Occurrence and Behavior of Methylsiloxanes in Urban Environment in Four Cities of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analytical Methods
2.3. Quality Assurance and Quality Control
2.4. Modeling Assessment
3. Results and Discussion
3.1. Concentrations of MSs in Four Cities
3.1.1. Air
3.1.2. Water
3.1.3. Soil and Sediment
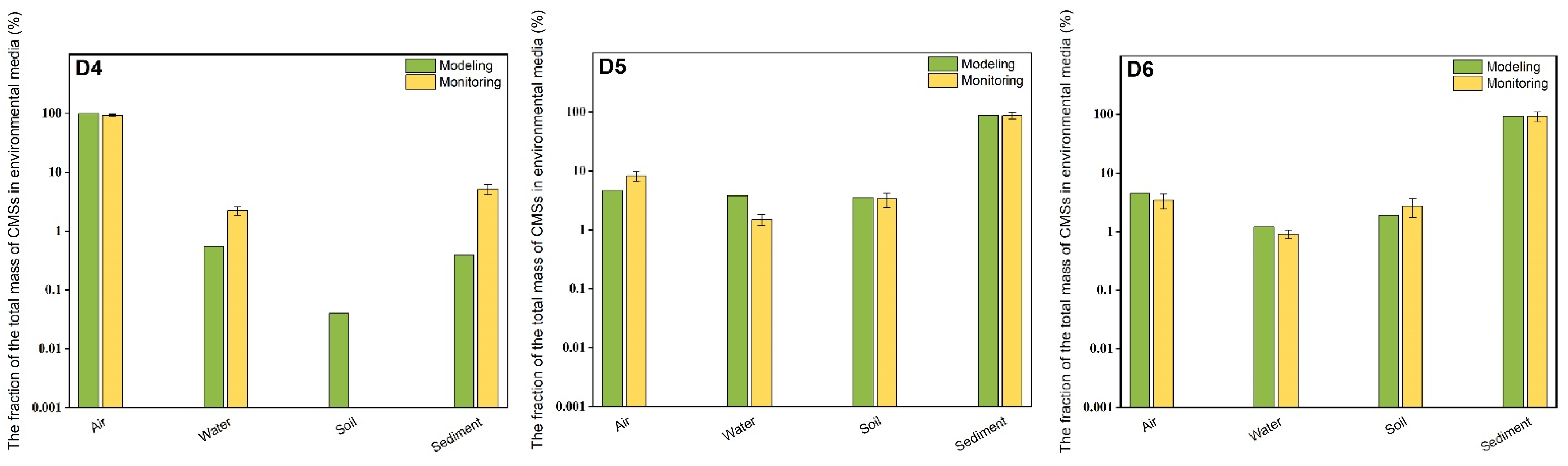
3.2. Model Simulation and Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Zhou, Y.; Wang, Y.; Chen, Y.; Zhang, B.; Zhang, J. Methylsiloxanes risk assessment combining external and internal exposure for college students. Sci. Total Environ. 2022, 845, 157379. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Kannan, K. Survey of organosilicones compounds, including cyclic and linear siloxanes, in personal-care and household products. Arch. Environ. Contam. Toxicol. 2008, 55, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Siliconces Environmental, Health, and Safety Center (SEHSC). 2011. Available online: http://sehsc.americanchemistry.com (accessed on 1 September 2022).
- Mojsiewicz-Pieńkowska, K.; Krenczkowska, D. Evolution of consciousness of exposure to siloxanes-review of publications. Chemosphere 2018, 191, 204–217. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (U.S. EPA). United States High Production Volume Challenge Program List. 2007. Available online: https://iaspub.epa.gov/sor_internet/registry/substreg/list/details.do?listId=74 (accessed on 28 August 2022).
- OECD (Organisation for Economic Co-operation and Development). Manual for the Assessment of Chemicals. 2007. Available online: http://www.oecd.org/env/ehs/risk-assessment/manualfortheassessmentofchemicals.htm (accessed on 1 September 2022).
- European Chemicals Agency (ECHA). UK Proposes Restriction on Octamethylcyclotetrasiloxane (D4) and Decamethylcyclopentasiloxane (D5) in Personal Care Products That Are Washed off in Normal Use; European Chemicals Agency: Helsinki, Finland, 2015. [Google Scholar]
- European Chemicals Agency (ECHA). Background Document to the Opinion on the Annex XV Dossier Proposing Restrictions on Octamethylcyclotetrasiloxane (D4) and Decamethylcyclopentasiloxane (D5); European Chemicals Agency: Helsinki, Finland, 2016. [Google Scholar]
- Wang, X.; Jasmin, S.; Jones, K.C.; Ping, G. Occurrence and spatial distribution of neutral perfluoroalkyl substances and cyclic volatile methylsiloxanes in the atmosphere of the Tibetan Plateau. Atmos. Chem. Phys. 2018, 18, 8745–8755. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Shi, Y.; Liu, N.; Cai, Y. Methyl siloxanes in environmental matrices and human plasma/fat from both general industries and residential areas in China. Sci. Total Environ. 2015, 505, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Buser, A.M.; Kierkegaard, A.; Bogdal, C.; MacLeod, M.; Scheringer, M.; Hungerbühler, K. Concentrations in ambient air and emissions of cyclic volatile methylsiloxanes in Zurich, Switzerland. Environ. Sci. Technol. 2013, 47, 7045–7051. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Cui, J.; Zhang, B.; Zhang, J. Assessment of volatile methylsiloxanes in environmental matrices and human plasma. Sci. Total Environ. 2019, 668, 1175–1182. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Sun, M.; Cui, J.; Zhang, B.; Zhang, J. Methylsiloxanes in plasma from potentially exposed populations and an assessment of the associated inhalation exposure risk. Environ. Int. 2020, 143, 105931. [Google Scholar] [CrossRef]
- Sparham, C.; Van Egmond, R.; O’Connor, S.; Hastie, C.; Whelan, M.; Kanda, R. Determination of decamethylcyclopentasiloxane in river water and final effluent by headspace gas chromatography/mass spectrometry. J. Chromatogr. A 2008, 1212, 124–129. [Google Scholar] [CrossRef]
- Zhang, Z.; Qi, H.; Ren, N.; Li, Y.; Gao, D.; Kannan, K. Survey of cyclic and linear siloxanes in sediment from Songhua river and in sewage sludge from wastewater treatment plants, Northeastern China. Arch. Environ. Contam. Toxicol. 2011, 60, 204–211. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Zhang, B.; Zhang, J. Distribution and evaluation of the fate of cyclic volatile methyl siloxanes in the largest lake of southwest China. Sci. Total Environ. 2019, 657, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Minomo, K.; Ohtsuka, N.; Motegi, M.; Nojiri, K.; Kannan, K. Distribution characteristics of volatile methylsiloxanes in Tokyo Bay watershed in Japan: Analysis of surface waters by purge and trap method. Sci. Total Environ. 2017, 586, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Brunete, C.; Miguel, E.; Albero, B.; Tadeo, J.L. Determination of cyclic and linear siloxanes in soil samples by ultrasonic-assisted extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 7024–7030. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Cabrerizo, A.; Galban-Malagon, C.; Barcelo, D.; Farre, M.; Dachs, J. Unexpected occurrence of volatile dimethylsiloxanes in Antarctic soils, vegetation, phytoplankton, and krill. Environ. Sci. Technol. 2015, 49, 4415–4424. [Google Scholar] [CrossRef] [PubMed]
- Sanchís, J.; Martínez, E.; Ginebreda, A.; Farré, M.; Barceló, D. Occurrence of linear and cyclic volatile methylsiloxanes in wastewater, surface water and sediments from Catalonia. Sci. Total Environ. 2013, 443, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, M.-K.; Lee, I.-S.; Choi, S.-D. Contamination characteristics of siloxanes in coastal sediment collected from industrialized bays in South Korea. Ecotox. Environ. Saf. 2019, 182, 109457. [Google Scholar] [CrossRef]
- Powell, D.E.; Schoyen, M.; Oxnevad, S.; Gerhards, R.; Bohmer, T.; Koerner, M. Bioaccumulation and trophic transfer of cyclic volatile methylsiloxanes (cVMS) in the aquatic marine food webs of the Oslofjord, Norway. Sci. Total Environ. 2018, 622–623, 127–139. [Google Scholar] [CrossRef]
- Hanssen, H.; Warner, N.A.; Braathen, T.; Odland, J.Ø.; Lund, E.; Nieboer, E.; Sandanger, T.M. Plasma concentrations of cyclic volatile methylsiloxanes (cVMS) in pregnant and postmenopausal Norwegian women and self-reported use of personal care products (PCPs). Environ. Int. 2013, 51, 82–87. [Google Scholar] [CrossRef]
- Wang, D.G.; de Solla, S.R.; Lebeuf, M.; Bisbicos, T.; Barrett, G.C.; Alaee, M. Determination of linear and cyclic volatile methylsiloxanes in blood of turtles, cormorants, and seals from Canada. Sci. Total Environ. 2017, 574, 1254–1260. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Wang, Y.; Zhang, B.; Zhang, J. Assessment of internal exposure to methylsiloxanes in children and associated non-dietary exposure risk. Environ. Int. 2021, 154, 106672. [Google Scholar] [CrossRef]
- Environment Canada, Health Canada. Screening Assessment for the Challenge: Decamethylcyclopentasiloxane (D5). 2008. Available online: http://ec.gc.ca/ese-ees/default.asp?lang=En&n=13CC261E-5FB0-4D33-8000 (accessed on 1 September 2022).
- Brooke, D.N.; Crookes, M.J.; Gray, D.; Robertson, S. Environmental Risk Assessment Report: Octamethylcyclotetrasiloxane; Environment Agency: Bristol, UK, 2009. [Google Scholar]
- Brooke, D.N.; Crookes, M.J.; Gray, D.; Robertson, S. Environmental Risk Assessment Report: Decamethylcyclopentasiloxane; Environment Agency: Bristol, UK, 2009. [Google Scholar]
- Brooke, D.N.; Crookes, M.J.; Gray, D.; Robertson, S. Environmental Risk Assessment Report: Dodecamethylcyclohexasiloxane; Environment Agency: Bristol, UK, 2009. [Google Scholar]
- Quinn, A.L.; Regan, J.M.; Tobin, J.M.; Marinik, B.J.; McMahon, J.M.; McNett, D.A.; Sushynski, C.M.; Crofoot, S.D.; Jean, P.A.; Plotzke, K.P. In vitro and in vivo evaluation of the estrogenic, androgenic, and progestagenic potential of two cyclic siloxanes. Toxicol. Sci. 2007, 96, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKim, J.M., Jr.; Wilga, P.C.; Breslin, W.J.; Plotzke, K.P.; Gallavan, R.H.; Meeks, R.G. Potential estrogenic and antiestrogenic activity of the cyclic siloxane octamethylcyclotetrasiloxane (D4) and the linear siloxane hexamethyldisiloxane (HMDS) in immature rats using the uterotrophic assay. Toxicol. Sci. 2001, 63, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeks, R.G.; Stump, D.G.; Siddiqui, W.H.; Holson, J.F.; Plotzke, K.P.; Reynolds, V.L. An inhalation reproductive toxicity study of octamethylcyclotetrasiloxane (D4) in female rats using multiple and single day exposure regimens. Reprod. Toxicol. 2007, 23, 192–201. [Google Scholar] [CrossRef]
- Granchi, D.; Cavedagna, D.; Ciapetti, G.; Stea, S.; Schiavon, P.; Giuliani, R.; Pizzoferrato, A. Silicone breast implants: The role of immune system on capsular contracture formation. J. Biomed. Mater. Res. 1995, 29, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.; Di Guardo, A.; Paterson, S.; Kicsi, G.; Cowan, C.E. Assessing the fate of new and existing chemicals: A five-stage process. Environ. Toxicol. Chem. 1996, 15, 1618–1626. [Google Scholar] [CrossRef]
- Mackay, D.; Di Guardo, A.; Paterson, S.; Cowan, C.E. Evaluating the environmental fate of a variety of types of chemicals using the EQC model. Environ. Toxicol. Chem. 1996, 15, 1627–1637. [Google Scholar] [CrossRef]
- Whelan, M.J. Evaluating the fate and behaviour of cyclic volatile methyl siloxanes in two contrasting North American lakes using a multi-media model. Chemosphere 2013, 91, 1566–1576. [Google Scholar] [CrossRef]
- Mackay, D.; Hughes, L.; Powell, D.E.; Kim, J. An updated Quantitative Water Air Sediment Interaction (QWASI) model for evaluating chemical fate and input parameter sensitivities in aquatic systems: Application to D5 (decamethylcyclopentasiloxane) and PCB-180 in two lakes. Chemosphere 2014, 111, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Shi, Y.; Cai, Y. Occurrence and fate of volatile siloxanes in a municipal Wastewater Treatment Plant of Beijing, China. Water Res. 2013, 47, 715–724. [Google Scholar] [CrossRef]
- Beijing Municipal Bureau of Statistics (BMBS). 2017. Available online: http://tjj.beijing.gov.cn/tjsj/yjdsj/rk/2017/201801/t20180119_391224.html (accessed on 5 August 2022).
- Genualdi, S.; Harner, T.; Cheng, Y. Global distribution of linear and cyclic volatile methyl siloxanes in air. Environ. Sci. Technol. 2011, 45, 3349–3354. [Google Scholar] [CrossRef]
- Yucuis, R.A.; Stanier, C.O.; Hornbucke, K.C. Cyclic siloxanes in air, including identification of high levels in Chicago and distinct diurnal variation. Chemosphere 2013, 92, 905–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kierkegaard, A.; McLachlan, M.S. Determination of linear and cyclic volatile methylsiloxanes in air at a regional background site in Sweden. Atmos. Environ. 2013, 80, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Krogseth, I.S.; Kierkegaard, A.; McLachlan, M.S.; Breivik, K.; Hansen, K.M.; Schlabach, M. Occurrence and seasonality of cyclic volatile methyl siloxanes in Arctic air. Environ. Sci. Technol. 2013, 47, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Kaj, L.; Schlabach, M.; Andersson, J.; Palm, C.A.; Schmidbauer, N.; Brorström-Lundén, E. Siloxanes in the Nordic Environment; Nordic Council of Ministers: Copenhagen, Denmark, 2005. [Google Scholar]
- Lu, Y.; Yuan, T.; Wang, W.H.; Kannan, K. Concentrations and assessment of exposure to siloxanes and synthetic musks in personal care products from China. Environ. Pollut. 2011, 159, 3522–3528. [Google Scholar] [CrossRef]
- Companioni-Damas, E.Y.; Santos, F.J.; Galceran, M.T. Analysis of linear and cyclic methylsiloxanes in sewage sludges and urban solls by concurrent solvent recondensation-large volume injection-gas chromatography-mass spectrometry. J. Chromatogr. A 2012, 1268, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Kierkegaard, A.; Van Egmond, R.; McLachlan, M.S. Cyclic Volatile Methylsiloxane Bioaccumulation in Flounder and Ragworm in the Humber Estuary. Environ. Sci. Technol. 2011, 45, 5936–5942. [Google Scholar] [CrossRef] [PubMed]
- Sparham, C.; Van Egmond, R.; Hastie, C. Determination of decamethylcyclopentasiloxane in river and estuarine sediments in the UK. J. Chromatogr. A 2011, 1218, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.E.; Kozerski, G.E. Cyclic methylsiloxane (cVMS) materials in surface sediments and cores for Lake Ontario. In HES Study No. 10724-108. Health and Environmental Sciences, Dow Corning Corporation, Auburn, Michigan: Study Submitted to Centre Europ6en des Silicones(CES); A Sector Group of the European Chemical Industry Council (Cefic): Brussels, Belgium, 2007. [Google Scholar]
- European Chemicals Agency (ECHA). Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and Council concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) With Regard to Octamethylcyclotetrasiloxane (D4) and Decamethylcyclopentasiloxane (D5); European Chemicals Agency: Brussels, Belgium, 2018. [Google Scholar]
- Horii, Y.; Kannan, K. Main Uses and Environmental Emissions of Volatile Methylsiloxanes. Volatile Methylsiloxanes in the Environment; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Kala, S.V.; Lykissa, E.D.; Lebovitz, R.M. Detection and Characterization of Poly(dimethylsiloxane)s in Biological Tissues by GC/AED and GC/MS. Anal. Chem. 1997, 69, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Kozerski, G.E. Assessment of the Fundamental Partitioning Properties of Permethylated Cyclosiloxanes; SETAC Europe: Porto, Portugal, 2007. [Google Scholar]
| Site | D4 | D5 | D6 | ΣL5–L8 |
|---|---|---|---|---|
| Beijing (urban) | 66.2 | 69.1 | 36.2 | <LOD 1 |
| Beijing (suburban) | 40.5 | 35.6 | 11.9 | <LOD |
| Kunming (urban) | 52.6 | 55.1 | 28.5 | <LOD |
| Kunming (suburban) | 32.9 | 30.6 | 7.7 | <LOD |
| Lijiang (urban) | 29.4 | 33.5 | 6.9 | <LOD |
| Lijiang (suburban) | 12.3 | 11.2 | 1.1 | <LOD |
| Zhangjiagang (urban) | 75.6 | 83.1 | 55.9 | <LOD |
| Zhangjiagang (suburban) | 45.1 | 47.2 | 19.3 | <LOD |
| Siloxane production plan (around) | 1.3 × 105 | 1.7 × 105 | 3.6 × 104 | 5.1 × 102 |
| Siloxane production plan (downwind) | 3.3 × 104 | 2.5 × 104 | 6.6 × 103 | 56.2 |
| Siloxane production plan (upwind) | 2.7 × 103 | 4.1 × 103 | 7.1 × 102 | <LOD |
| City | Site | D4 | D5 | D6 | ΣL3–L5 |
|---|---|---|---|---|---|
| Beijing | River a (1) | <LOD 1 | 17.1 | <LOD | <LOD |
| River a (2) | <LOD | 15.6 | <LOD | <LOD | |
| River b (1) | <LOD | 12.8 | <LOD | <LOD | |
| River b (2) | <LOD | 13.1 | <LOD | <LOD | |
| River c (1) | <LOD | 19.9 | 18.8 | <LOD | |
| River c (2) | <LOD | 20.5 | 19.5 | <LOD | |
| River d (1) | 20.2 | 132 | 105 | <LOD | |
| River d (2) | 10.8 | 62.3 | 38.5 | <LOD | |
| River e (1) | 15.3 | 113 | 69.2 | <LOD | |
| River e (2) | 9.5 | 44.3 | 30.3 | <LOD | |
| River f (1) | 16.5 | 122 | 88.1 | <LOD | |
| River f (2) | 10.2 | 52.7 | 45.6 | <LOD | |
| Kunming | River g (1) | <LOD | 13.3 | <LOD | <LOD |
| River g (2) | <LOD | 12.6 | <LOD | <LOD | |
| River h (1) | 15.3 | 108 | 65.3 | <LOD | |
| River h (2) | <LOD | 35.3 | 23.1 | <LOD | |
| River j (1) | 12.2 | 85.3 | 51.5 | <LOD | |
| River j (2) | <LOD | 29.1 | 20.6 | <LOD | |
| Lijiang | River k (1) | <LOD | 25.2 | 19.6 | <LOD |
| River k (2) | 10.2 | 31.5 | 23.4 | <LOD | |
| River m (1) | <LOD | <LOD | <LOD | <LOD | |
| River m (2) | <LOD | <LOD | <LOD | <LOD | |
| River n (1) | <LOD | <LOD | <LOD | <LOD | |
| River n (2) | <LOD | <LOD | <LOD | <LOD | |
| Zhangjiagang | River p (1) | <LOD | 25.1 | 21.2 | <LOD |
| River p (2) | <LOD | 21.7 | 19.1 | <LOD | |
| River q (1) | 10.6 | 56.9 | 36.1 | <LOD | |
| River q (2) | 9.9 | 52.2 | 34.5 | <LOD | |
| River r (1) | 145 | 191 | 176 | 59.6 | |
| River r (2) | 11.5 | 61.3 | 39.2 | <LOD |
| City | Site | Soil | Sediment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| D4 | D5 | D6 | ΣL5–L11 | D4 | D5 | D6 | ΣL5–L11 | ||
| Beijing | River a (1) | <LOD 1 | 17.1 | <LOD | <LOD | 0.88 | 102 | 136 | 115 |
| River a (2) | <LOD | <LOD | <LOD | <LOD | 0.81 | 91.8 | 128 | 109 | |
| River b (1) | <LOD | <LOD | <LOD | <LOD | 0.65 | 78.1 | 98.5 | 101 | |
| River b (2) | <LOD | <LOD | <LOD | <LOD | 0.71 | 85.7 | 107 | 108 | |
| River c (1) | <LOD | <LOD | <LOD | <LOD | 0.96 | 156 | 192 | 150 | |
| River c (2) | <LOD | <LOD | <LOD | <LOD | 0.98 | 172 | 206 | 166 | |
| River d (1) | <LOD | 1.10 | 0.94 | <LOD | 8.50 | 1582 | 1759 | 1012 | |
| River d (2) | <LOD | <LOD | <LOD | <LOD | 1.20 | 355 | 426 | 223 | |
| River e (1) | <LOD | 0.82 | 0.73 | <LOD | 6.60 | 1195 | 1429 | 764 | |
| River e (2) | <LOD | <LOD | <LOD | <LOD | 1.10 | 262 | 314 | 197 | |
| River f (1) | <LOD | 0.85 | 0.78 | <LOD | 7.10 | 1321 | 1462 | 832 | |
| River f (2) | <LOD | <LOD | <LOD | <LOD | 1.10 | 325 | 401 | 210 | |
| Kunming | River g (1) | <LOD | <LOD | <LOD | <LOD | 0.89 | 87.1 | 106 | 106 |
| River g (2) | <LOD | <LOD | <LOD | <LOD | 0.82 | 85.2 | 103 | 85.2 | |
| River h (1) | <LOD | 1.10 | 0.89 | <LOD | 6.90 | 1039 | 1250 | 684 | |
| River h (2) | <LOD | <LOD | <LOD | <LOD | 1.10 | 247 | 295 | 166 | |
| River j (1) | <LOD | 0.81 | 0.72 | <LOD | 5.70 | 1005 | 1209 | 636 | |
| River j (2) | <LOD | <LOD | <LOD | <LOD | 0.97 | 203 | 258 | 141 | |
| Lijiang | River k (1) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| River k (2) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| River m (1) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| River m (2) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| River n (1) | <LOD | <LOD | <LOD | <LOD | 0.64 | 38.2 | 51.3 | 37.9 | |
| River n (2) | <LOD | <LOD | <LOD | <LOD | 0.62 | 33.6 | 46.5 | 35.1 | |
| Zhangjiagang | River p (1) | <LOD | <LOD | <LOD | <LOD | 0.69 | 74.2 | 91.6 | 95.5 |
| River p (2) | <LOD | <LOD | <LOD | <LOD | 0.75 | 85.1 | 104 | 99.3 | |
| River q (1) | <LOD | <LOD | <LOD | <LOD | 0.91 | 106 | 135 | 117 | |
| River q (2) | <LOD | <LOD | <LOD | <LOD | 0.83 | 101 | 127 | 108 | |
| River r (1) | 29.6 | 96.7 | 85.3 | 102 | 55.3 | 1965 | 2241 | 1694 | |
| River r (2) | <LOD | <LOD | 0.76 | <LOD | 1.20 | 366 | 415 | 283 | |
| Chemical | Temperature (°C) | Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|---|
| D4 | 0 | 92.0 | 2.8 | 0.02 | 5.2 |
| 15 | 97.7 | 0.88 | 0.03 | 1.4 | |
| 25 | 99.2 | 0.40 | 0.04 | 0.41 | |
| 35 | 99.6 | 0.20 | 0.09 | 0.09 | |
| D5 | 0 | 5.2 | 4.8 | 3.0 | 87.0 |
| 15 | 5.7 | 4.3 | 4.0 | 86.0 | |
| 25 | 8.3 | 4.3 | 6.3 | 81.2 | |
| 35 | 17.6 | 4.5 | 12.0 | 65.9 | |
| D6 | 0 | 1.4 | 1.3 | 3.9 | 93.5 |
| 15 | 5.2 | 1.2 | 3.0 | 90.6 | |
| 25 | 6.2 | 1.3 | 2.6 | 90.0 | |
| 35 | 7.5 | 1.6 | 2.9 | 88.1 |
| Chemical | Temperature (°C) | Total Residence Time | Advection Residence Time | Reaction Residence Time |
|---|---|---|---|---|
| D4 | 0 | 4.4 | 4.5 | 134 |
| 15 | 3.5 | 4.3 | 20.6 | |
| 25 | 2.5 | 4.2 | 6.3 | |
| 35 | 1.6 | 4.2 | 2.5 | |
| D5 | 0 | 68.1 | 70.8 | 1773 |
| 15 | 51.2 | 66.3 | 225 | |
| 25 | 20.4 | 47.1 | 48.7 | |
| 35 | 6.9 | 22.9 | 9.9 | |
| D6 | 0 | 243 | 249 | 9738 |
| 15 | 61.8 | 76.0 | 329 | |
| 25 | 34.4 | 64.0 | 74.5 | |
| 35 | 16.0 | 53.1 | 22.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Guo, J.; Zhou, Y.; Zhang, B.; Zhang, J. Occurrence and Behavior of Methylsiloxanes in Urban Environment in Four Cities of China. Int. J. Environ. Res. Public Health 2022, 19, 13869. https://doi.org/10.3390/ijerph192113869
Jiang Y, Guo J, Zhou Y, Zhang B, Zhang J. Occurrence and Behavior of Methylsiloxanes in Urban Environment in Four Cities of China. International Journal of Environmental Research and Public Health. 2022; 19(21):13869. https://doi.org/10.3390/ijerph192113869
Chicago/Turabian StyleJiang, Yao, Junyu Guo, Ying Zhou, Boya Zhang, and Jianbo Zhang. 2022. "Occurrence and Behavior of Methylsiloxanes in Urban Environment in Four Cities of China" International Journal of Environmental Research and Public Health 19, no. 21: 13869. https://doi.org/10.3390/ijerph192113869






