Perspectives on the Therapeutic Effects of Pelvic Floor Electrical Stimulation: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
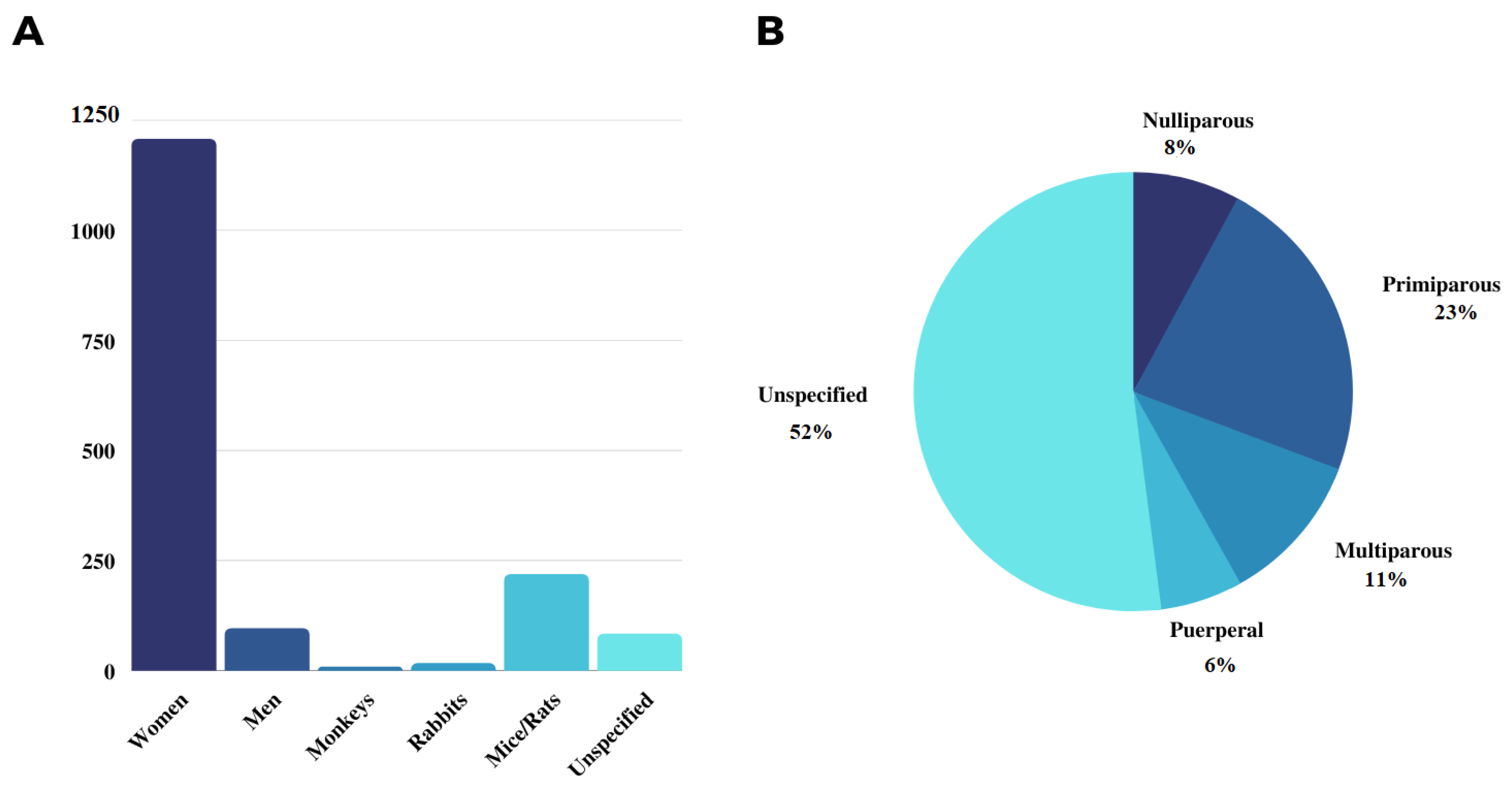
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghaderi, F.; Bastani, P.; Hajebrahimi, S.; Jafarabadi, M.A.; Berghmans, B. Pelvic floor rehabilitation in the treatment of women with dyspareunia: A randomized controlled clinical trial. Int. Urogynecol. J. 2019, 30, 1849–1855. [Google Scholar] [CrossRef]
- Deng, K.; Balog, B.M.; Lin, D.L.; Hanzlicek, B.; Song, Q.-X.; Zhu, H.; Damaser, M.S. Daily bilateral pudendal nerve electrical stimulation improves recovery from stress urinary incontinence. Interface Focus 2019, 9, 20190020. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Li, B.; Hong, S.; Min, J.; Hu, M.; Tang, J.; Wang, T.; Yang, L.; Hong, L. Electrical stimulation activates calpain 2 and subsequently upregulates collagens via the integrin β1/TGF-β1 signaling pathway. Cell Signal. 2019, 59, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.S.; Lapitan, M.C.M. Prevalence of Urinary Incontinence in Men, Women, and Children—Current Evidence: Findings of the Fourth International Consultation on Incontinence. Urology 2010, 76, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Havton, L.A.; Christe, K.L.; Edgerton, V.R.; Gad, P.N. Noninvasive spinal neuromodulation to map and augment lower urinary tract function in rhesus macaques. Exp. Neurol. 2019, 322, 113033. [Google Scholar] [CrossRef] [PubMed]
- Albers, L.L.; Sedler, K.D.; Bedrick, E.J.; Teaf, D.; Peralta, P. Factors Related to Genital Tract Trauma in Normal Spontaneous Vaginal Births. Birth 2006, 33, 94–100. [Google Scholar] [CrossRef]
- Saad, L.H.C.; Coy, C.S.R.; Fagundes, J.J.; Ariyzono, M.D.L.; Shoji, N.; Góes, J.R.N. Quantificacao da funcao esfincteriana pela medida da capacidade de sus- tentacao da pressao de contracao voluntaria do canal anal. Arq. Gastroenterol. 2002, 39, 233–239. [Google Scholar] [CrossRef]
- Laurienzo, C.E.; Magnabosco, W.J.; Jabur, F.; Faria, E.F.; Gameiro, M.O.; Sarri, A.J.; Kawano, P.R.; Yamamoto, H.A.; Reis, L.O.; Amaro, J.L. Pelvic floor muscle training and electrical stimulation as rehabilitation after radical prostatectomy: A randomized controlled trial. J. Phys. Ther. Sci. 2018, 30, 825–831. [Google Scholar] [CrossRef]
- Goode, P.S.; Burgio, K.L.; Johnson, T.M.; Clay, O.; Roth, D.L.; Markland, A.D.; Burkhardt, J.H.; Issa, M.M.; Lloyd, L.K. Behavioral Therapy With or Without Biofeedback and Pelvic Floor Electrical Stimulation for Persistent Postprostatectomy Incontinence. JAMA 2011, 305, 151–159. [Google Scholar] [CrossRef]
- Filocamo, M.; Limarzi, V.; Del Popolo, G.; Cecconi, F.; Marzocco, M.; Tosto, A.; Nicita, G. Effectiveness of Early Pelvic Floor Rehabilitation Treatment for Post-Prostatectomy Incontinence. Eur. Urol. 2005, 48, 734–738. [Google Scholar] [CrossRef]
- Berghmans; Hendriks; Bø; Smith, H.; Bie, D.; Van Doorn, V.W. Conservative treatment of stress urinary incontinence in women: A systematic review of randomized clinical trials. Br. J. Urol. 1998, 82, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Melling, C.V.; Goyal, A. Current pharmacological management of idiopathic overactive bladder in children in the UK: A national survey of practice. J. Pediatr. Urol. 2020, 16, 37.e1–37.e8. [Google Scholar] [CrossRef] [PubMed]
- Alós, R.; Solana, A.; Ruiz, M.D.; Moro, D.; García-Armengol, J.; Roig-Vila, J.V. Novel techniques in the treatment of anal incontinece. Cir. Esp. 2005, 78, S41–S49. [Google Scholar] [CrossRef]
- Zhao, S.; Mehta, A.S.; Zhao, M. Biomedical applications of electrical stimulation. Cell. Mol. Life Sci. 2020, 77, 2681–2699. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.M.; da Silva, J.B.; Rocha, A.P.R.; Liebano, R.E.; Driusso, P. Intravaginal electrical stimulation associated with pelvic floor muscle training for women with stress urinary incontinence: Study protocol for a randomized controlled trial with economic evaluation. Trials 2021, 22, 823. [Google Scholar] [CrossRef]
- Jha, S.; Walters, S.J.; Bortolami, O.; Dixon, S.; Alshreef, A. Impact of pelvic floor muscle training on sexual function of women with urinary incontinence and a comparison of electrical stimulation versus standard treatment (IPSU trial): A randomised controlled trial. Physiotherapy 2018, 104, 91–97. [Google Scholar] [CrossRef]
- De Sousa, H.A.; Silva, M.D.G.D.; Barbosa, K.D.P.; Vianna, L.M.D.S.; Pacheco, Y.G.; De Godoy, J.R.P.; Kuckelhaus, S.A.S. Electrical stimulation structurally affects the tissues of the rectum and anus of nulliparous rats. J. Anat. 2017, 231, 398–404. [Google Scholar] [CrossRef]
- Hernandez-Reynoso, A.G.; Corona-Quintanilla, D.L.; López-García, K.; Horbovetz, A.A.; Castelán, F.; Zimmern, P.; Martínez-Gómez, M.; Romero-Ortega, M.I. Targeted neuromodulation of pelvic floor nerves in aging and multiparous rabbits improves continence. Sci. Rep. 2021, 11, 10615. [Google Scholar] [CrossRef]
- Mallmann, S.; Ferla, L.; Rodrigues, M.P.; Paiva, L.L.; Sanches, P.R.; Ferreira, C.F.; Ramos, J.G.L. Comparison of parasacral transcutaneous electrical stimulation and transcutaneous posterior tibial nerve stimulation in women with overactive bladder syndrome: A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 250, 203–208. [Google Scholar] [CrossRef]
- Pagani, R.N.; Kovaleski, J.L.; de Resende, L.M.M. Avanços na composição da Methodi Ordinatio para revisão sistemática de literatura. Ciência Da Inf. 2018, 46, 161–187. [Google Scholar] [CrossRef]
- Yang, S.; Sang, W.; Feng, J.; Zhao, H.; Li, X.; Li, P.; Fan, H.; Tang, Z.; Gao, L. The effect of rehabilitation exercises combined with direct vagina low voltage low frequency electric stimulation on pelvic nerve electrophysiology and tissue function in primiparous women: A randomised controlled trial. J. Clin. Nurs. 2017, 26, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, R.; Yıldız, N.; Alkan, H. Efficacy of percutaneous and transcutaneous tibial nerve stimulation in women with idiopathic overactive bladder: A prospective randomised controlled trial. Ann. Phys. Rehabil. Med. 2021, 65, 101486. [Google Scholar] [CrossRef] [PubMed]
- Jacomo, R.H.; Alves, A.T.; Lucio, A.; Garcia, P.A.; Lorena, D.C.R.; de Sousa, J.B. Transcutaneous tibial nerve stimulation versus parasacral stimulation in the treatment of overactive bladder in elderly people: A triple-blinded randomized controlled trial. Clinics 2020, 75, e1477. [Google Scholar] [CrossRef] [PubMed]
- Antônio, F.I.; Bø, K.; Pena, C.C.; Bueno, S.M.; Mateus-Vasconcelos, E.C.L.; Fernandes, A.C.N.L.; Ferreira, C.H.J. Intravaginal electrical stimulation increases voluntarily pelvic floor muscle contractions in women who are unable to voluntarily contract their pelvic floor muscles: A randomised trial. J. Physiother. 2022, 68, 37–42. [Google Scholar] [CrossRef]
- Feng, X.; Lv, J.; Li, M.; Lv, T.; Wang, S. Short-term Efficacy and Mechanism of Electrical Pudendal Nerve Stimulation Versus Pelvic Floor Muscle Training Plus Transanal Electrical Stimulation in Treating Post-radical Prostatectomy Urinary Incontinence. Urology 2022, 160, 168–175. [Google Scholar] [CrossRef]
- Del Río-Gonzalez, S.; Aragon, I.M.; Castillo, E.; Milla-España, F.; Galacho, A.; Machuca, J.; Lara, M.F.; Herrera-Imbroda, B. Percutaneous Tibial Nerve Stimulation Therapy for Overactive Bladder Syndrome: Clinical Effectiveness, Urodynamic, and Durability Evaluation. Urology 2017, 108, 52–58. [Google Scholar] [CrossRef]
- Zhong, F.; Miao, W.; Yu, Z.; Hong, L.; Deng, N. Clinical effect of electrical stimulation biofeedback therapy combined with pelvic floor functional exercise on postpartum pelvic organ prolapse. Am. J. Transl. Res. 2021, 13, 6629–6637. [Google Scholar]
- Hwang, U.-J.; Lee, M.-S.; Jung, S.-H.; Ahn, S.-H.; Kwon, O.-Y. Which pelvic floor muscle functions are associated with improved subjective and objective symptoms after 8 weeks of surface electrical stimulation in women with stress urinary incontinence? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 16–21. [Google Scholar] [CrossRef]
- Li, W.; Hu, Q.; Zhang, Z.; Shen, F.; Xie, Z. Effect of different electrical stimulation protocols for pelvic floor rehabilitation of postpartum women with extremely weak muscle strength: Randomized control trial. Medicine 2020, 99, e19863. [Google Scholar] [CrossRef]
- Elena, S.; Dragana, Z.; Ramina, S.; Evgeniia, A.; Orazov, M. Electromyographic Evaluation of the Pelvic Muscles Activity After High-Intensity Focused Electromagnetic Procedure and Electrical Stimulation in Women With Pelvic Floor Dysfunction. Sex. Med. 2020, 8, 282–289. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Oliveira, M.; Silva, H.; Gomes, A.; Nascimento, G.; Marini, G.; Micussi, M.T. Evaluation of satisfaction of pelvic floor muscle training isolated and associated with tibial nerve stimulation in women with mixed urinary incontinence: A randomized, single-blinded clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 265, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Mateus-Vasconcelos, E.C.L.; Brito, L.G.O.; Driusso, P.; Silva, T.D.; Antônio, F.I.; Ferreira, C.H. Effects of three interventions in facilitating voluntary pelvic floor muscle contraction in women: A randomized controlled trial. Braz. J. Phys. Ther. 2018, 22, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Brose, S.W.; Bourbeau, D.J.; Gustafson, K.J. Genital nerve stimulation is tolerable and effective for bladder inhibition in sensate individuals with incomplete SCI. J. Spinal Cord Med. 2018, 41, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Feng, X.; Lv, J.; Cai, T.; Wang, S. Short-term Clinical Efficacy of Electric Pudendal Nerve Stimulation on Neurogenic Lower Urinary Tract Disease: A Pilot Research. Urology 2018, 112, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Ness, T.J.; DeWitte, C.; McNaught, J.; Clodfelder-Miller, B.; Su, X. Spinal mechanisms of pudendal nerve stimulation-induced inhibition of bladder hypersensitivity in rats. Neurosci. Lett. 2018, 686, 181–185. [Google Scholar] [CrossRef]
- Hwang, U.-J.; Lee, M.-S.; Jung, S.-H.; Ahn, S.-H.; Kwon, O.-Y. Pelvic Floor Muscle Parameters Affect Sexual Function After 8 Weeks of Transcutaneous Electrical Stimulation in Women with Stress Urinary Incontinence. Sex. Med. 2019, 7, 505–513. [Google Scholar] [CrossRef]
- Min, J.; Li, B.; Liu, C.; Hong, S.; Tang, J.; Hu, M.; Liu, Y.; Li, S.; Hong, L. Therapeutic Effect and Mechanism of Electrical Stimulation in Female Stress Urinary Incontinence. Urology 2017, 104, 45–51. [Google Scholar] [CrossRef]
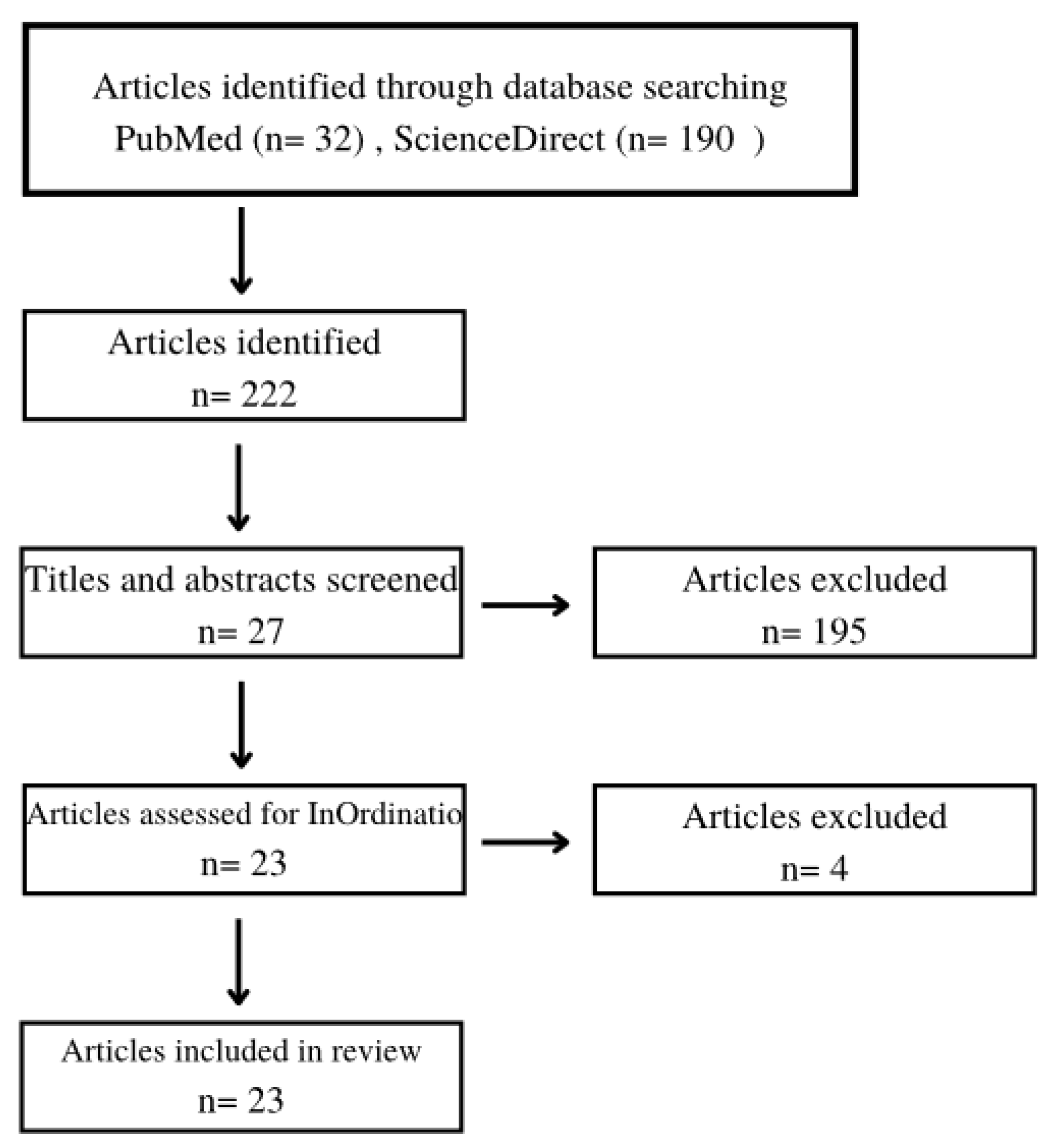
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | Humans and animals submitted to electrical stimulation against pelvic and perineal dysfunctions. | Pregnant population |
| Intervention | Electrical stimulation isolated or in combination with pelvic and perineal exercises | Other interventions |
| Type of study | Randomized and experimental studies | Literature reviews, encyclopedia, case reports, book chapters. |
| Language | English | Other languages |
| Year | 2017–2022 | Other period |
| Classification | Authors | InOrdinatio Value |
|---|---|---|
| [1] | Yang S et al., 2017 | 68.04 |
| [2] | Jha S et al., 2018 | 63.36 |
| [3] | Sonmez R et al., 2021 | 59.92 |
| [4] | Jacomo RH et al., 2020 | 57.36 |
| [5] | Antônio, F.I et al., 2022 | 57 |
| [6] | Feng X et al., 2022 | 52.64 |
| [7] | Del Río-Gonzalez S et al., 2017 | 51.64 |
| [8] | Havton LA et al., 2019 | 50.33 |
| [9] | Zhong F et al., 2021 | 50.1 |
| [10] | Hernandez AG et al., 2021 | 49.38 |
| [11] | Mallmann S et al., 2020 | 48.43 |
| [12] | Hwang UJ et al., 2020 | 48.43 |
| [13] | Li W et al., 2020 | 47.89 |
| [14] | Elena S et al., 2020 | 47.49 |
| [15] | Hwang UJ et al., 2019 | 47.49 |
| [16] | Oliveira MC et al., 2021 | 47.43 |
| [17] | Li Yang et al., 2019 | 47.31 |
| [18] | Min J et al., 2017 | 46.64 |
| [19] | Mateus–Vasconcelos E et al., 2018 | 46.38 |
| [20] | Ness TJ et al., 2018 | 46.05 |
| [21] | Brose SW et al., 2018 | 45.98 |
| [22] | Li T et al., 2018 | 37.65 |
| [23] | De Souza HA et al., 2017 | 33.92 |
| No. | Authors | Site of Intervention | Electric Current |
|---|---|---|---|
| [1] | Yang S et al., 2017 | Vagina | 60–80 Hz |
| [2] | Jha S et al., 2018 | Unspecified | Unspecified |
| [3] | Sonmez R et al., 2021 | Tibial nerve | 20 Hz |
| [4] | Jacomo RH et al., 2020 | Tibial nerve and para-sacral region | 10 Hz |
| [5] | Antônio, F.I et al., 2022 | Intravaginal | 50 Hz |
| [6] | Feng X et al., 2022 | Pudendal nerve and transanal region | 2.5 Hz |
| [7] | Del Río-Gonzalez S et al., 2017 | Tibial nerve | 20 Hz |
| [8] | Havton LA et al., 2019 | Spinal cord | 1 Hz |
| [9] | Zhong F et al., 2021 | Intravaginal | 35–80 Hz |
| [10] | Hernandez AG et al., 2021 | Bulbospongious and pubococcygeus nerves | 2–20 Hz |
| [11] | Mallmann S et al., 2020 | Tibial nerve and para-sacral region | 20 Hz |
| [12] | Hwang UJ et al., 2020 | Perivaginal and sacral | 25 Hz |
| [13] | Li W et al., 2020 | Intravaginal | 50 Hz |
| [14] | Elena S et al., 2020 | Vagina | 2.5 T |
| [15] | Hwang UJ et al., 2019 | Perivaginal and sacral | 25 Hz |
| [16] | Oliveira MC et al., 2021 | Tibial nerve | 20 Hz |
| [17] | Li Yang et al., 2019 | Vagina | 50 Hz |
| [18] | Min J et al., 2017 | Vagina | 20–50 Hz |
| [19] | Mateus–Vasconcelos E et al., 2018 | Intravaginal | 50 Hz |
| [20] | Ness TJ et al., 2018 | Pudendal nerve | 10 Hz |
| [21] | Brose SW et al., 2018 | Anogenital region | 20 Hz |
| [22] | Li T et al., 2018 | Pudendal nerve and anogenital region | 2.5–3.5 Hz |
| [23] | De Souza HA et al., 2017 | Vagina and rectum | 50 Hz |
| Author, Reference Number | Effect |
|---|---|
| Yang S et al., 2017 [21] | Improvement in incontinence score and Oxford grading for pelvic floor muscle strength. Synergic effect when associated with pelvic rehabilitation exercises. |
| Jha S et al., 2018 [16] | No significant improvement was observed after electrostimulation and physiotherapy against urinary incontinence and sexual dysfunction. |
| Sonmez R et al., 2021 [22] | Significant reduction in incontinence score, urination frequency, nocturia, number of absorbents used, and life quality obtained by electrical stimulation. |
| Jacomo RH et al., 2020 [23] | Electrical stimulation had beneficial effects on the treatment of hyperactive bladder, decreasing incontinence and nocturia scores. |
| Antônio, F.I et al., 2022 [24] | A 36% increase in the ability to contract pelvic floor muscles and improvement in incontinence score after electrical stimulation. |
| Feng X et al., 2022 [25] | An improvement in the urinary incontinence score was observed in man with urinary incontinence post-radical prostatectomy. |
| Del Río-Gonzalez S et al., 2017 [26] | Improvement in clinical and urodynamic parameters with durability up to 24 months after electrical stimulation. |
| Havton LA et al., 2019 [5] | Improvement in the urinary control in a less invasive way. |
| Zhong F et al., 2021 [27] | Significant improvement in stage I pelvic organ prolapes, life quality, and sexual quality. |
| Hernandez AG et al., 2021 [18] | Improvement in axonal composition, diameter, and regeneration of pelvic and perianal nerves. |
| Mallmann S et al., 2020 [19] | Improvement in life quality, discomfort level, and incontinence score. |
| Hwang UJ et al., 2020 [28] | Improvement in the power, strength, and resistance of pelvic floor muscles, urinary loss, and incontinence score. |
| Li W et al., 2020 [29] | Improvement in pelvic muscle contraction and muscle strength. |
| Elena S et al., 2020 [30] | Improvement in the recovery of pelvic floor muscle strength and sexual dysfunction-associated urinary incontinence. |
| Hwang UJ et al., 2019 [36] | Improvement in sexual function (desire, excitement, orgasm), besides the power, strength, and resistance of pelvic muscles. |
| Oliveira MC et al., 2021 [31] | Inconsistent results regarding urinary incontinence improvement. |
| Li Yang et al., 2019 [3] | Electrical stimulation activated collagen expression, increased the intracellular concentration of calcium, and suppressed apoptosis. |
| Min J et al., 2017 [37] | Electrical stimulation increased maximum bladder capacity and urodynamic aspects. It also increased collagen and calcium channel, assisting the treatment of urinary incontinence. |
| Mateus–Vasconcelos et al., 2018 [32] | Decrease in urinary incontinence score. |
| Ness TJ et al., 2018 [35] | Electrical stimulation induced neuromodulatory effects in pelvic sensorial systems treating bladder painful disorders. |
| Brose SW et al., 2018 [33] | The treatment decreased detrusor hyperactivity and pelvic pain. |
| Li T et al., 2018 [34] | Improvement in life quality and residual urine volume. |
| De Souza HA et al., 2017 [17] | Electrical stimulation improved the morphology of rectum and anus tissues with hyperplasia and hypertrophy, which consequently improved the anal sphincter function. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmento, A.L.C.; Sá, B.S.; Vasconcelos, A.G.; Arcanjo, D.D.R.; Durazzo, A.; Lucarini, M.; Leite, J.R.d.S.d.A.; Sousa, H.A.; Kückelhaus, S.A.S. Perspectives on the Therapeutic Effects of Pelvic Floor Electrical Stimulation: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14035. https://doi.org/10.3390/ijerph192114035
Sarmento ALC, Sá BS, Vasconcelos AG, Arcanjo DDR, Durazzo A, Lucarini M, Leite JRdSdA, Sousa HA, Kückelhaus SAS. Perspectives on the Therapeutic Effects of Pelvic Floor Electrical Stimulation: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(21):14035. https://doi.org/10.3390/ijerph192114035
Chicago/Turabian StyleSarmento, Ana Lúcia Carneiro, Bruno Silva Sá, Andreanne Gomes Vasconcelos, Daniel Dias Rufino Arcanjo, Alessandra Durazzo, Massimo Lucarini, José Roberto de Souza de Almeida Leite, Hugo Alves Sousa, and Selma Aparecida Souza Kückelhaus. 2022. "Perspectives on the Therapeutic Effects of Pelvic Floor Electrical Stimulation: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 21: 14035. https://doi.org/10.3390/ijerph192114035
APA StyleSarmento, A. L. C., Sá, B. S., Vasconcelos, A. G., Arcanjo, D. D. R., Durazzo, A., Lucarini, M., Leite, J. R. d. S. d. A., Sousa, H. A., & Kückelhaus, S. A. S. (2022). Perspectives on the Therapeutic Effects of Pelvic Floor Electrical Stimulation: A Systematic Review. International Journal of Environmental Research and Public Health, 19(21), 14035. https://doi.org/10.3390/ijerph192114035











