Mortality Risk Factors for Coronavirus Infection in Hospitalized Adults in Brazil: A Retrospective Cohort Study
Abstract
:1. Background
2. Material and Methods
2.1. Design
2.2. Context
2.3. Data Source
2.4. Population
2.5. Variables
2.5.1. Outcome
2.5.2. Exposure Variables
2.6. Statistical Analysis
2.7. Ethical Aspects
2.8. Patient and Public Involvement
2.9. Data Availability
3. Results
3.1. Population Characteristics
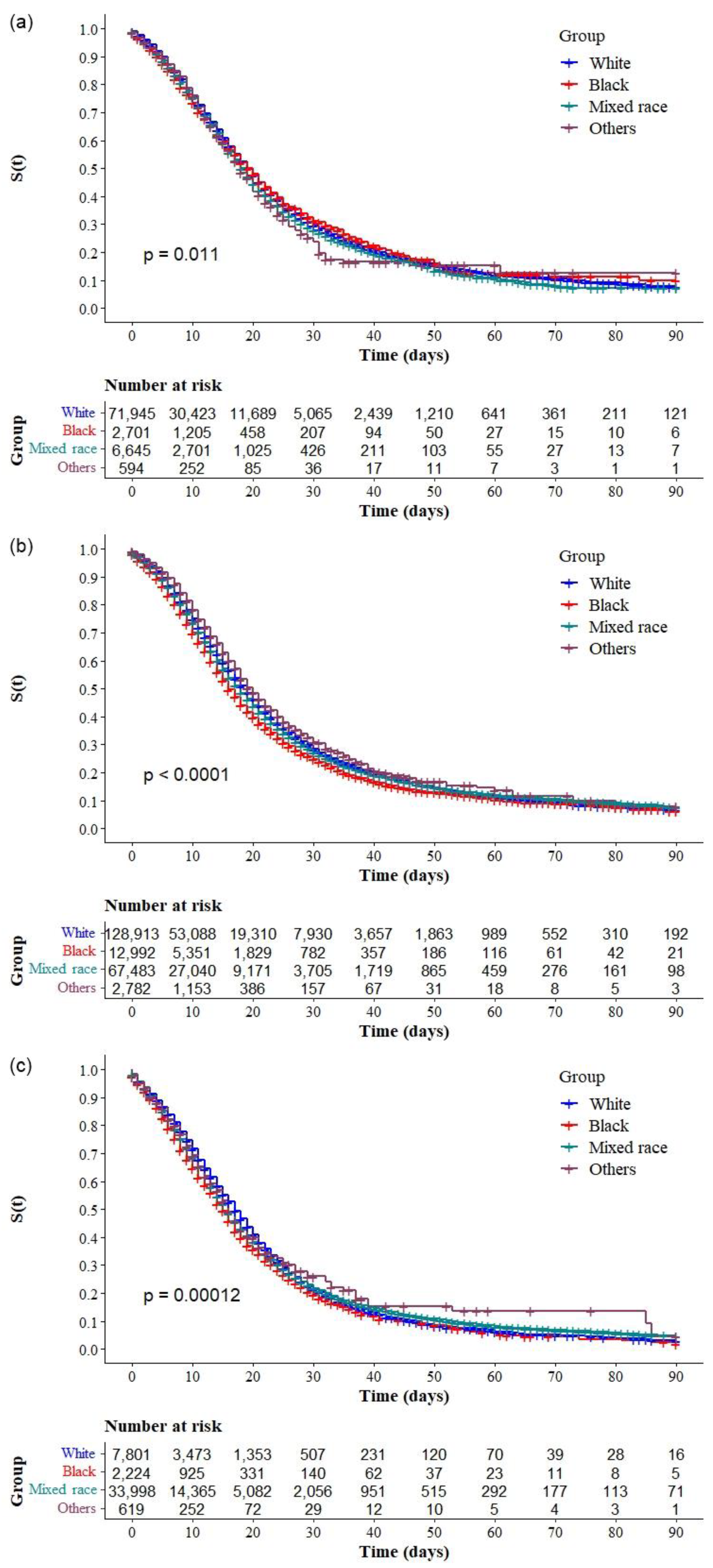
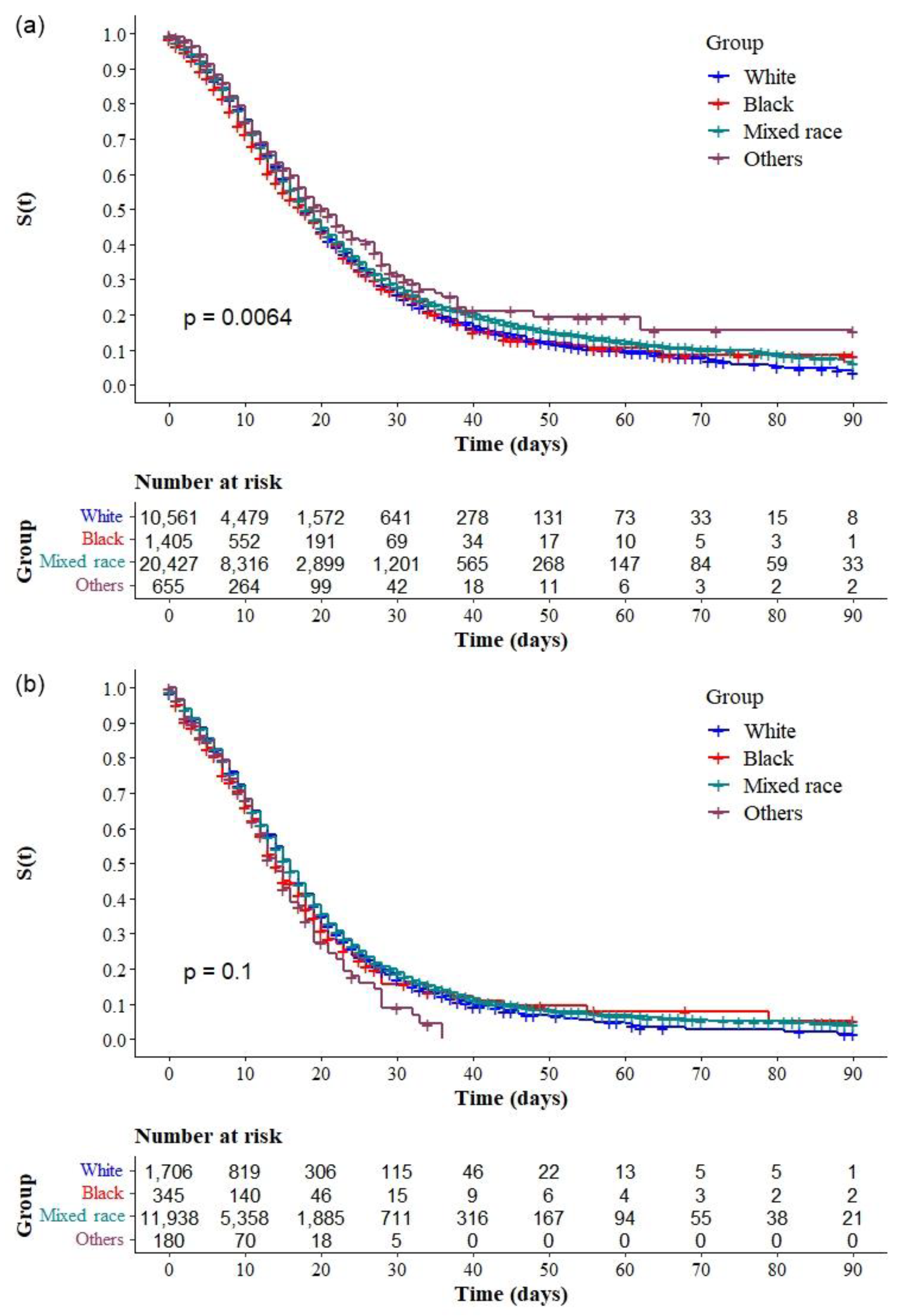
3.2. Risk Factors for COVID-19 Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baqui, P.; Bica, I.; Marra, V.; Ercole, A.; van der Schaar, M. Ethnic and Regional Variations in Hospital Mortality from COVID-19 in Brazil: A Cross-Sectional Observational Study. Lancet Glob. Health 2020, 8, e1018–e1026. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Aghae, M.; Agha, R. The Socio-Economic Implications of the Coronavirus Pandemic (COVID-19): A Review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 24 April 2021).
- Meo, S.A.; Abukhalaf, A.A.; Alomar, A.A.; AlMutairi, F.J.; Usmani, A.M.; Klonoff, D.C. Impact of Lockdown on COVID-19 Prevalence and Mortality during 2020 Pandemic: Observational Analysis of 27 Countries. Eur. J. Med. Res. 2020, 25, 56. [Google Scholar] [CrossRef] [PubMed]
- BRASIL Guia de Vigilância Epidemiológica Emergência de Saúde Pública de Importância Nacional Pela Doença Pelo Coronavírus 2019. Available online: https://www.conasems.org.br/wp-content/uploads/2021/03/Guia-de-vigilancia-epidemiologica-da-covid_19_15.03_2021.pdf (accessed on 4 May 2021).
- Alcantara, L.C.J.; Nogueira, E.; Shuab, G.; Tosta, S.; Fristch, H.; Pimentel, V.; Souza-Neto, J.A.; Coutinho, L.L.; Fukumasu, H.; Sampaio, S.C.; et al. SARS-CoV-2 Epidemic in Brazil: How the Displacement of Variants Has Driven Distinct Epidemic Waves. Virus Res. 2022, 315, 198785. [Google Scholar] [CrossRef] [PubMed]
- Khateeb, J.; Li, Y.; Zhang, H. Emerging SARS-CoV-2 Variants of Concern and Potential Intervention Approaches. Crit. Care 2021, 25, 244. [Google Scholar] [CrossRef]
- Gramacho, W.; Turgeon, M.; Kennedy, J.; Stabile, M.; Mundim, P.S. Political Preferences, Knowledge, and Misinformation About COVID-19: The Case of Brazil. Front. Polit. Sci. 2021, 3, 1–13. [Google Scholar] [CrossRef]
- Da Fonseca, E.M.; Shadlen, K.C.; Bastos, F.I. The Politics of COVID-19 Vaccination in Middle-Income Countries: Lessons from Brazil. Soc. Sci. Med. 2021, 281, 114093. [Google Scholar] [CrossRef]
- De Andrade, C.L.T.; de Aguiar Pereira, C.C.; Martins, M.; Lima, S.M.L.; Portela, M.C. COVID-19 Hospitalizations in Brazil’s Unified Health System (SUS). PLoS ONE 2020, 15, e0243126. [Google Scholar] [CrossRef]
- De Menezes Soares, R.C.; Mattos, L.R.; Raposo, L.M. Risk Factors for Hospitalization and Mortality Due to COVID-19 in Espírito Santo State, Brazil. Am. J. Trop. Med. Hyg. 2020, 103, 1184–1190. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Chinn, J.; de Ferrante, M.; Kirby, K.A.; Hohmann, S.F.; Amin, A. Male Gender Is a Predictor of Higher Mortality in Hospitalized Adults with COVID-19. PLoS ONE 2021, 16, e0254066. [Google Scholar] [CrossRef]
- Ge, E.; Li, Y.; Wu, S.; Candido, E.; Wei, X. Association of Pre-Existing Comorbidities with Mortality and Disease Severity among 167,500 Individuals with COVID-19 in Canada: A Population-Based Cohort Study. PLoS ONE 2021, 16, e0258154. [Google Scholar] [CrossRef] [PubMed]
- Concepción-Zavaleta, M.J.; Coronado-Arroyo, J.C.; Zavaleta-Gutiérrez, F.E.; Concepción-Urteaga, L.A. Does Level of Education Influence Mortality of SARS-CoV-2 in a Developing Country? Int. J. Epidemiol. 2020, 49, 2091–2093. [Google Scholar] [CrossRef] [PubMed]
- Reiner, R.C.; Wiens, K.E.; Deshpande, A.; Baumann, M.M.; Lindstedt, P.A.; Blacker, B.F.; Troeger, C.E.; Earl, L.; Munro, S.B.; Abate, D.; et al. Department of Error: Mapping Geographical Inequalities in Childhood Diarrhoeal Morbidity and Mortality in Low-Income and Middle-Income Countries, 2000–2017: Analysis for the Global Burden of Disease Study 2017 (The Lancet (2020) 395(10239) (1779–1801), S01. Lancet 2020, 395, 1762. [Google Scholar] [CrossRef]
- Menon, T.; Gandhi, S.A.Q.; Tariq, W.; Sharma, R.; Sardar, S.; Arshad, A.M.; Adhikari, R.; Ata, F.; Kataria, S.; Singh, R. Impact of Chronic Kidney Disease on Severity and Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Cureus 2021, 13, e14279. [Google Scholar] [CrossRef]
- Ng, W.H.; Tipih, T.; Makoah, N.A.; Vermeulen, J.G.; Goedhals, D.; Sempa, J.B.; Burt, F.J.; Taylor, A.; Mahalingam, S. Comorbidities in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis. MBio 2021, 12, e03647-20. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- De Souza, F.S.H.; Hojo-Souza, N.S.; de Oliveira Batista, B.D.; da Silva, C.M.; Guidoni, D.L. On the Analysis of Mortality Risk Factors for Hospitalized COVID-19 Patients: A Data-Driven Study Using the Major Brazilian Database. PLoS ONE 2021, 16, e0248580. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística IBGE Cidades. Available online: https://cidades.ibge.gov.br/ (accessed on 6 June 2022).
- United Nations Development Programme Human Development Indicators. Available online: http://hdr.undp.org/en/countries/profiles/BRA (accessed on 18 October 2021).
- Ministério do Planejamento Orçamento e Gestão; Instituto Brasileiro de Geografia e Estatística; Diretoria de Pesquisas. Coordenação de População e Indicadores Sociais Características Étnico-Raciais Da População: Um Estudo Das Categorias de Classificação de Cor Ou Raça 2008. Available online: http://biblioteca.ibge.gov.br/visualizacao/livros/liv49891.pdf (accessed on 20 June 2022).
- Johnston, M.C.; Crilly, M.; Black, C.; Prescott, G.J.; Mercer, S.W. Defining and Measuring Multimorbidity: A Systematic Review of Systematic Reviews. Eur. J. Public Health 2019, 29, 182–189. [Google Scholar] [CrossRef]
- Woolcott, O.O.; Castilla-Bancayán, J.P. The Effect of Age on the Association between Diabetes and Mortality in Adult Patients with COVID-19 in Mexico. Sci. Rep. 2021, 11, 8386. [Google Scholar] [CrossRef]
- Rosenthal, N.; Cao, Z.; Gundrum, J.; Sianis, J.; Safo, S. Risk Factors Associated with In-Hospital Mortality in a US National Sample of Patients with COVID-19. JAMA Netw. Open 2020, 3, e2029058. [Google Scholar] [CrossRef]
- Antos, A.; Kwong, M.L.; Balmorez, T.; Villanueva, A.; Murakami, S. Unusually High Risks of COVID-19 Mortality with Age-Related Comorbidities: An Adjusted Meta-Analysis Method to Improve the Risk Assessment of Mortality Using the Comorbid Mortality Data. Infect. Dis. Rep. 2021, 13, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Dessie, Z.G.; Zewotir, T. Mortality-Related Risk Factors of COVID-19: A Systematic Review and Meta-Analysis of 42 Studies and 423,117 Patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, L.A.; Noonan, J.; Wang, X.; Peter, K. Higher Mortality of COVID-19 in Males: Sex Differences in Immune Response and Cardiovascular Comorbidities. Cardiovasc. Res. 2020, 116, 2197–2206. [Google Scholar] [CrossRef]
- Bwire, G.M. Coronavirus: Why Men Are More Vulnerable to Covid-19 Than Women? SN Compr. Clin. Med. 2020, 2, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Pereira, R.H.M.; Prete, C.A.; Zarebski, A.E.; Emanuel, L.; Alves, P.J.H.; Peixoto, P.S.; Braga, C.K.V.; de Souza Santos, A.A.; de Souza, W.M.; et al. Higher Risk of Death from COVID-19 in Low-Income and Non-White Populations of São Paulo, Brazil. BMJ Glob. Health 2021, 6, e004959. [Google Scholar] [CrossRef] [PubMed]
- Asch, D.A.; Islam, M.N.; Sheils, N.E.; Chen, Y.; Doshi, J.A.; Buresh, J.; Werner, R.M. Patient and Hospital Factors Associated with Differences in Mortality Rates among Black and White US Medicare Beneficiaries Hospitalized with COVID-19 Infection. Annu. Rev. Plant Biol. 2021, 4, e2112842. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Bastos, L.S.L.; Gelli, J.G.M.; Marchesi, J.F.; Baião, F.; Hamacher, S.; Bozza, F.A. Characterisation of the First 250,000 Hospital Admissions for COVID-19 in Brazil: A Retrospective Analysis of Nationwide Data. Lancet Respir. Med. 2021, 9, 407–418. [Google Scholar] [CrossRef]
- Sabino, E.C.; Buss, L.F.; Carvalho, M.P.S.; Prete, C.A.; Crispim, M.A.E.; Fraiji, N.A.; Pereira, R.H.M.; Parag, K.V.; da Silva Peixoto, P.; Kraemer, M.U.G.; et al. Resurgence of COVID-19 in Manaus, Brazil, despite High Seroprevalence. Lancet 2021, 397, 452–455. [Google Scholar] [CrossRef]
- Gregg, E.W.; Sophiea, M.K.; Weldegiorgis, M. Diabetes and Covid-19: Population Impact 18 Months into the Pandemic. Diabetes Care 2021, 44, 1916–1923. [Google Scholar] [CrossRef]
- Galiero, R.; Pafundi, P.C.; Simeon, V.; Rinaldi, L.; Perrella, A.; Vetrano, E.; Caturano, A.; Alfano, M.; Beccia, D.; Nevola, R.; et al. Impact of Chronic Liver Disease upon Admission on COVID-19 in-Hospital Mortality: Findings from COVOCA Study. PLoS ONE 2020, 15, e0243700. [Google Scholar] [CrossRef]
- Do Prado, M.F.; De Paula Antunes, B.B.; Dos Santos Lourenço Bastos, L.; Peres, I.T.; De Araújo Batista Da Silva, A.; Dantas, L.F.; Baião, F.A.; Maçaira, P.; Hamacher, S.; Bozza, F.A. Analysis of COVID-19 under-Reporting in Brazil. Rev. Bras. Ter. Intensiv. 2020, 32, 224–228. [Google Scholar] [CrossRef] [PubMed]




| Variables | |
|---|---|
| Age (years), median (IQR)—(n = 468,266) * | 53 (41–75) |
| Age group (years), n (%)—(n = 468,266) * | |
| 18–29 | 17,741 (3.8) |
| 30–39 | 53,527 (11.4) |
| 40–49 | 84,678 (18.1) |
| 50–59 | 105,905 (22.6) |
| 60–69 | 95,361 (20.4) |
| ≥70 | 111,014 (23.7) |
| Sex, n (%)—(n = 468,184) * | |
| Male | 261,227 (55.8) |
| Female | 206,957 (44.2) |
| Race, n (%)—(n = 385,914) * | |
| White | 220,926 (57.2) |
| Mixed race | 140,491 (36.4) |
| Black | 19,667 (5.1) |
| Others (Asian or Indigenous) | 4830 (1.3) |
| Education, n (%) (n = 166,220) * | |
| Illiterate | 7660 (4.6) |
| Elementary School (ES-1) | 41,077 (24.7) |
| Elementary School (ES-2) | 32,379 (19.5) |
| High School | 58,675 (35.3) |
| Higher Education | 26,429 (15.9) |
| Region country, n (%) (n = 468,226) * | |
| South | 93,676 (20.0) |
| Southeast | 255,258 (54.5) |
| Central-West | 43,856 (9.4) |
| Northeast | 59,981 (12.8) |
| North | 15,455 (3.3) |
| Variables | |
|---|---|
| Comorbidities, n (%) | |
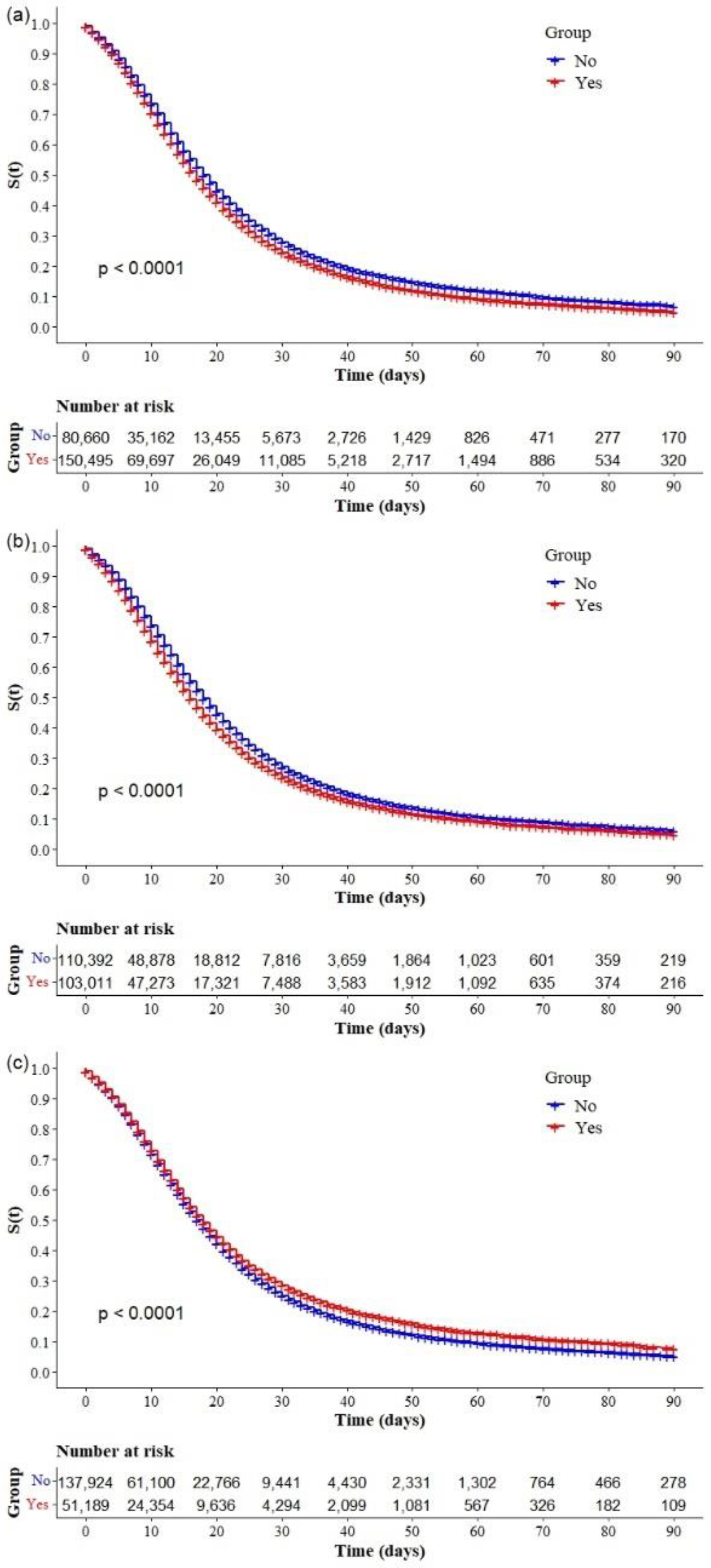
| Cardiopathy (231,155) * | 150,495 (65.1) |
| Diabetes mellitus (n = 213,403) * | 103,011 (48.3) |
| Obesity (n = 189,113) * | 51,189 (27.1) |
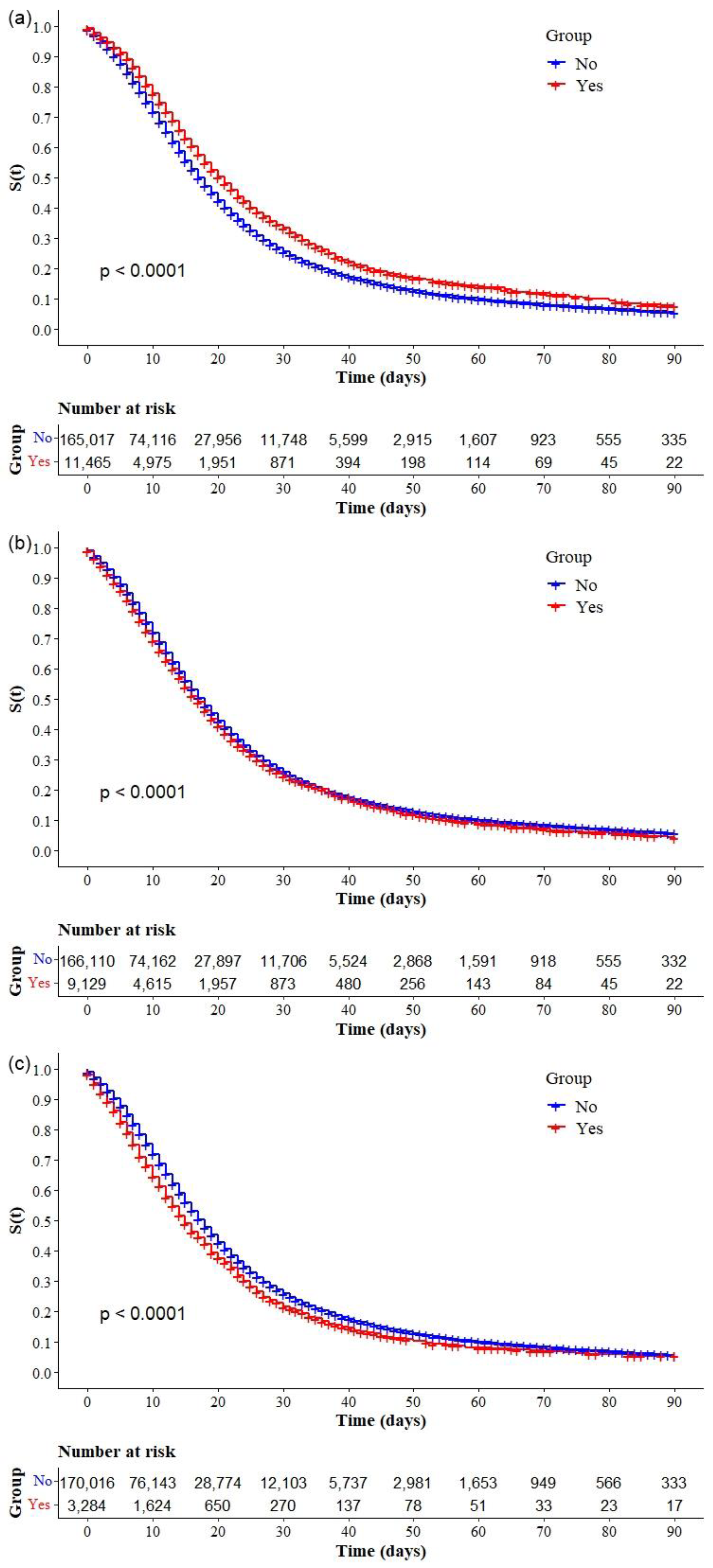
| Chronic kidney disease (n = 176,998) * | 13,581 (7.7) |
| Chronic neurological disease (n = 176,881) * | 13,046 (7.4) |
| Lung disease (n = 177,147) * | 12,480 (7.0) |
| Asthma (n = 176,482) * | 11,465 (6.5) |
| Immunodepression (n = 175,239) * | 9129 (5.2) |
| Chronic liver disease (n = 173,330) * | 3284 (1.9) |
| Chronic hematological disease (n = 173,647) * | 2561 (1.5) |
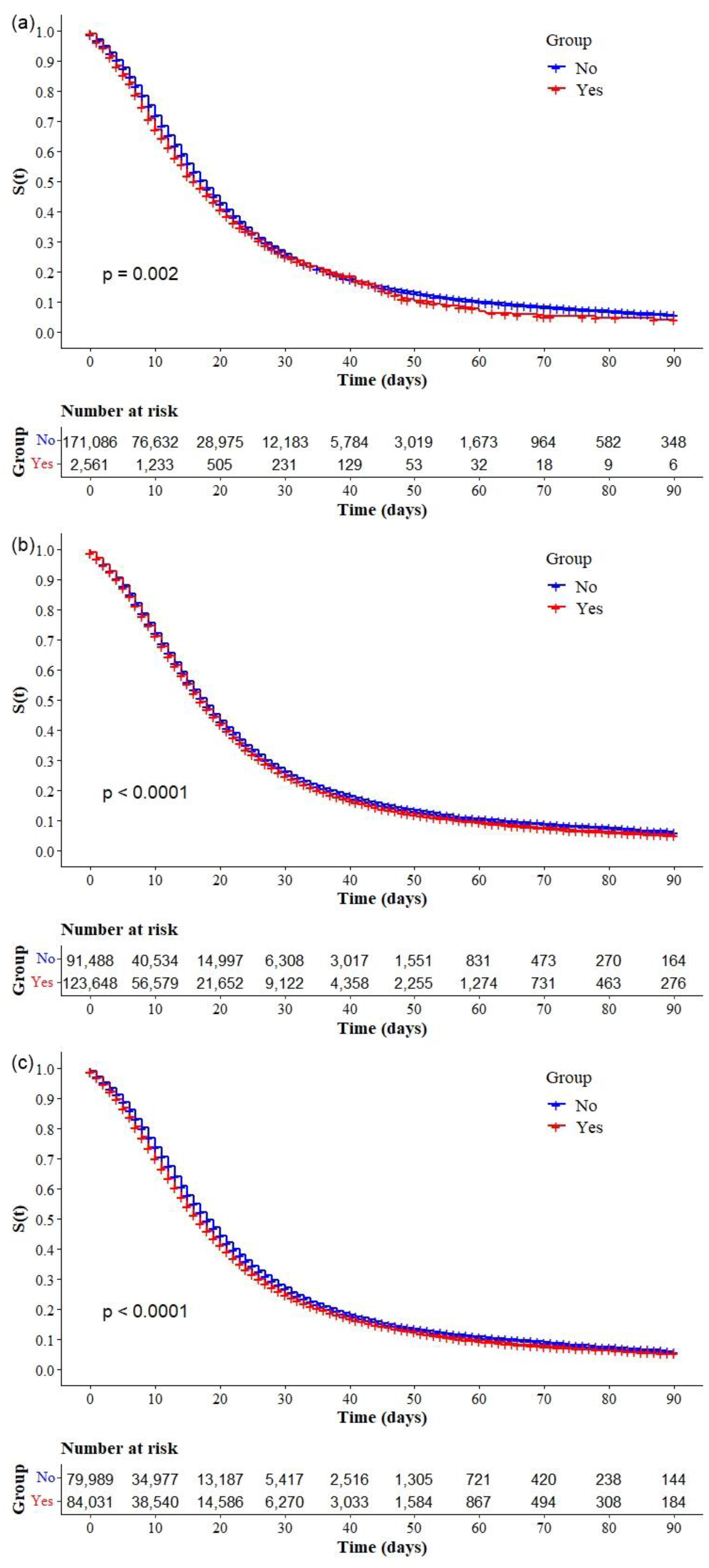
| Other (n = 215,136) * | 123,648 (57.5) |
| Multimorbidity, n (%) (n = 164,020) * | 84,031 (51.2) |
| Health assistance, n (%) | |
| UTI admission, (n = 432,133) * | |
| No | 257,539 (59.6) |
| Yes | 174,594 (40.4) |
| Ventilatory support, (n = 422,061) * | |
| No | 47,834 (13.7) |
| Yes, not invasive | 259,547 (61.5) |
| Yes, invasive | 104,680 (24.8) |
| Signs or symptoms, n (%) | |
| Fever (n = 389,388) * | 258,970 (66.5) |
| Cough (n = 407,117) * | 321,678 (79.0) |
| Sore throat (326,378) * | 77,772 (23.8) |
| Dyspnea (n = 418,016) * | 348,344 (83.3) |
| Respiratory distress (382,136) * | 269,783 (70.6) |
| Oxygen < 95% saturation (n = 406,401) * | 330,397 (81.3) |
| Diarrhea (n = 323,410) * | 62,319 (19.3) |
| Vomit (n = 315,985) * | 36,705 (11.6) |
| Abdominal pain (n = 309,346) * | 26,393 (8.5) |
| Fatigue (n = 332,710) * | 131,105 (39.4) |
| Alterations in smell (n = 313,009) * | 45,969 (14.6) |
| Alterations in taste (n = 313,712) * | 46,859 (14.9) |
| Variables | aHR | 95% CI | β | p-Value * |
|---|---|---|---|---|
| Age group (years) | ||||
| 18–29 | 1.00 | |||
| 30–39 | 1.07 | 0.96–1.19 | 0.053 | 0.206 |
| 40–49 | 1.13 | 1.02–1.25 | 0.040 | 0.019 |
| 50–59 | 1.20 | 1.09–1.33 | 0.049 | <0.001 |
| 60–69 | 1.43 | 1.30–1.57 | 0.049 | <0.001 |
| ≥70 | 2.00 | 1.82–2.20 | 0.049 | <0.001 |
| Sex | ||||
| Male | 1.00 | |||
| Female | 0.94 | 0.93–0.97 | 0.011 | <0.001 |
| Race | ||||
| White | 1.00 | |||
| Mixed race | 1.07 | 1.02–1.12 | 0.023 | 0.005 |
| Black | 1.06 | 1.03–1.10 | 0.015 | <0.001 |
| Others (Asian or Indigenous) | 0.96 | 0.85–1.09 | 0.065 | 0.548 |
| Education | ||||
| Illiterate | 1.00 | |||
| Elementary School (ES-1) | 0.94 | 0.84–0.98 | 0.024 | 0.009 |
| Elementary School (ES-2) | 0.83 | 0.79–0.88 | 0.025 | <0.001 |
| High School | 0.77 | 0.73–0.80 | 0.025 | <0.001 |
| Higher Education | 0.64 | 0.60–0.68 | 0.028 | <0.001 |
| Region country | ||||
| South | 1.00 | |||
| Southeast | 1.17 | 1.14–1.20 | 0.014 | <0.001 |
| Central-West | 1.28 | 1.21–1.34 | 0.025 | <0.001 |
| Northeast | 1.05 | 1.00–1.10 | 0.024 | 0.026 |
| North | 1.37 | 1.29–1.46 | 0.031 | <0.001 |
| Comorbidities | ||||
| Diabetes mellitus | ||||
| No | 1.00 | |||
| Yes | 1.08 | 1.06–1.10 | 0.011 | <0.001 |
| Obesity | ||||
| No | 1.00 | |||
| Yes | 1.04 | 1.00–1.07 | 0.015 | 0.018 |
| Chronic kidney disease | ||||
| No | 1.00 | |||
| Yes | 1.14 | 1.09–1.19 | 0.022 | <0.001 |
| Chronic neurological disease | ||||
| No | 1.00 | |||
| Yes | 1.18 | 1.13–1.24 | 0.023 | <0.001 |
| Pneumopathy | ||||
| No | 1.00 | |||
| Yes | 1.06 | 1.01–1.11 | 0.024 | 0.012 |
| Asthma | ||||
| No | 1.00 | |||
| Yes | 0.90 | 0.84–0.95 | 0.030 | <0.001 |
| Immunodepression | ||||
| No | 1.00 | |||
| Yes | 1.11 | 1.06–1.18 | 0.027 | <0.001 |
| Chronic liver disease | ||||
| No | 1.00 | |||
| Yes | 1.22 | 1.12–1.33 | 0.043 | <0.001 |
| Health assistance | ||||
| Ventilatory support | ||||
| No | 1.00 | |||
| Yes, not invasive | 1.32 | 1.26–1.39 | 0.026 | <0.001 |
| Yes, invasive | 2.20 | 2.08–2.32 | 0.028 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, R.A.; Pinheiro, R.S.; Paula, H.d.S.C.d.; Araújo, L.A.d.; Gonçalves, I.A.d.J.; Pedroso, C.F.; Guilarde, A.O.; Oliveira, G.A.d.; Batista, K.d.A. Mortality Risk Factors for Coronavirus Infection in Hospitalized Adults in Brazil: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 14074. https://doi.org/10.3390/ijerph192114074
Guimarães RA, Pinheiro RS, Paula HdSCd, Araújo LAd, Gonçalves IAdJ, Pedroso CF, Guilarde AO, Oliveira GAd, Batista KdA. Mortality Risk Factors for Coronavirus Infection in Hospitalized Adults in Brazil: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(21):14074. https://doi.org/10.3390/ijerph192114074
Chicago/Turabian StyleGuimarães, Rafael Alves, Raquel Silva Pinheiro, Hellen da Silva Cintra de Paula, Lyriane Apolinário de Araújo, Ingrid Aline de Jesus Gonçalves, Charlise Fortunato Pedroso, Adriana Oliveira Guilarde, Geraldo Andrade de Oliveira, and Karla de Aleluia Batista. 2022. "Mortality Risk Factors for Coronavirus Infection in Hospitalized Adults in Brazil: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 19, no. 21: 14074. https://doi.org/10.3390/ijerph192114074





