High Frequency of Antibiotic Resistance Genes (ARGs) in the Lerma River Basin, Mexico
Abstract
:1. Introduction
2. Materials and Methods
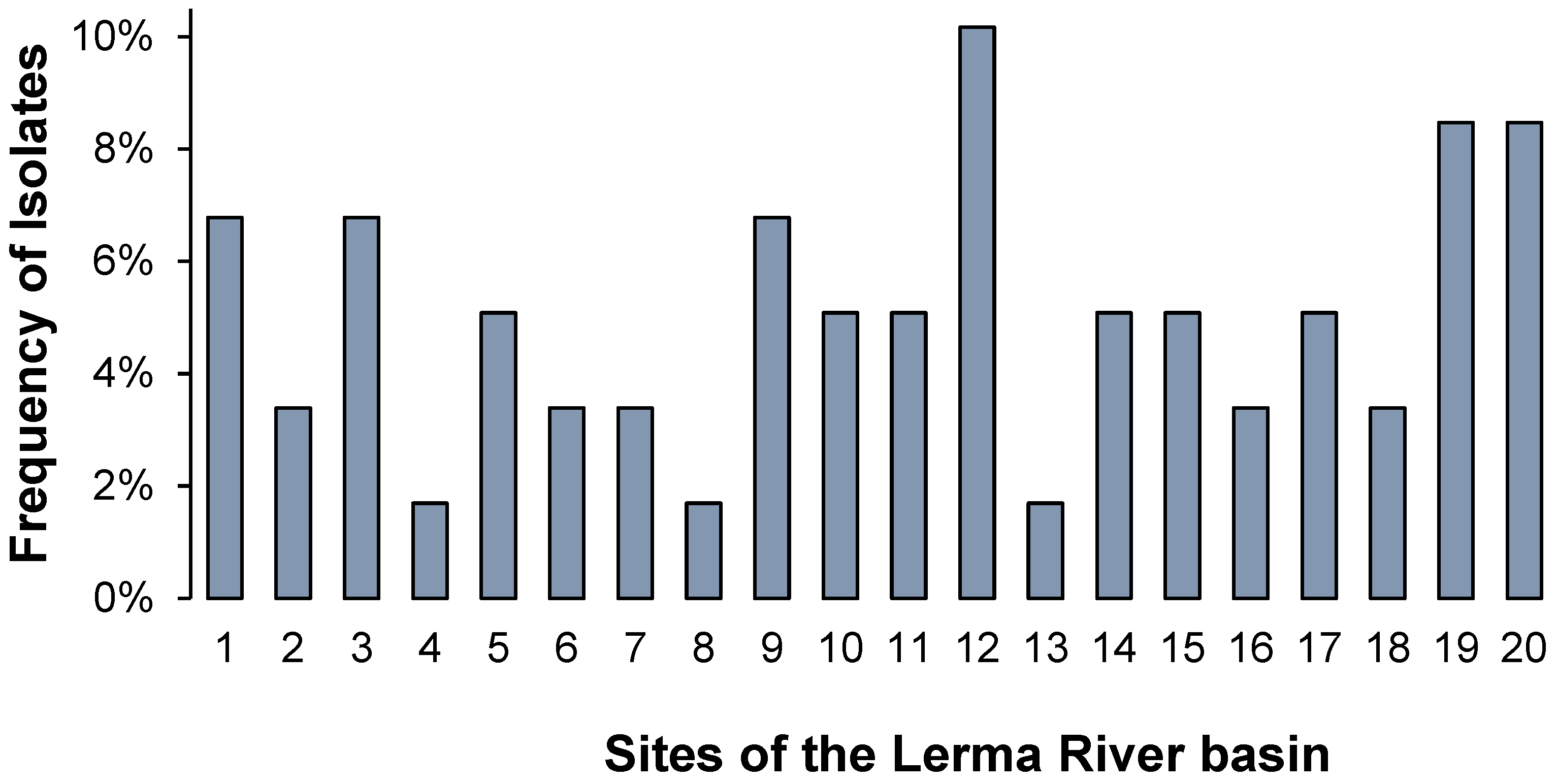
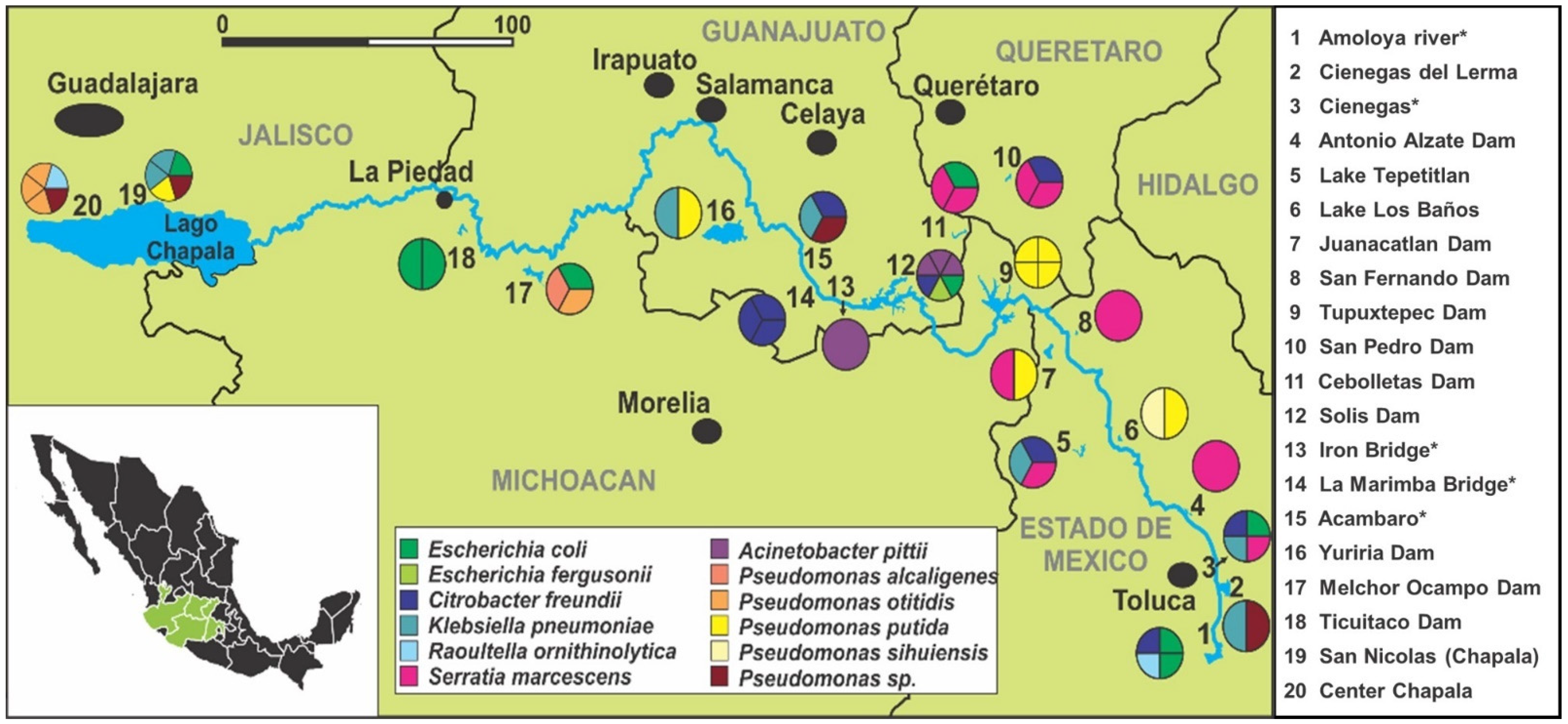
2.1. Study Sites and Sample Collection
2.2. Antimicrobial Susceptibility
2.3. Molecular Detection of Bacterial Species
2.4. Detection of Resistance Genes
2.5. Whole Genome Sequencing
2.6. Bioinformatic Analysis
3. Results
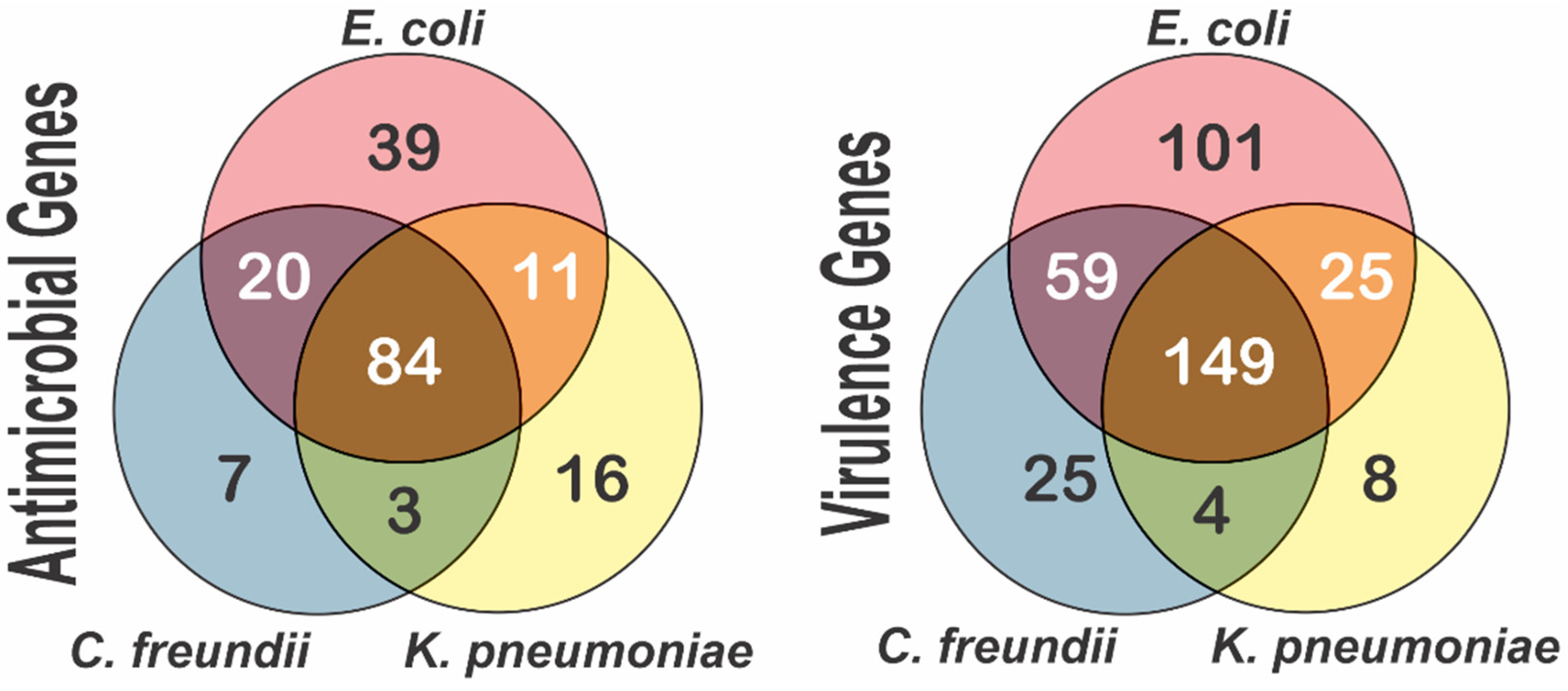
3.1. Bacterial Distribution and Characterization
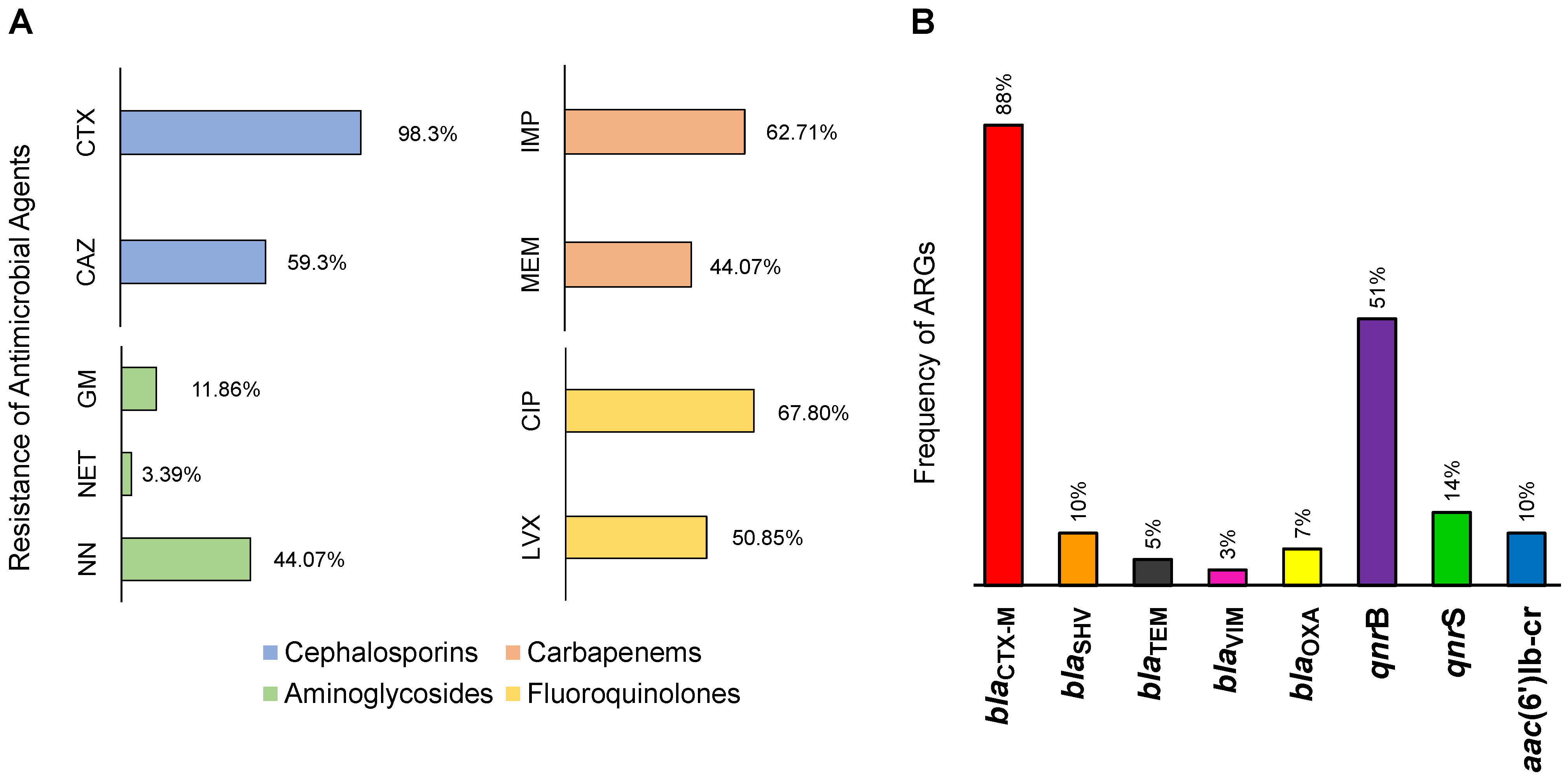
3.2. Antimicrobial Resistance in the Lerma River
3.3. Multidrug Resistance in the Lerma River Basin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhullar, K.; Waglechner, N.; Pawlowski, A.; Koteva, K.; Banks, E.D.; Johnston, M.D.; Barton, H.A.; Wright, G.D. Antibiotic Resistance Is Prevalent in an Isolated Cave Microbiome. PLoS ONE 2012, 7, e34953. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.T.; Chen, Q.L.; He, J.Z.; Hu, H.W. Microbial regulation of natural antibiotic resistance: Understanding the pro-tist-bacteria interactions for evolution of soil resistome. Sci. Total Environ. 2020, 705, 135882. [Google Scholar] [CrossRef] [PubMed]
- Laroche-Ajzenberg, E.; Ribeiro, A.F.; Bodilis, J.; Riah, W.; Buquet, S.; Chaftar, N.; Pawlak, B. Conjugative multiple-antibiotic resistance plasmids in Escherichia coli isolated from environmental waters contaminated by human faecal wastes. J. Appl. Microbiol. 2014, 118, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.; Bergeron, N.; Krauss, R.M.; Jansson, J.K.; Tiedje, J.M. Metagenomic Insights into the Degradation of Resistant Starch by Human Gut Microbiota. Appl. Environ. Microbiol. 2018, 84, 23. [Google Scholar] [CrossRef] [Green Version]
- Garza-González, E.; Morfín-Otero, R.; Mendoza-Olazarán, S.; Bocanegra-Ibarias, P.; Flores-Treviño, S.; Rodríguez-Noriega, E.; Ponce-De-León, A.; Sanchez-Francia, D.; Franco-Cendejas, R.; Arroyo-Escalante, S.; et al. A snapshot of antimicrobial resistance in Mexico. Results from 47 centers from 20 states during a six-month period. PLoS ONE 2019, 14, e0209865. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Vivas, R.; Barbosa, A.A.T.; Dolabella, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef]
- Finton, M.D.; Meisal, R.; Porcellato, D.; Brandal, L.T.; Lindstedt, B.-A. Whole Genome Sequencing and Characterization of Multidrug-Resistant (MDR) Bacterial Strains Isolated from a Norwegian University Campus Pond. Front. Microbiol. 2020, 11, 1273. [Google Scholar] [CrossRef]
- Fernandez-Lopez, R.; De Toro, M.; Moncalian, G.; Garcillan-Barcia, M.P.; De la Cruz, F. Comparative Genomics of the Conju-gation Region of F-like Plasmids: Five Shades of F. Front. Mol. Biosci. 2016, 3, 71. [Google Scholar] [CrossRef]
- Yang, Q.-E.; Sun, J.; Li, L.; Deng, H.; Liu, B.-T.; Fang, L.-X.; Liao, X.-P.; Liu, Y.-H. IncF plasmid diversity in multi-drug resistant Escherichia coli strains from animals in China. Front. Microbiol. 2015, 6, 964. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.O.; Cardoso, A.M.; Coutinho, F.H.; Pinto, L.H.; Vieira, R.P.; Albano, R.M.; Clementino, M.M. Diversity and anti-biotic resistance profiles of Pseudomonads from a hospital wastewater treatment plant. J. Appl. Microbiol. 2015, 119, 1527–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Duin, D.; Paterson, D.L. Multidrug-resistant bacteria in the community: Trends and lessons learned. Infect. Dis. Clin. 2016, 30, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Mourkas, E.; Florez-Cuadrado, D.; Pascoe, B.; Calland, J.K.; Bayliss, S.C.; Mageiros, L.; Méric, G.; Hitchings, M.D.; Quesada, A.; Porrero, C.; et al. Gene pool transmission of multidrug resistance among Campylobacter from livestock, sewage and human disease. Environ. Microbiol. 2019, 21, 4597–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas IV, J.C.; Oladeinde, A.; Kieran, T.J.; Finger, J.W., Jr.; Bayona-Vásquez, N.J.; Cartee, J.C.; Beasley, J.C.; Seaman, J.C.; McArthur, J.V.; Rhodes, O.E.; et al. Co-occurrence of antibiotic, biocide, and heavy metal resistance genes in bacteria from metal and radionuclide contaminated soils at the Savannah River Site. Microb. Biotechnol. 2020, 13, 1179–1200. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Lartigue, M.F.; Decousser, J.W.; Nordmann, P. ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob. Agents Chemother. 2005, 49, 447–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castanheira, M.; Mendes, R.E.; Jones, R.N.; Sader, H.S. Changes in the Frequencies of β-Lactamase Genes among Enterobacte-riaceae Isolates in U.S. Hospitals, 2012 to 2014: Activity of Ceftazidime-Avibactam Tested against β-Lactamase-Producing Isolates. Antimicrob. Agents Chemother. 2016, 60, 4770–4777. [Google Scholar] [CrossRef] [Green Version]
- Brechet, C.; Plantin, J.; Sauget, M.; Thouverez, M.; Talon, D.; Cholley, P.; Guyeux, C.; Hocquet, D.; Bertrand, X. Wastewater treatment plants release large amounts of extended-spectrum b-lactamase-producing Escherichia coli into the environment. Clin. Infect. Dis. 2014, 58, 1658–1665. [Google Scholar] [CrossRef] [Green Version]
- Hocquet, D.; Muller, A.; Bertrand, X. What happens in hospitals does not stay in hospitals: Antibiotic-resistant bacteria in hospital wastewater systems. J. Hosp. Infect. 2016, 93, 395–402. [Google Scholar] [CrossRef]
- Guenther, S.; Aschenbrenner, K.; Stamm, I.; Bethe, A.; Semmler, T.; Stubbe, A.; Stubbe, M.; Batsajkhan, N.; Glupczynski, Y.; Wieler, L.H.; et al. Comparable high rates of extended-spectrum-β-lactamase-producing Escherichia coli in birds of prey from Germany and Mongolia. PLoS ONE 2012, 7, e53039. [Google Scholar] [CrossRef]
- Veldman, K.; van Tulden, P.; Kant, A.; Testerink, J.; Mevius, D. Characteristics of Cefotaxime-Resistant Escherichia coli from Wild Birds in The Netherlands. Appl. Environ. Microbiol. 2013, 79, 7556–7561. [Google Scholar] [CrossRef] [Green Version]
- Stedt, J.; Bonnedahl, J.; Hernandez, J.; Waldenström, J.; McMahon, B.J.; Tolf, C.; Olsen, B.; Drobni, M. Carriage of CTX-M type extended spectrum β-lactamases (ESBLs) in gulls across Europe. Acta Veter. Scand. 2015, 57, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, E.M.; Barrón, M.D.L.C.; Caretta, C.A.; Goñi-Urriza, M.; Andrade, L.H.; Cuevas-Rodríguez, G.; Malm, O.; Torres, J.P.; Simon, M.; Guyoneaud, R. Impact of hydrocarbons, PCBs and heavy metals on bacterial communities in Lerma River, Salamanca, Mexico: Investigation of hydrocarbon degradation potential. Sci. Total Environ. 2015, 521–522, 1–10. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, M.; Ramos-Espinosa, M.G.; Carranza-Fraser, J. Análisis multimétrico para evaluar contaminación en el río Lerma y lago de Chapala, México. Hidrobiologica 2007, 17, 17–30. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, M.C.F.; Ahrenfeldt, J.; Cisneros, J.L.B.; Jurtz, V.; Larsen, M.V.; Hasman, H.; Aarestrup, F.M.; Lund, O. A bacterial analysis platform: An integrated system for analysing bacteri-al whole genome sequencing data for clinical diagnostics and surveillance. PLoS ONE 2016, 11, e0157718. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xiong, Z.; Sun, L.; Yang, J.; Jin, Q. VFDB 2012 update: Toward the genetic diversity and molecular evolution of bacterial virulence factors. Nucleic Acids Res. 2011, 40, D641–D645. [Google Scholar] [CrossRef]
- Böger, B.; Surek, M.; de O Vilhena, R.; Fachi, M.M.; Junkert, A.M.; Santos, J.M.; Domingos, E.L.; Cobre, A.D.F.; Momade, D.R.; Pontarolo, R. Occurrence of antibiotics and antibiotic resistant bacteria in subtropical urban rivers in Brazil. J. Hazard. Mater. 2020, 402, 123448. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Santos, A.; Tacao, M.; Alves, A.; Henriques, I.; Correia, A. Genetic diversity and antimicrobial resistance of Esch-erichia coli from Tagus estuary (Portugal). Sci. Total Environ. 2013, 461, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, D.; He, S.; Ye, H.; Zhang, L.; Wenhui, Z.; Zhang, W.; Shuchang, C.; Chen, S. Prevalence of Antibiotic-Resistant Escherichia coli in Drinking Water Sources in Hangzhou City. Front. Microbiol. 2017, 8, 1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spindler, A.; Otton, L.M.; Fuentefria, D.B.; Corçao, G. Beta-lactams resistance and presence of class 1 integron in Pseu-domonas spp. isolated from untreated hospital effluents in Brazil. Antonie Leeuwenhoek 2012, 102, 73–81. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, L.; Zhang, Y.; Zhang, H.; Yu, X.; Zhang, S. Occurrence and variations of five classes of antibiotic resistance genes along the Jiulong River in southeast China. J. Environ. Biol. 2013, 34, 345–351. [Google Scholar]
- Zurfluh, K.; Hachler, H.; Nüesch-Inderbinen, M.; Stephan, R. Characteristics of Extended-Spectrum β-lactamase- and Car-bapenemase-producing Enterobacteriaceae Isolates from rivers and lakes in Switzerland. Appl. Environ. Microbiol. 2013, 79, 3021–3026. [Google Scholar] [CrossRef] [Green Version]
- Khan, G.A.; Berglund, B.; Khan, K.M.; Lindgren, P.-E.; Fick, J. Occurrence and Abundance of Antibiotics and Resistance Genes in Rivers, Canal and near Drug Formulation Facilities—A Study in Pakistan. PLoS ONE 2013, 8, e62712. [Google Scholar] [CrossRef]
- Czekalski, N.; Diez, E.G.; Bürgmann, H. Wastewater as a point source of antibiotic-resistance genes in the sediment of a freshwater lake. ISME J. 2014, 8, 1381–1390. [Google Scholar] [CrossRef]
- Laht, M.; Karkman, A.; Voolaid, V.; Ritz, C.; Tenson, T.; Virta, M.; Kisand, V. Abundances of Tetracycline, Sulphonamide and Beta-Lactam Antibiotic Resistance Genes in Conventional Wastewater Treatment Plants (WWTPs) with Different Waste Load. PLoS ONE 2014, 9, e103705. [Google Scholar] [CrossRef] [Green Version]
- Devarajan, N.; Laffite, A.; Graham, N.D.; Meijer, M.; Prabakar, K.; Mubedi, J.I.; Elongo, V.; Mpiana, P.T.; Ibelings, B.W.; Wildi, W.; et al. Accumulation of Clinically Relevant Antibiotic-Resistance Genes, Bacterial Load, and Metals in Freshwater Lake Sediments in Central Europe. Environ. Sci. Technol. 2015, 49, 6528–6537. [Google Scholar] [CrossRef] [PubMed]
- Djenadi, K. Antibiotic Resistance Bacteria from Rivers Water in Algeria. J. Bacteriol. Parasitol. 2017, 8, 1–3. [Google Scholar] [CrossRef]
- Mondragón, V.A.; Llamas-Pérez, D.F.; González-Guzmán, G.E.; Márquez-González, A.R.; Padilla-Noriega, R.; Durán-Avelar, M.D.J.; Franco, B. Identification of Enterococcus faecalis bacteria resistant to heavy metals and antibiotics in surface waters of the Mololoa River in Tepic, Nayarit, Mexico. Environ. Monit. Assess. 2011, 183, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.D.; Gutierrez, S.; Sahagun, D.; Gomez, J.; Mendoza, J.; Ellis, C.C.; Bauer, S.; Blattner, J.; Lee, W.-Y.; Alvarez, M.; et al. Assessment of Antibiotic Levels, Multi-Drug Resistant Bacteria and Genetic Biomarkers in the Waters of the Rio Grande River Between the United States-Mexico Border. J. Health Pollut. 2019, 9, 190912. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Gardea, M.C.; Tamez-Guerra, P.; Gomez-Flores, R.; Zavala-Díaz de la Serna, F.J.; Eroza-de la Vega, G.; Nevárez-Moorillón, G.V.; Pérez-Recoder, M.C.; Sánchez-Ramírez, B.; González-Horta, M.C.; Infante-Ramírez, R. Multi-Drug-Resistant Bacteria Isolated from Surface Water in Bassaseachic Falls National Park, Mexico. Int. J. Environ. Res. Public Health 2016, 13, 597. [Google Scholar] [CrossRef] [Green Version]
- Canizalez-Roman, A.; Velazquez-Roman, J.; Valdez-Flores, M.A.; Flores-Villaseñor, H.; Vidal, J.E.; Muro-Amador, S.; Guadrón-Llanos, A.M.; Gonzalez-Nuñez, E.; Medina-Serrano, J.; Tapia-Pastrana, G.; et al. Detection of antimi-crobial-resistance diarrheagenic Escherichia coli strains in surface water used to irrigate food products in the northwest of Mexico. Int. J. Food Microbiol. 2019, 304, 1–10. [Google Scholar] [CrossRef]
- Tacao, M.; Correia, A.; Henriques, I. Low prevalence of carbapenem-resistant~bacteria in river water: Resistance is mostly related to intrinsic mechanisms. Microb. Drug Resist. 2015, 21, 497–506. [Google Scholar] [CrossRef]
- Arora, S.; Gautam, V.; Rana, S.; Ray, P. Novel chromogenic medium for detection of extended-spectrum be-ta-lactamase-producing Enterobacteriaceae, methicillin resistant Staphylococcus aureus and vancomycin resistant Entero-coccus. J. Med. Investig. Pract. 2014, 9, 98. [Google Scholar]
- Nnadozie, C.F.; Odume, O.N. Freshwater environments as reservoirs of antibiotic resistant bacteria and their role in the dis-semination of antibiotic resistance genes. Environ. Pollut. 2019, 245, 113067. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Q.; Zhang, S.; Zhang, J.; Yang, G.; Wang, H.; Huang, J.; Chen, M.; Xue, L.; Wang, J. Antibiotic-resistant extended spectrum ß-lactamase- and plasmid-mediated AmpC-producing enterobacteriaceae isolated from retail food products and the pearl River in Guangzhou, China. Front. Microbiol. 2017, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Harmon, D.E.; Miranda, O.A.; McCarley, A.; Eshaghian, M.; Carlson, N.; Ruiz, C. Prevalence and characterization of car-bapenem-resistant bacteria in water bodies in the Los Angeles-Southern California area. Microbiol. Open 2019, 8, e00692. [Google Scholar] [CrossRef] [Green Version]
- Girlich, D.; Poirel, L.; Nordmann, P. Diversity of clavulanic acid-inhibited extended-spectrum b-lactamases in Aeromonas spp. from the seine river, Paris, France. Antimicrob. Agents Chemother. 2011, 55, 1256–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacão, M.; Correia, A.; Henriques, I. Resistance to Broad-Spectrum Antibiotics in Aquatic Systems: Anthropogenic Activities Modulate the Dissemination of bla CTX-M -Like Genes. Appl. Environ. Microbiol. 2012, 78, 4134–4140. [Google Scholar] [CrossRef]
- Ribeiro, A.F.; Laroche, E.; Hanin, G.; Fournier, M.; Quillet, L.; Dupont, J.P.; Pawlak, B. Antibiotic-resistant Escherichia coli in karstic systems: A biological indicator of the origin of fecal contamination? FEMS Microbiol. Ecol. 2012, 81, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Lu, J.; Tong, Y.; Li, S.; Wang, X. Distribution of antibiotic resistance genes in Bosten Lake, Xinjiang, China. Water Sci. Technol. 2014, 70, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Czekalski, N.; Sigdel, R.; Birtel, J.; Matthews, B.; Bürgmann, H. Does human activity impact the natural antibiotic resistance background? Abundance of antibiotic resistance genes in 21 Swiss lakes. Environ. Int. 2015, 81, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hao, L.; Guo, X.; Wang, N.; Ye, B. Prevalence of antibiotic resistance genes of wastewater and surface water in livestock farms of Jiangsu Province, China. Environ. Sci. Pollut. Res. 2015, 22, 13950–13959. [Google Scholar] [CrossRef]
- Coque, T.M.; Baquero, F.; Cantón, R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Eurosurveillance 2008, 13, 19044. [Google Scholar] [CrossRef]
- Poirel, L.; Le Thomas, I.; Naas, T.; Nordmann, P. Biochemical sequence analyses of GES1, a novel class A extended-spectrum beta-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2000, 44, 622–632. [Google Scholar] [CrossRef] [Green Version]
- Bush, K.; Jacoby, G.A. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Khalili, H.; Izadpanah, M. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: An evidence-based literature review. J. Res. Pharm. Pract. 2015, 4, 105–114. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Antibiotic Resistance Threats in the United States. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 1 July 2022).
- Centers for Disease Control and Prevention. 2019. Antibiotic Resistance Threats in the United States. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 1 July 2022).
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.-H. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Belachew, T.; Mihret, A.; Legesse, T.; Million, Y.; Desta, K. High level of drug resistance by gram-negative bacteria from selected sewage polluted urban rivers in Addis Ababa, Ethiopia. BMC Res. Notes 2018, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Tafoukt, R.; Touati, A.; Leangapichart, T.; Bakour, S.; Rolain, J.M. Characterization of OXA-48-like-producing Enterobacteri-aceae isolated from river water in Algeria. Water Res. 2017, 120, 185–189. [Google Scholar] [CrossRef]
- Flerlage, T.; De Cardenas, J.N.B.; Garner, C.D.; Hasan, N.A.; Karathia, H.; Qudeimat, A.; Maron, G.; Hayden, R. Multiple NDM-5-Expressing Escherichia Coli Isolates from an Immunocompromised Pediatric Host. Open Forum Infect. Dis. 2020, 7, ofaa018. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, B.; Mukherjee, M. Epidemiologic and molecular characterization of β-lactamase-producing multi-drug-resistant uropathogenic Escherichia coli isolated from asymptomatic hospitalized patients. Int. Microbiol. 2022, 25, 27–45. [Google Scholar] [CrossRef]
- Prah, I.; Ayibieke, A.; Mahazu, S.; Sassa, C.T.; Hayashi, T.; Yamaoka, S.; Suzuki, T.; Iwanaga, S.; Ablordey, A.; Saito, R. Emergence of oxacillinase-181 carbapenemase-producing diarrheagenic Escherichia coli in Ghana. Emerg. Microbes Infect. 2021, 10, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Klockgether, J.; Fischer, S.; Trcek, J.; Tümmler, B.; Römling, U. Why?—Successful Pseudomonas aeruginosa clones with a focus on clone C. FEMS Microbiol. Rev. 2020, 44, 740–762. [Google Scholar] [CrossRef] [PubMed]
- Lyimo, B.; Buza, J.; Subbiah, M.; Temba, S.; Kipasika, H.; Smith, W.; Call, D.R. IncF Plasmids Are Commonly Carried by Antibiotic Resistant Escherichia coli Isolated from Drinking Water Sources in Northern Tanzania. Int. J. Microbiol. 2016, 2016, 3103672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahube, T.; Eviana, L.S.; Koraimann, G.; Yost, C.K. Characterization and comparative analysis of antibiotic resistance plasmids isolated from a wastewater treatment plant. Front. Microbiol. 2014, 5, 558. [Google Scholar] [CrossRef] [Green Version]
- Fall, C.; Hinojosa-Peña, A.; Carreño-De-León, M. Design of a monitoring network and assessment of the pollution on the Lerma river and its tributaries by wastewaters disposal. Sci. Total Environ. 2007, 373, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment e a review e Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, N.; Laffite, A.; Mulaji, C.K.; Otomonga, J.P.; Mpiana, P.T.; Mubedi, J.I.; Prabakar, K.; Ibelings, B.W.; Poté, J. Oc-currence of antibiotic resistance genes and bacterial markers in a tropical river receiving hospital and urban wastewaters. PLoS ONE 2016, 11, e0149211. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, K.; Kumar, G.N.; Bhardwaj, A.K. Characterization of Vibrio fluvialis qnrVC5 Gene in Native and Heterologous Hosts: Synergy of qnrVC5 with other Determinants in Conferring Quinolone Resistance. Front. Microbiol. 2016, 7, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelstein, M.; Pimkin, M.; Palagin, I.; Edelstein, I.; Stratchounski, L. Prevalence and molecular epidemiology of CTX-M ex-tended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob. Agents Chemother. 2003, 47, 3724–3732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galani, I.; Xirouchaki, E.; Kanellakopoulou, K.; Petrikkos, G.; Giamarellou, H. Transferable plasmid mediating resistance to multiple antimicrobial agents in Klebsiella pneumoniae isolates in Greece. Clin. Microbiol. Infect. 2002, 8, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Quinteros, M.; Radice, M.; Gardella, N.; Rodriguez, M.M.; Costa, N.; Korbenfeld, D.; Couto, E.; Gutkind, G. Extended-Spectrum B-Lactamases in Enterobacteriaceae in Buenos Aires, Argentina, Public Hospitals. Antimicrob. Agents Chemother. 2003, 47, 2864–2867. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.S.; Kim, K.; Huh, J.Y.; Jung, B.; Kang, M.S.; Hong, S.G. Multiplex PCR for Rapid Detection of Genes Multiplex PCR for Rapid Detection of Genes Encoding Class A Carbapenemases. Ann. Lab. Med. 2012, 32, 359–361. [Google Scholar] [CrossRef] [Green Version]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Wang, M.; Sahm, D.F.; Jacoby, G.A.; Hooper, D.C. Emerging Plasmid-Mediated Quinolone Resistance Associated with the qnr Gene in Klebsiella pneumoniae Clinical Isolates in the United States. Antimicrob. Agents Chemother. 2004, 48, 1295–1299. [Google Scholar] [CrossRef]
- Cai, X.; Li, C.; Huang, J.; Li, Y. Prevalence of plasmid-mediated quinolone resistance qnr genes in Central China. Afr. J. Microbiol. Res. 2011, 5, 975–978. [Google Scholar]

| Site | 1 | 2 | 3 | 4 | 5 | 8 | 9 | 10 | 11 | 12 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | LR12-01CF | LR13-01EC | LR14-01EC | LR16-02KP | LR17-02PSP | LR18-03CF | LR25-03EC | LR26-03KP | LR27-03SM | LR28-04SM | LR29-05CF | LR31-05SM | LR39-08SM | LR40-09PP | LR41-09PP | LR42-09PP | LR43-09PP | LR44-10CF | LR46-10SM | LR47-11EC | LR48-11SM | LR49-11SM | LR51-12AP | LR54-12CF | LR56-12EF | LR58-14CF | LR60-14CF | LR33-15CF | LR61-15KP | LR62-15PSP | LR63-16KP | LR64-16PP | LR34-17EC | LR35-18EC | LR69-19KP | LR53-19KP | LR76-20RO | |
| Antimicrobial category | Agent | |||||||||||||||||||||||||||||||||||||
| β-lactams | ceftazidime | |||||||||||||||||||||||||||||||||||||
| cefotaxime | ||||||||||||||||||||||||||||||||||||||
| carbapenems | meropenem | |||||||||||||||||||||||||||||||||||||
| imipenem | ||||||||||||||||||||||||||||||||||||||
| aminoglycosides | trobamycin | |||||||||||||||||||||||||||||||||||||
| netilmicin | ||||||||||||||||||||||||||||||||||||||
| gentamicine | ||||||||||||||||||||||||||||||||||||||
| fluoroquinolones | levofloxacin | |||||||||||||||||||||||||||||||||||||
| ciprofloxacin | ||||||||||||||||||||||||||||||||||||||
| ARGs | ||||||||||||||||||||||||||||||||||||||
| β-lactams | blaCTX-M | |||||||||||||||||||||||||||||||||||||
| blaSHV | ||||||||||||||||||||||||||||||||||||||
| blaTEM | ||||||||||||||||||||||||||||||||||||||
| carbapenems | blaOXA | |||||||||||||||||||||||||||||||||||||
| blaVIM | ||||||||||||||||||||||||||||||||||||||
| aminoglycosides | aac(6′)-lb-cr | |||||||||||||||||||||||||||||||||||||
| fluoroquinolones | qnrB | |||||||||||||||||||||||||||||||||||||
| qnrS | ||||||||||||||||||||||||||||||||||||||
| C. freundii | E. coli | S. marcescens | K. pneumoniae | E. fergusonii | ||||||||||||||||||||||||||||||||||
| A. pittii | P. putida | Pseudomonas sp. | R. ornithinolytica | |||||||||||||||||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia-Arreola, A.K.; Ruiz-Garcia, D.A.; Rodulfo, H.; Sharma, A.; De Donato, M. High Frequency of Antibiotic Resistance Genes (ARGs) in the Lerma River Basin, Mexico. Int. J. Environ. Res. Public Health 2022, 19, 13988. https://doi.org/10.3390/ijerph192113988
Tapia-Arreola AK, Ruiz-Garcia DA, Rodulfo H, Sharma A, De Donato M. High Frequency of Antibiotic Resistance Genes (ARGs) in the Lerma River Basin, Mexico. International Journal of Environmental Research and Public Health. 2022; 19(21):13988. https://doi.org/10.3390/ijerph192113988
Chicago/Turabian StyleTapia-Arreola, Ana K., Daniel A. Ruiz-Garcia, Hectorina Rodulfo, Ashutosh Sharma, and Marcos De Donato. 2022. "High Frequency of Antibiotic Resistance Genes (ARGs) in the Lerma River Basin, Mexico" International Journal of Environmental Research and Public Health 19, no. 21: 13988. https://doi.org/10.3390/ijerph192113988







