Pesticides in the Indoor Environment of Residential Houses: A Case Study in Strasbourg, France
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sampling Campaigns
Passive Samplers
2.3. Analytical Procedure
2.3.1. Reagents and Chemicals
2.3.2. Pesticides Extraction from Dust Samples
2.3.3. Pesticides Analysis from Air and Dust Samples
2.4. QA/QC
3. Results
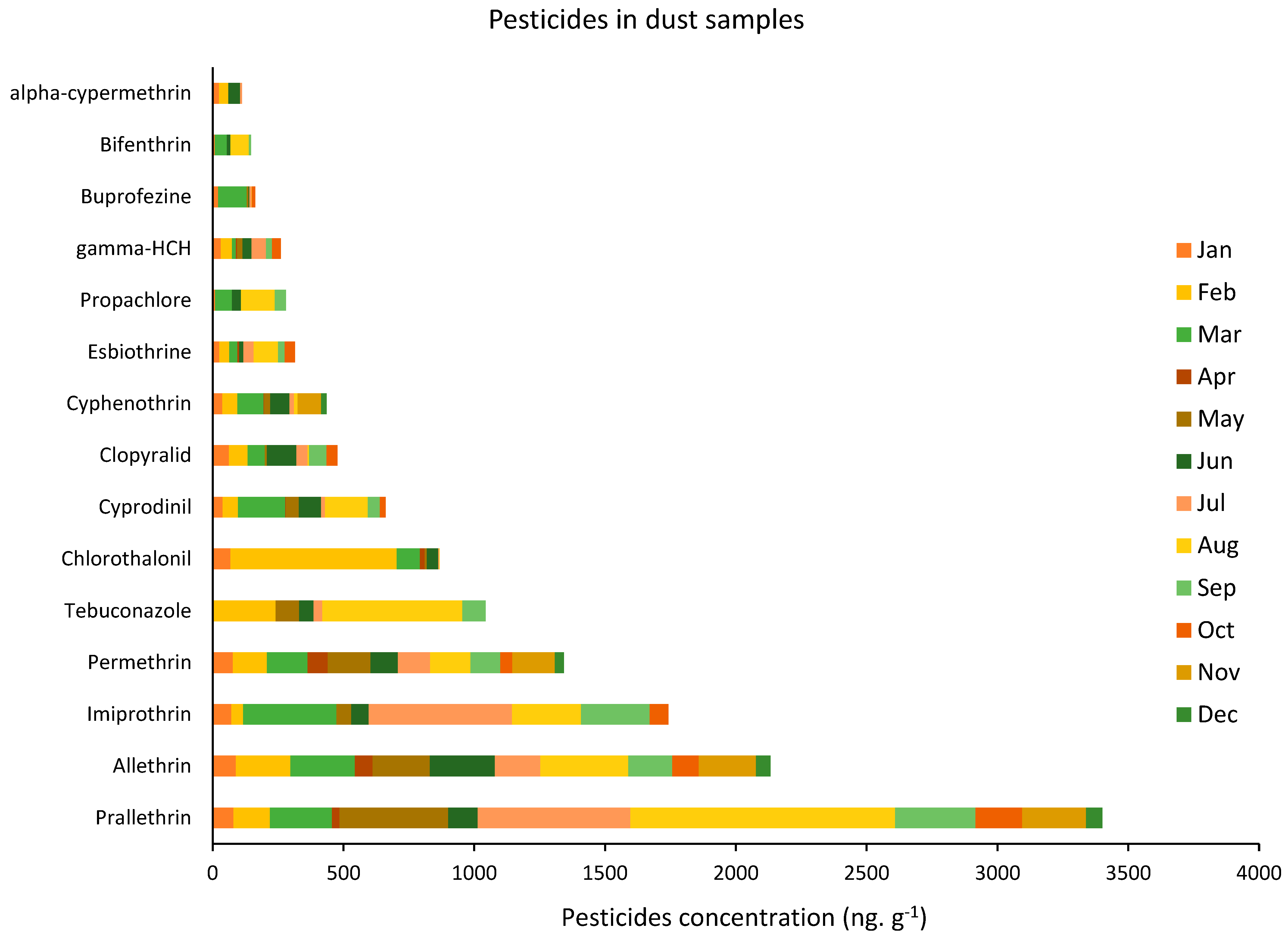
3.1. Pesticides in Dust Samples
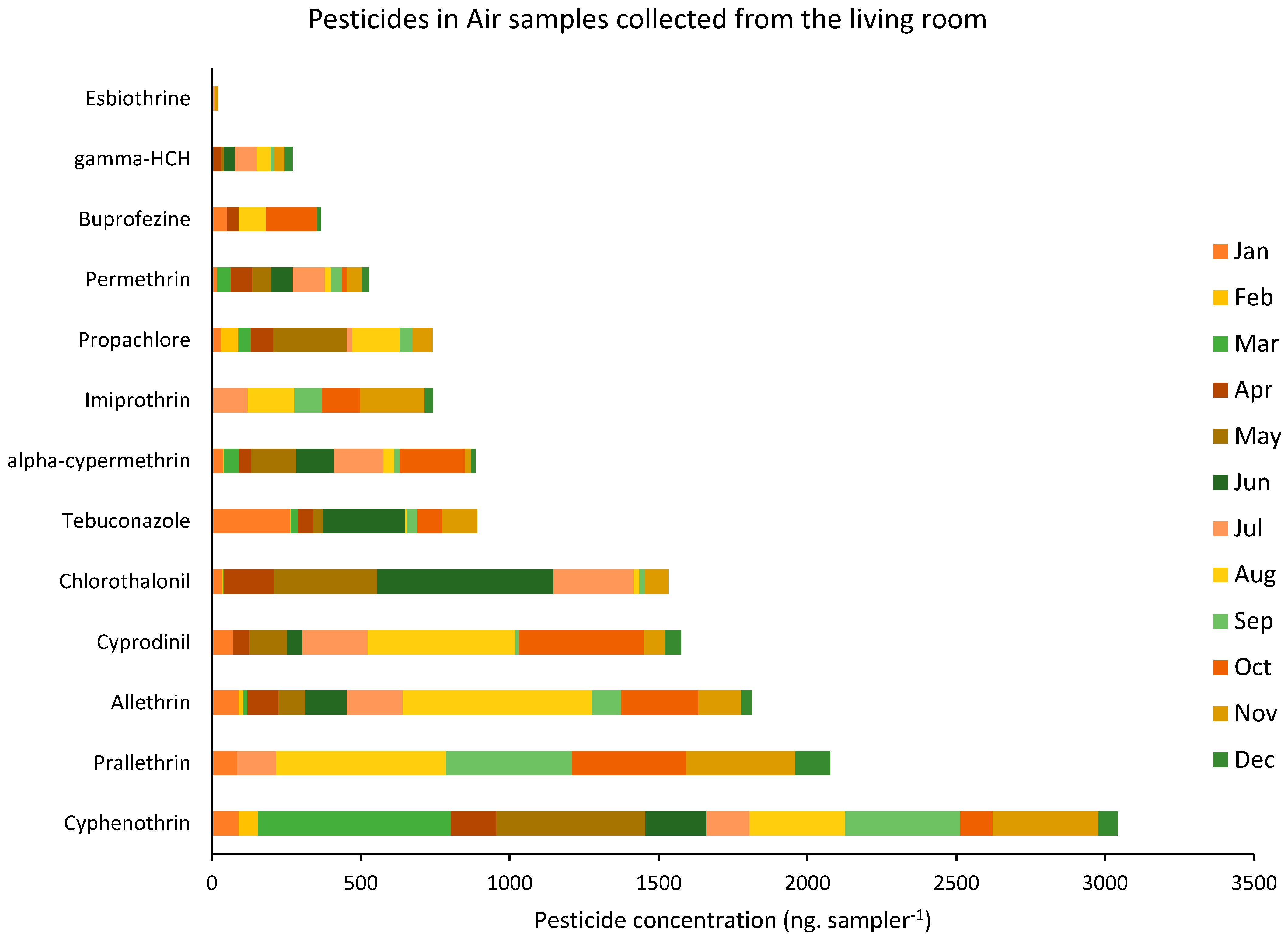
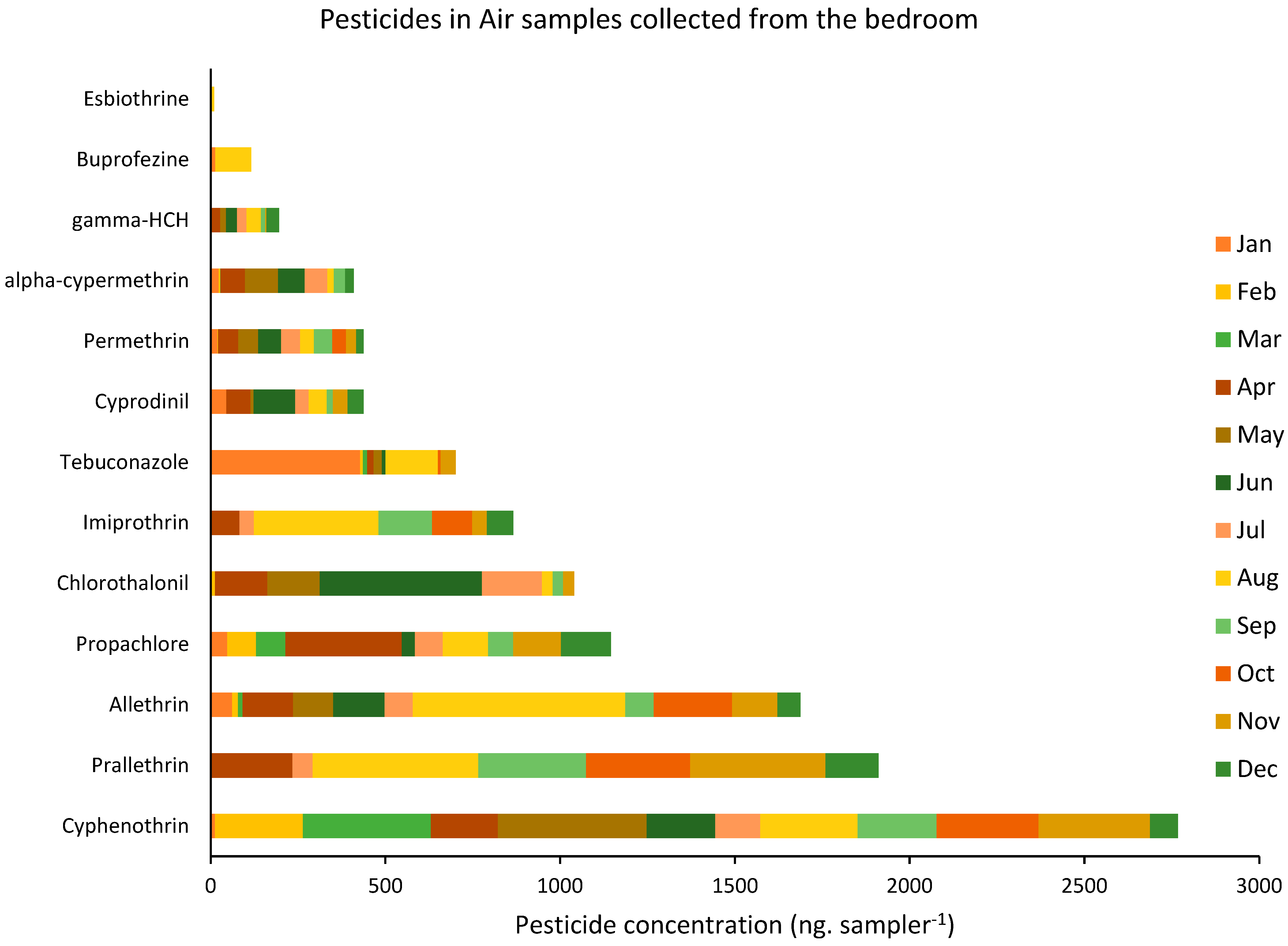
3.2. Pesticides in Air Samples
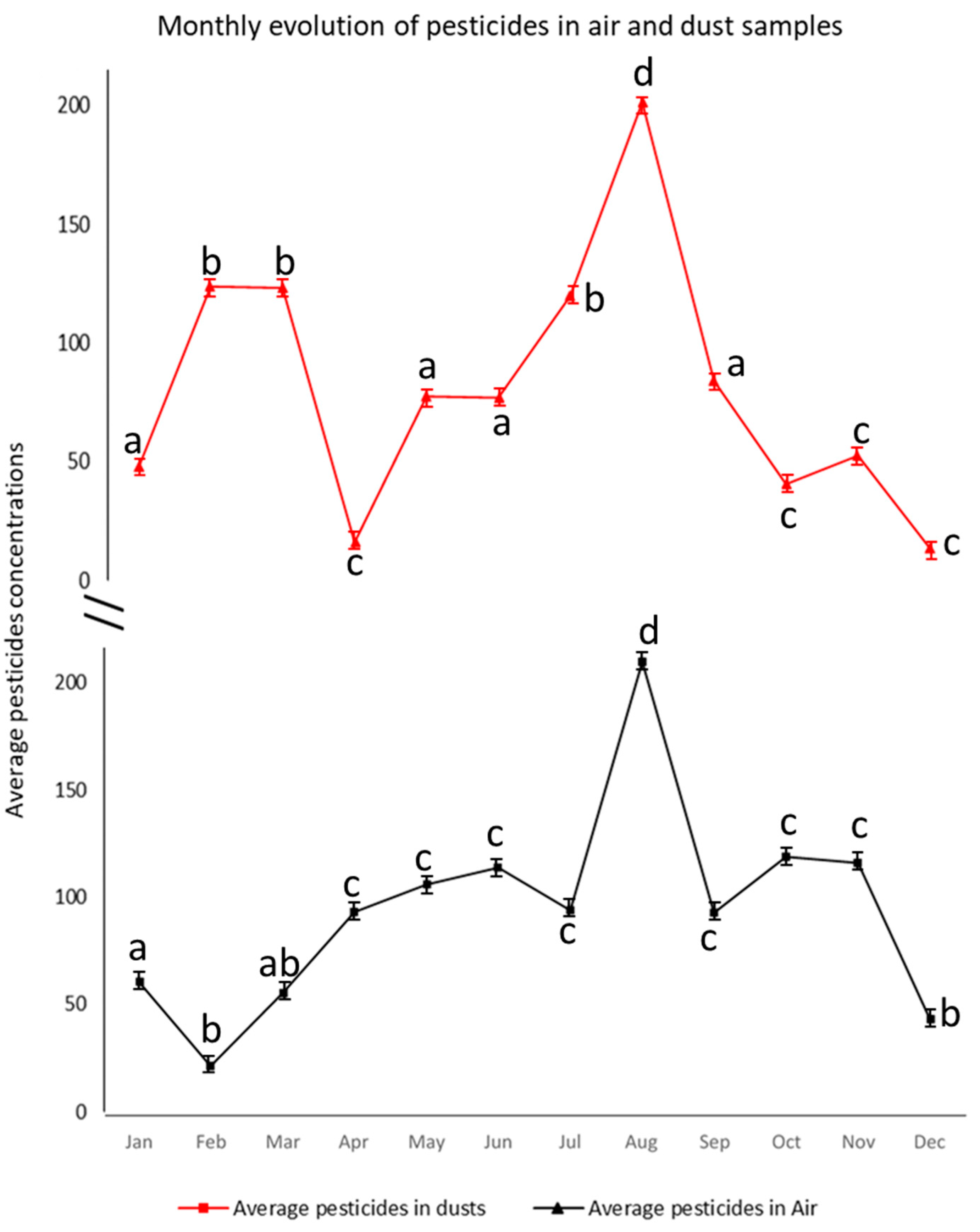
3.3. Pesticides in Dust and Air Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalimeri, K.K.; Bartzis, J.G.; Sakellaris, I.A.; de Oliveira Fernandes, E. Investigation of the PM2.5, NO2 and O3 I/O ratios for office and school microenvironments. Environ. Res. 2019, 179, 108791. [Google Scholar] [CrossRef]
- Alves, C.A.; Vicente, E.D.; Evtyugina, M.; Vicente, A.M.; Nunes, T.; Lucarelli, F.; Calzolai, G.; Nava, S.; Calvo, A.I.; Alegre, C.d.B.; et al. Indoor and outdoor air quality: A university cafeteria as a case study. Atmos. Pollut. Res. 2020, 11, 531–544. [Google Scholar] [CrossRef]
- Lucattini, L.; Poma, G.; Covaci, A.; de Boer, J.; Lamoree, M.H.; Leonards, P.E.G. A review of semi-volatile organic compounds (SVOCs) in the indoor environment: Occurrence in consumer products, indoor air and dust. Chemosphere 2018, 201, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-d.; Li, X.; Fan, L.; Li, L.; Wang, J.; Yang, W.-J.; Wang, L.; Yao, X.-Y.; Wang, X.-L. Indoor air quality in the primary school of China—Results from CIEHS 2018 study. Environ. Pollut. 2021, 291, 118094. [Google Scholar] [CrossRef]
- Wang, X.; Banks, A.P.W.; He, C.; Drage, D.S.; Gallen, C.L.; Li, Y.; Li, Q.; Thai, P.K.; Mueller, J.F. Polycyclic aromatic hydrocarbons, polychlorinated biphenyls and legacy and current pesticides in indoor environment in Australia—Occurrence, sources and exposure risks. Sci. Total Environ. 2019, 693, 133588. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Yusà, V.; Villoldo, E.; Corpas-Burgos, F.; Coscollà, C. Indoor air pesticide in dwellings of breastfeeding mothers of the Valencian Region (Spain): Levels, exposure and risk assessment. Atmos. Environ. 2021, 248, 118231. [Google Scholar] [CrossRef]
- He, C.; Wang, X.; Thai, P.; Baduel, C.; Gallen, C.; Banks, A.; Bainton, P.; English, K.; Mueller, J.F. Organophosphate and brominated flame retardants in Australian indoor environments: Levels, sources, and preliminary assessment of human exposure. Environ. Pollut. 2018, 235, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Mandin, C.; Blanchard, O.; Mercier, F.; Pelletier, M.; Le Bot, B.; Glorennec, P.; Ramalho, O. Semi-volatile organic compounds in French dwellings: An estimation of concentrations in the gas phase and particulate phase from settled dust. Sci. Total Environ. 2019, 650, 2742–2750. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Adamkiewicz, G.; Attfield, K.R.; Kapp, M.; Spengler, J.D.; Tao, L.; Xie, S.H. Household pesticide contamination from indoor pest control applications in urban low-income public housing dwellings: A community-based participatory research. Environ. Sci. Technol. 2013, 47, 2018–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). TrAC Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Tan, J.; Cheng, S.M.; Loganath, A.; Chong, Y.S.; Obbard, J.P. Selected organochlorine pesticide and polychlorinated biphenyl residues in house dust in Singapore. Chemosphere 2007, 68, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Chandra Yadav, I.; Devi, N.L.; Li, J.; Zhang, G. Polychlorinated biphenyls and organochlorines pesticides in indoor dust: An exploration of sources and health exposure risk in a rural area (Kopawa) of Nepal. Ecotoxicol. Environ. Saf. 2020, 195, 110376. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kim, H.-H.; Lee, J.-I.; Lee, J.-H.; Kang, H.; Lee, J.-Y. Indoor contamination from pesticides used for outdoor insect control. Sci. Total Environ. 2018, 625, 994–1002. [Google Scholar] [CrossRef]
- Garcia-Jares, C.; Regueiro, J.; Barro, R.; Dagnac, T.; Llompart, M. Analysis of industrial contaminants in indoor air. Part 2. Emergent contaminants and pesticides. J. Chromatogr. A 2009, 1216, 567–597. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 2833. [Google Scholar] [CrossRef]
- Butte, W. Sources and impacts of pesticides in indoor environments. In The Handbook of Environmental Chemistry; Springer: Berlin, Germany, 2004; pp. 89–116. [Google Scholar]
- Gallart-Mateu, D.; Armenta, S.; de la Guardia, M. Indoor and outdoor determination of pesticides in air by ion mobility spectrometry. Talanta 2016, 161, 632–639. [Google Scholar] [CrossRef]
- Weschler, C.J.; Nazaroff, W.W. Semivolatile organic compounds in indoor environments. Atmos. Environ. 2008, 42, 9018–9040. [Google Scholar] [CrossRef]
- Butte, W.; Heinzow, B. Pollutants in house dust as indicators of indoor contamination. Rev. Environ. Contam. Toxicol. 2002, 175, 1–46. [Google Scholar]
- Wania, F.; Shen, L.; Lei, Y.D.; Teixeira, C.; Muir, D.C. Development and calibration of a resin-based passive sampling system for monitoring persistent organic pollutants in the atmosphere. Environ. Sci. Technol. 2003, 37, 1352–1359. [Google Scholar] [CrossRef]
- Sonnette, A.; Delhomme, O.; Alleman, L.Y.; Coddeville, P.; Millet, M. A versatile method for the quantification of 100 SVOCs from various families: Application to indoor air, dust and bioaccessibility evaluation. Microchem. J. 2021, 169, 106574. [Google Scholar] [CrossRef]
- Al-Alam, J.; Lévy, M.; Ba, H.; Pham-Huu, C.; Millet, M. Passive air samplers based on ceramic adsorbent for monitoring of organochlorine pesticides, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in outdoor air. Environ. Technol. Innov. 2020, 20, 101094. [Google Scholar] [CrossRef]
- Schummer, C.; Tuduri, L.; Briand, O.; Appenzeller, B.M.; Millet, M. Application of XAD-2 resin-based passive samplers and SPME–GC–MS/MS analysis for the monitoring of spatial and temporal variations of atmospheric pesticides in Luxembourg. Environ. Pollut. 2012, 170, 88–94. [Google Scholar] [CrossRef]
- Lemley, A.; Hedge, A.; Obendorf, S.; Hong, S.; Kim, J.; Muss, T.; Varner, C. Selected pesticide residues in house dust from farmers’ homes in central New York state, USA. Bull. Environ. Contam. Toxicol. 2002, 69, 155–163. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Kadokami, K. Occurrence and exposure risk assessment of organic micropollutants in indoor dust from Malaysia. Chemosphere 2022, 287, 132340. [Google Scholar] [CrossRef]
- Raeppel, C.; Salquèbre, G.; Millet, M.; Appenzeller, B.M.R. Pesticide detection in air samples from contrasted houses and in their inhabitants’ hair. Sci. Total Environ. 2016, 544, 845–852. [Google Scholar] [CrossRef]
- Schummer, C.; Mothiron, E.; Appenzeller, B.M.; Rizet, A.-L.; Wennig, R.; Millet, M. Temporal variations of concentrations of currently used pesticides in the atmosphere of Strasbourg, France. Environ. Pollut. 2010, 158, 576–584. [Google Scholar] [CrossRef]
- Abbatt, J.P.; Wang, C. The atmospheric chemistry of indoor environments. Environ. Sci. Process. Impacts 2020, 22, 25–48. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, A.; Vijayalakshmi, A. Monitoring of allethrin, deltamethrin, esbiothrin, prallethrin and transfluthrin in air during the use of household mosquito repellents. J. Environ. Monit. 2001, 3, 191–193. [Google Scholar] [CrossRef]
- Egeghy, P.; Sheldon, L.; Fortmann, R.; Stout, D.; Tulve, N.; Cohel-Hubal, E.; Melnyk, L.; Morgan, M.; Jones, P.; Whitaker, D. Important Exposure Factors for Children: An Analysis of Laboratory and Observations on Data Characterizing Cumulative Exposure to Pesticides; National Exposure Research Laboratory Office of Research and Development: Research Triangle Park, NC, USA, 2007. [Google Scholar]
- Blanchard, O.; Glorennec, P.; Mercier, F.; Bonvallot, N.; Chevrier, C.; Ramalho, O.; Mandin, C.; Bot, B.L. Semivolatile organic compounds in indoor air and settled dust in 30 French dwellings. Environ. Sci. Technol. 2014, 48, 3959–3969. [Google Scholar] [CrossRef]
- Quirós-Alcalá, L.; Bradman, A.; Nishioka, M.; Harnly, M.E.; Hubbard, A.; McKone, T.E.; Ferber, J.; Eskenazi, B. Pesticides in house dust from urban and farmworker households in California: An observational measurement study. Environ. Health 2011, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Pfeil, R. Pesticide Residues: Pyrethroids. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 31–34. [Google Scholar]
- Glorennec, P.; Serrano, T.; Fravallo, M.; Warembourg, C.; Monfort, C.; Cordier, S.; Viel, J.-F.; Le Gléau, F.; Le Bot, B.; Chevrier, C. Determinants of children’s exposure to pyrethroid insecticides in western France. Environ. Int. 2017, 104, 76–82. [Google Scholar] [CrossRef]
- Roinestad, K.S.; Louis, J.B.; Rosen, J.D. Determination of pesticides in indoor air and dust. J. AOAC Int. 1993, 76, 1121–1126. [Google Scholar] [CrossRef]
- Rudel, R.A.; Camann, D.E.; Spengler, J.D.; Korn, L.R.; Brody, J.G. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003, 37, 4543–4553. [Google Scholar] [CrossRef]
- Msibi, S.S.; Chen, C.-Y.; Chang, C.-P.; Chen, C.-J.; Chiang, S.-Y.; Wu, K.-Y. Indoor Air Exposure to Multiple Agricultural Pesticides Potentially Posing the Highest Risk to Young Children. Aerosol Air Qual. Res. 2021, 21, 210062. [Google Scholar] [CrossRef]
- Whyatt, R.M.; Garfinkel, R.; Hoepner, L.A.; Holmes, D.; Borjas, M.; Williams, M.K.; Reyes, A.; Rauh, V.; Perera, F.P.; Camann, D.E. Within-and between-home variability in indoor-air insecticide levels during pregnancy among an inner-city cohort from New York City. Environ. Health Perspect. 2007, 115, 383–389. [Google Scholar] [CrossRef]
- Morgan, M.K. Children’s exposures to pyrethroid insecticides at home: A review of data collected in published exposure measurement studies conducted in the United States. Int. J. Environ. Res. Public Health 2012, 9, 2964–2985. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-K.; Lee, H.-J.; Song, E.; Kim, Y.; Kim, D.Y.; Lee, J.-H.; Yoo, H.J.; Oh, J.-E.; Kwon, J.-H. Exposure to permethrin used as a home insecticide: A case study comparing model predictions and excretion of metabolites. Environ. Int. 2021, 155, 106581. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Alam, J.; Sonnette, A.; Delhomme, O.; Alleman, L.Y.; Coddeville, P.; Millet, M. Pesticides in the Indoor Environment of Residential Houses: A Case Study in Strasbourg, France. Int. J. Environ. Res. Public Health 2022, 19, 14049. https://doi.org/10.3390/ijerph192114049
Al-Alam J, Sonnette A, Delhomme O, Alleman LY, Coddeville P, Millet M. Pesticides in the Indoor Environment of Residential Houses: A Case Study in Strasbourg, France. International Journal of Environmental Research and Public Health. 2022; 19(21):14049. https://doi.org/10.3390/ijerph192114049
Chicago/Turabian StyleAl-Alam, Josephine, Alexandre Sonnette, Olivier Delhomme, Laurent Y. Alleman, Patrice Coddeville, and Maurice Millet. 2022. "Pesticides in the Indoor Environment of Residential Houses: A Case Study in Strasbourg, France" International Journal of Environmental Research and Public Health 19, no. 21: 14049. https://doi.org/10.3390/ijerph192114049







