111–0.806 | 0.595 | 0.264–1.341 | 0.0503 |
| Menopause | ||||||||
| Yes | 1.0 | 0.878 | 0.561–1.373 | 0.653 | 0.346–1.233 | 0.777 | 0.382–1.580 | 0.5041 |
| No | 1.0 | 0.427 | 0.103–1.778 | 0.810 | 0.194–3.381 | 0.664 | 0.090–4.919 | 0.6711 |
| Follow-up time | ||||||||
| <9.2 | 1.0 | 0.593 | 0.439–0.801 | 0.552 | 0.392–0.779 | 0.480 | 0.373–0.619 | <0.0001 |
| ≥9.2 | 1.0 | 1.435 | 0.6520–3.157 | 1.072 | 0.440–2.614 | 0.868 | 0.471–1.601 | 0.7175 |
| Hypertension | ||||||||
| Yes | 1.0 | 0.943 | 0.689–1.292 | 0.526 | 0.319–0.868 | 0.645 | 0.378–1.102 | 0.0351 |
| No | 1.0 | 0.718 | 0.262–1.966 | - | - | 0.800 | 0.111–5.758 | 0.9276 |
| Hyperlipidemia | ||||||||
| Yes | 1.0 | 0.798 | 0.578–1.101 | 0.449 | 0.267–0.753 | 0.606 | 0.354–1.038 | 0.0041 |
| No | 1.0 | 1.418 | 0.630–3.189 | 0.360 | 0.050–2.571 | 0.472 | 0.066–3.367 | 0.5061 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.-J.; Yin, M.-C.; Chen, P.-Y.; Lin, M.-H.; Peng, Y.-H.; Ho, W.-C.; Chen, P.-C.; Hsu, C.Y. Association between Statin Use and Diabetes Risk in Patients with Transient Ischemic Attack. Int. J. Environ. Res. Public Health 2022, 19, 13770. https://doi.org/10.3390/ijerph192113770
Chen F-J, Yin M-C, Chen P-Y, Lin M-H, Peng Y-H, Ho W-C, Chen P-C, Hsu CY. Association between Statin Use and Diabetes Risk in Patients with Transient Ischemic Attack. International Journal of Environmental Research and Public Health. 2022; 19(21):13770. https://doi.org/10.3390/ijerph192113770
Chicago/Turabian StyleChen, Fu-Jun, Ming-Chien Yin, Pei-Yun Chen, Min-Hua Lin, Yi-Hao Peng, Wen-Chao Ho, Pau-Chung Chen, and Chung Y. Hsu. 2022. "Association between Statin Use and Diabetes Risk in Patients with Transient Ischemic Attack" International Journal of Environmental Research and Public Health 19, no. 21: 13770. https://doi.org/10.3390/ijerph192113770
APA StyleChen, F.-J., Yin, M.-C., Chen, P.-Y., Lin, M.-H., Peng, Y.-H., Ho, W.-C., Chen, P.-C., & Hsu, C. Y. (2022). Association between Statin Use and Diabetes Risk in Patients with Transient Ischemic Attack. International Journal of Environmental Research and Public Health, 19(21), 13770. https://doi.org/10.3390/ijerph192113770







