Adsorption of Heavy Metal Ions Copper, Cadmium and Nickel by Microcystis aeruginosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Species
2.2. Preparation of Algal Powder
2.3. Adsorption Experiment
2.4. Removal Rate
2.5. Analysis Method
2.6. Statistical Analysis Method
3. Results
3.1. Optimization Analysis of Uniform Experiment Process
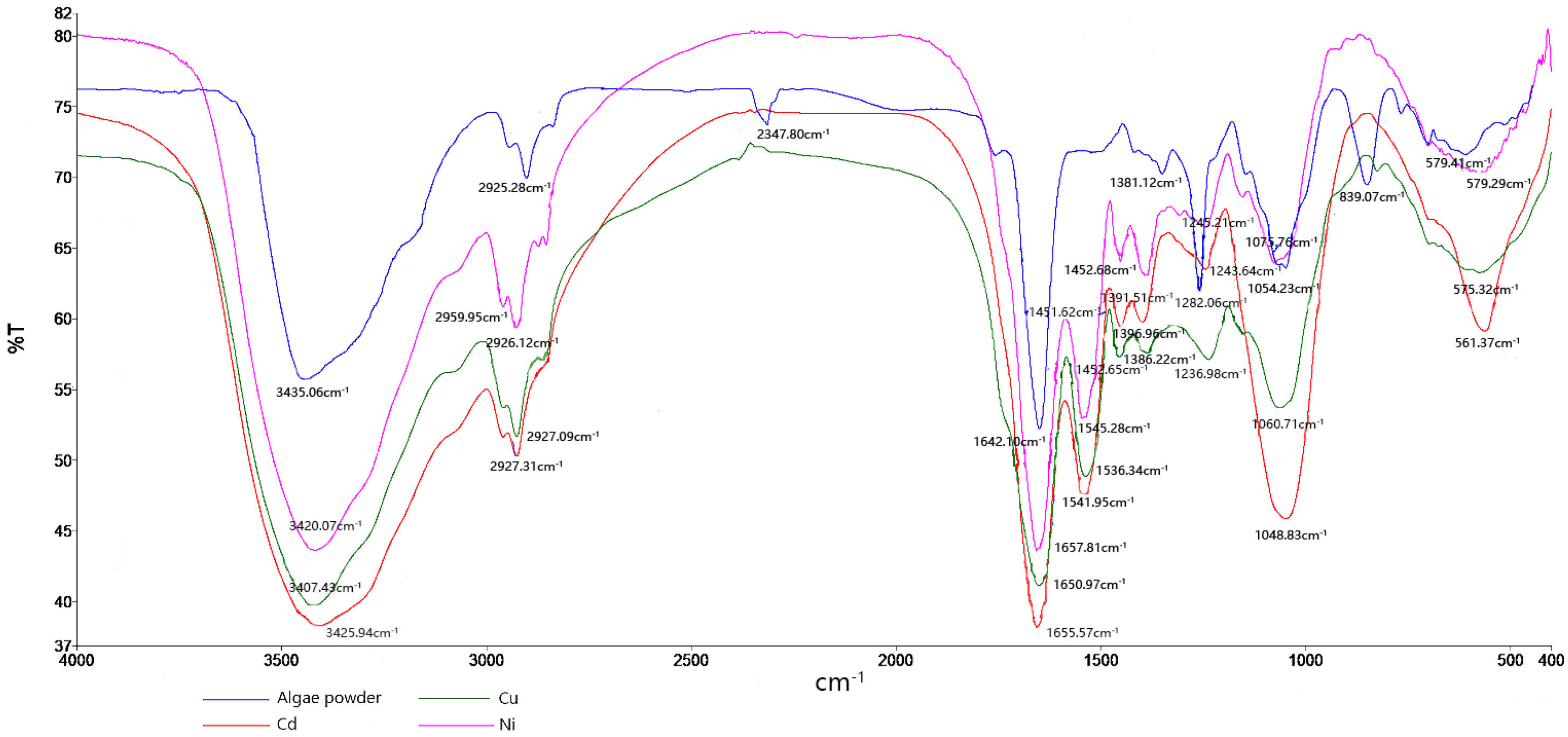
3.2. FTIR Analysis
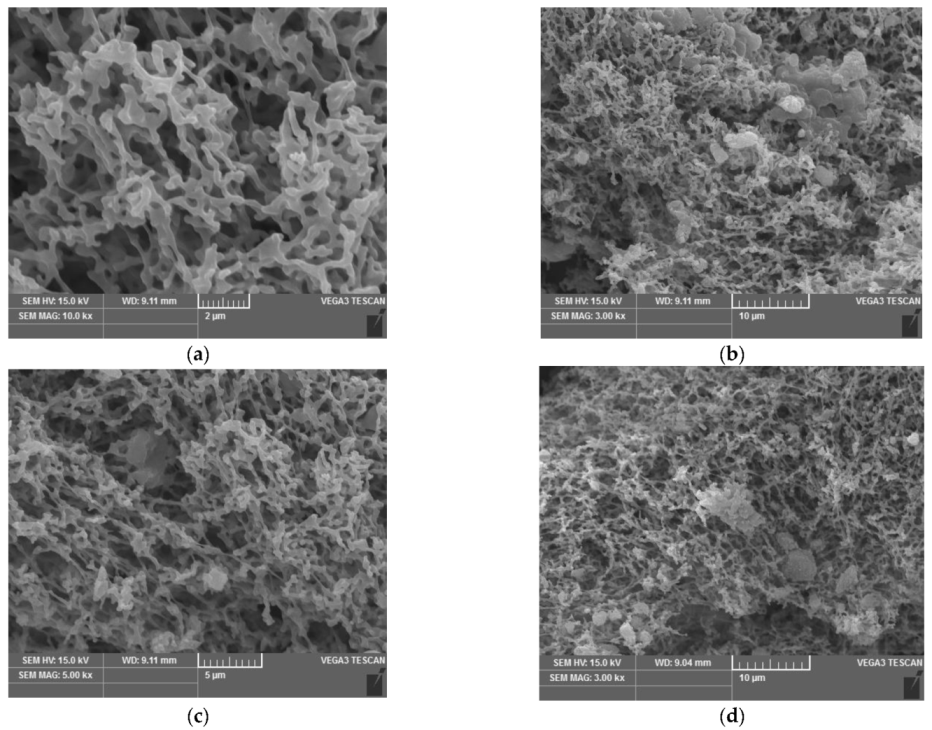
3.3. SEM Analysis
3.4. TG-DSC Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.; Ng, H.S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Calderón, O.A.; Abdeldayem, O.M.; Pugazhendhi, A.; Rene, E.R. Current updates and perspectives of biosorption technology: An alternative for the removal of heavy metals from wastewater. Curr. Pollut. Rep. 2020, 6, 8–27. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Yang, A.; Zhu, Q.; Sun, H.; Sun, P.; Yao, B.; Zang, Y.; Du, X.; Dong, L. Xanthate-Modified Magnetic Fe3O4@ SiO2-Based Polyvinyl Alcohol/Chitosan Composite Material for Efficient Removal of Heavy Metal Ions from Water. Polymers 2022, 14, 1107. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Liakopoulou-Kyriakides, M. Bioremoval of heavy metals by bacterial biomass. Environ. Monit. Assess. 2015, 187, 4173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Show, P.L.; Lau, B.F.; Chang, J.S.; Ling, T.C. New prospects for modified algae in heavy metal adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef]
- Chen, X.; Cui, J.; Xu, X.; Sun, B.; Zhang, L.; Dong, W.; Chen, C.; Sun, D. Bacterial cellulose/attapulgite magnetic composites as an efficient adsorbent for heavy metal ions and dye treatment. Carbohydr. Polym. 2020, 229, 115512. [Google Scholar] [CrossRef]
- Omar, H.H. Adsorption of zinc ions by Scenedesmus obliquus and S. quadricauda and its effect on growth and metabolism. Biol. Plant. 2002, 45, 261–266. [Google Scholar] [CrossRef]
- Rocha, G.S.; Parrish, C.C.; Espíndola, E.L. Shifts in photosynthetic parameters and lipid production of the freshwater microalga Selenastrum gracile (Chlorophyceae) under cadmium exposure. J. Appl. Phycol. 2020, 32, 4047–4055. [Google Scholar] [CrossRef]
- Chu, L.; Hou, X.; Song, X.; Zhao, X. Toxicological effects of different ionic liquids on growth, photosynthetic pigments, oxidative stress, and ultrastructure of Nostoc punctiforme and the combined toxicity with heavy metals. Chemosphere 2022, 298, 134273. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef]
- Chen, J.Z.; Tao, X.C.; Xu, J.; Zhang, T.; Liu, Z.L. Biosorption of lead, cadmium and mercury by immobilized Microcystis aeruginosa in a column. Process Biochem. 2005, 40, 3675–3679. [Google Scholar] [CrossRef]
- Tao, Y.; Xue, B.; Yang, Z.; Yao, S.; Li, S. Effects of heavy metals on the sorption of polycyclic aromatic hydrocarbons by Microcystis aeruginosa. J. Environ. Qual. 2014, 43, 1953–1962. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, D.; Ji, Y.; Wu, Q. Comparison of heavy metal accumulation by a bloom-forming cyanobacterium, Microcystis aeruginosa. Chin. Sci. Bull. 2012, 57, 3790–3797. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Fu, D.; Hu, W.; Lu, X.; Wu, Y.; Bryan, H. Physiological responses and accumulation ability of Microcystis aeruginosa to zinc and cadmium: Implications for bioremediation of heavy metal pollution. Bioresour. Technol. 2020, 303, 122963. [Google Scholar] [CrossRef]
- Ni, L.; Su, L.; Li, S.; Wang, P.; Li, D.; Ye, X.; Li, Y.; Li, Y.; Li, Y.; Wang, C. The characterization of dissolved organic matter extracted from different sources and their influence on cadmium uptake by Microcystis aeruginosa. Environ. Toxicol. Chem. 2017, 36, 1856–1863. [Google Scholar] [CrossRef]
- Rzymski, P.; Poniedzialek, B.; Niedzielski, P.; Tabaczewski, P.; Wiktorowicz, K. Cadmium and lead toxicity and bioaccumulation in Microcystis aeruginosa. Front. Environ. Sci. Eng. 2014, 8, 427–432. [Google Scholar] [CrossRef]
- Ribeiro, R.F.L.; Magalhães, S.M.S.; Barbosa, F.A.R. Evaluation of the Potential of Microalgae Microcystis novacekii in the Removal of Pb2+ from an Aqueous Medium. J. Hazard. Mater. 2010, 179, 947–953. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Biosorption of heavy metal ions by green alga Neochloris oleoabundans: Effects of metal ion properties and cell wall structure. J. Hazard. Mater. 2021, 418, 126336. [Google Scholar] [CrossRef]
- Balaji, S.; Kalaivani, T.; Rajasekaran, C.; Shalini, M.; Siva, R.; Singh, R.K.; Akthar, M.A. Arthrospira (Spirulina) species as bioadsorbents for lead, chromium, and cadmium—A comparative study. CLEAN–Soil Air Water 2014, 42, 1790–1797. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Freire, S.J.; Santos, L.V.S.; Palladino, F.; Jacob, R.S. Biosorption of copper ions from aqueous solution using Chlorella pyrenoidosa: Optimization, equilibrium and kinetics studies. Microchem. J. 2019, 145, 119–129. [Google Scholar] [CrossRef]
- Ahmad, S.; Pandey, A.; Pathak, V.V.; Tyagi, V.V.; Kothari, R. Phycoremediation: Algae as eco-friendly tools for the removal of heavy metals from wastewaters. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Cham, Switzerland, 2020; pp. 53–76. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhu, X.; Jia, Q.; Wang, Y. Effect of biosorption of heavy metal zinc in water by immobilized Sargassum thunbergii. Chin. J. Environ. Eng. 2017, 11, 2812–2818. [Google Scholar] [CrossRef]
- Salih, S.S.; Mahdi, A.; Kadhom, M.; Ghosh, T.K. Competitive adsorption of As (III) and As (V) onto chitosan/diatomaceous earth adsorbent. J. Environ. Chem. Eng. 2019, 7, 103407. [Google Scholar] [CrossRef]
- Harja, M.; Buema, G.; Bulgariu, L.; Bulgariu, D.; Sutiman, D.M.; Ciobanu, G. Removal of cadmium (II) from aqueous solution by adsorption onto modified algae and ash. Korean J. Chem. Eng. 2015, 32, 1804–1811. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Munoz, J.A. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Tuzun, I.; Celik, G.; Yilmaz, M.; Arica, M.Y. Biosorption of mercury (II), cadmium (II) and lead (II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Int. J. Miner. Process. 2006, 81, 35–43. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Doshi, H.; Ray, A.; Kothari, I.L.; Gami, B. Spectroscopic and scanning electron microscopy studies of bioaccumulation of pollutants by algae. Curr. Microbiol. 2006, 53, 148–157. [Google Scholar] [CrossRef]
- Cirik, Y.; Molu Bekci, Z.; Buyukates, Y.; Ak, İ.; Merdivan, M. Heavy metals uptake from aqueous solutions using marine algae (Colpomenia sinuosa): Kinetics and isotherms. Chem. Ecol. 2012, 28, 469–480. [Google Scholar] [CrossRef]
- Schmitt, D.; Müller, A.; Csögör, Z.; Frimmel, F.H.; Posten, C. The adsorption kinetics of metal ions onto different microalgae and siliceous earth. Water Res. 2001, 35, 779–785. [Google Scholar] [CrossRef]
- Sun, R.; Mo, Y.; Feng, X.; Zhang, L.; Jin, L.; Li, Q. Effects of typical algae species (Aphanizomenon flosaquae and Microcystis aeruginosa) on photoreduction of Hg2+ in water body. J. Environ. Sci. 2019, 85, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.W.; Benemann, J.R. Bioremoval of heavy metals by the use of microalgae. Biotechnol. Adv. 1993, 11, 781–812. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Zhang, X.; Ye, N.; Xing, F. Pyrolytic characteristics and kinetic studies of three kinds of red algae. Biomass Bioenergy 2011, 35, 1765–1772. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.; Wang, S. Purification of algal calcium-chelating peptide and its physical chemical properties. J. Aquat. Food Prod. Technol. 2018, 27, 518–530. [Google Scholar] [CrossRef]
- Liu, C.; Duan, X.; Chen, Q.; Chao, C.; Lu, Z.; Lai, Q.; Megharaj, M. Investigations on pyrolysis of microalgae Diplosphaera sp. MM1 by TG-FTIR and Py-GC/MS: Products and kinetics. Bioresour. Technol. 2019, 294, 122126. [Google Scholar] [CrossRef]


| Variable Sequence | Mass Concentration of Heavy Metals (mg/L) | Temperature (°C) | pH | Adsorption Time (h) | ||
|---|---|---|---|---|---|---|
| Cu2+ | Cd2+ | Ni2+ | ||||
| 1 | 20 | 20 | 20 | 15 | 6 | 1 |
| 2 | 15 | 15 | 15 | 35 | 7 | 1 |
| 3 | 5 | 5 | 5 | 30 | 4 | 2 |
| 4 | 25 | 25 | 25 | 20 | 4 | 4 |
| 5 | 10 | 10 | 10 | 25 | 5 | 5 |
| 6 | 10 | 10 | 10 | 20 | 8 | 3 |
| 7 | 15 | 15 | 15 | 15 | 7 | 5 |
| 8 | 25 | 25 | 25 | 30 | 8 | 5 |
| 9 | 25 | 25 | 25 | 35 | 5 | 3 |
| 10 | 5 | 5 | 5 | 35 | 8 | 4 |
| 11 | 20 | 20 | 20 | 35 | 6 | 5 |
| 12 | 25 | 25 | 25 | 25 | 8 | 2 |
| Variable Sequence | Cu2+ | Cd2+ | Ni2+ | ||||
|---|---|---|---|---|---|---|---|
| Initial Metal Concentration | Final Metal Concentration | Removal Rate | Final Metal Concentration | Removal Rate | Final Metal Concentration | Removal Rate | |
| 1 | 20 | 17.01 | 14.95% | 15.60 | 22.00% | 19.40 | 3.00% |
| 2 | 15 | 13.10 | 12.67% | 3.67 | 75.53% | 1.70 | 88.67% |
| 3 | 5 | 4.94 | 1.20% | 2.10 | 58.00% | 4.90 | 2.00% |
| 4 | 25 | 19.69 | 21.24% | 19.00 | 24.00% | 20.80 | 16.80% |
| 5 | 10 | 8.98 | 10.20% | 7.60 | 24.00% | 8.60 | 14.00% |
| 6 | 10 | 9.49 | 5.10% | 2.50 | 75.00% | 8.50 | 15.00% |
| 7 | 15 | 13.56 | 9.60% | 7.70 | 48.66% | 12.50 | 16.67% |
| 8 | 25 | 4.19 | 83.24% | 5.06 | 79.76% | 18.40 | 26.40% |
| 9 | 25 | 22.93 | 8.28% | 16.60 | 33.60% | 24.10 | 3.60% |
| 10 | 5 | 3.62 | 27.60% | 0.40 | 92.00% | 4.80 | 4.00% |
| 11 | 20 | 4.92 | 75.40% | 9.10 | 54.50% | 15.90 | 20.50% |
| 12 | 25 | 22.47 | 10.12% | 3.60 | 85.60% | 22.10 | 11.60% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, G.; He, Y.; Liang, D.; Wang, F.; Luo, Y.; Yang, H.; Wang, Q.; Wang, J.; Gao, P.; Wen, X.; et al. Adsorption of Heavy Metal Ions Copper, Cadmium and Nickel by Microcystis aeruginosa. Int. J. Environ. Res. Public Health 2022, 19, 13867. https://doi.org/10.3390/ijerph192113867
Zeng G, He Y, Liang D, Wang F, Luo Y, Yang H, Wang Q, Wang J, Gao P, Wen X, et al. Adsorption of Heavy Metal Ions Copper, Cadmium and Nickel by Microcystis aeruginosa. International Journal of Environmental Research and Public Health. 2022; 19(21):13867. https://doi.org/10.3390/ijerph192113867
Chicago/Turabian StyleZeng, Guoming, Yu He, Dong Liang, Fei Wang, Yang Luo, Haodong Yang, Quanfeng Wang, Jiale Wang, Pei Gao, Xin Wen, and et al. 2022. "Adsorption of Heavy Metal Ions Copper, Cadmium and Nickel by Microcystis aeruginosa" International Journal of Environmental Research and Public Health 19, no. 21: 13867. https://doi.org/10.3390/ijerph192113867






