Improvement of In Vivo Fluorescence Tools for Fast Monitoring of Freshwater Phytoplankton and Potentially Harmful Cyanobacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Setup of Monoalgal Cultures
2.2. Sample Preparation for Intercalibration Procedures
2.3. Determination of Chl-a Using In Vivo Fluorescence
2.4. Determination of Chl-a Using Extraction Methods
2.4.1. Solvent 1: 90% Acetone
2.4.2. Solvent 2: 90% Ethanol
2.4.3. Solvent 3: Dimethyl Sulfoxide
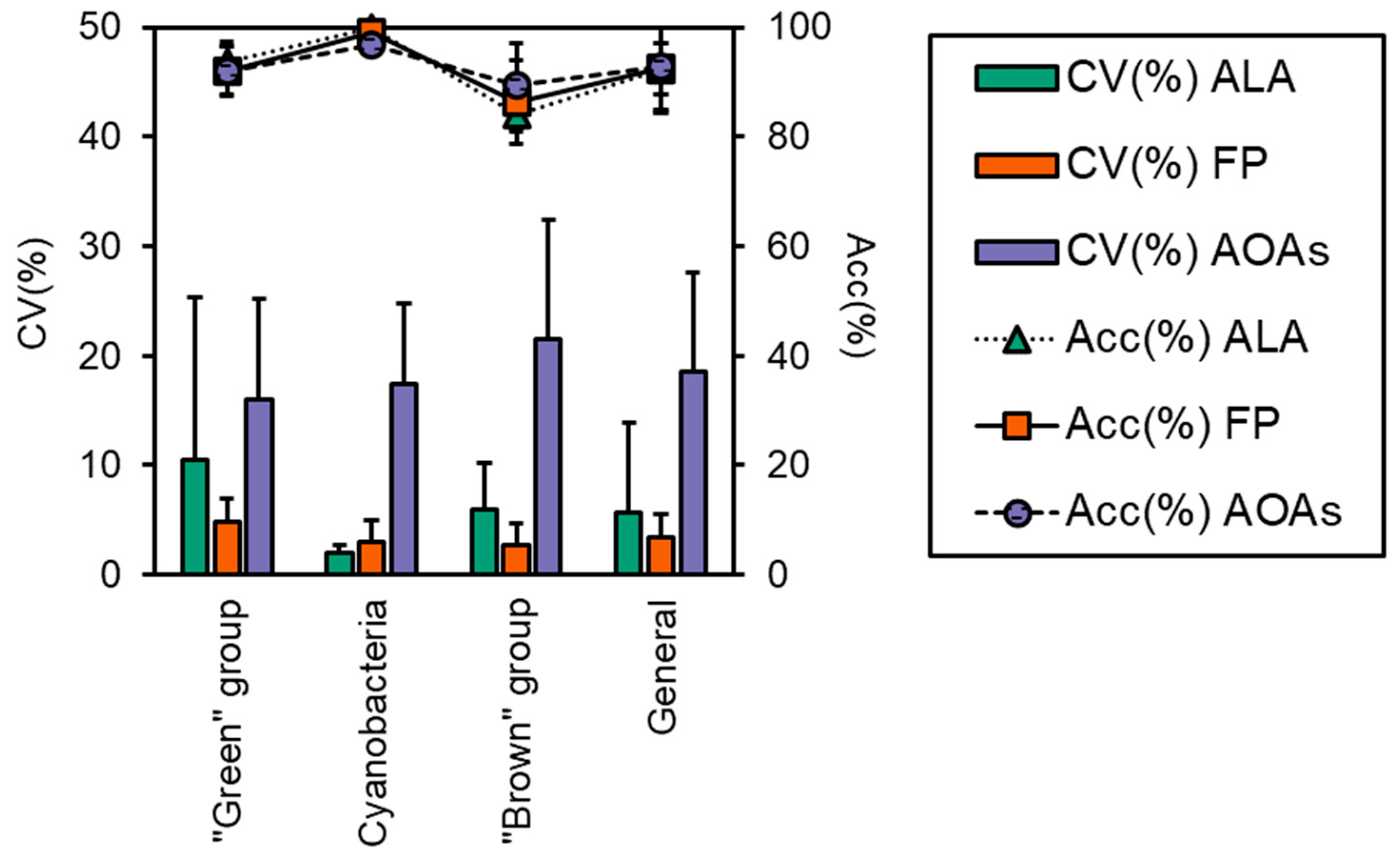
2.5. Performance Evaluation of the Probes
2.6. Application of Probes to a Field Freshwater Sample
2.7. Data Analysis
3. Results
3.1. Evaluation of Different Solvents for Chl-a Extraction
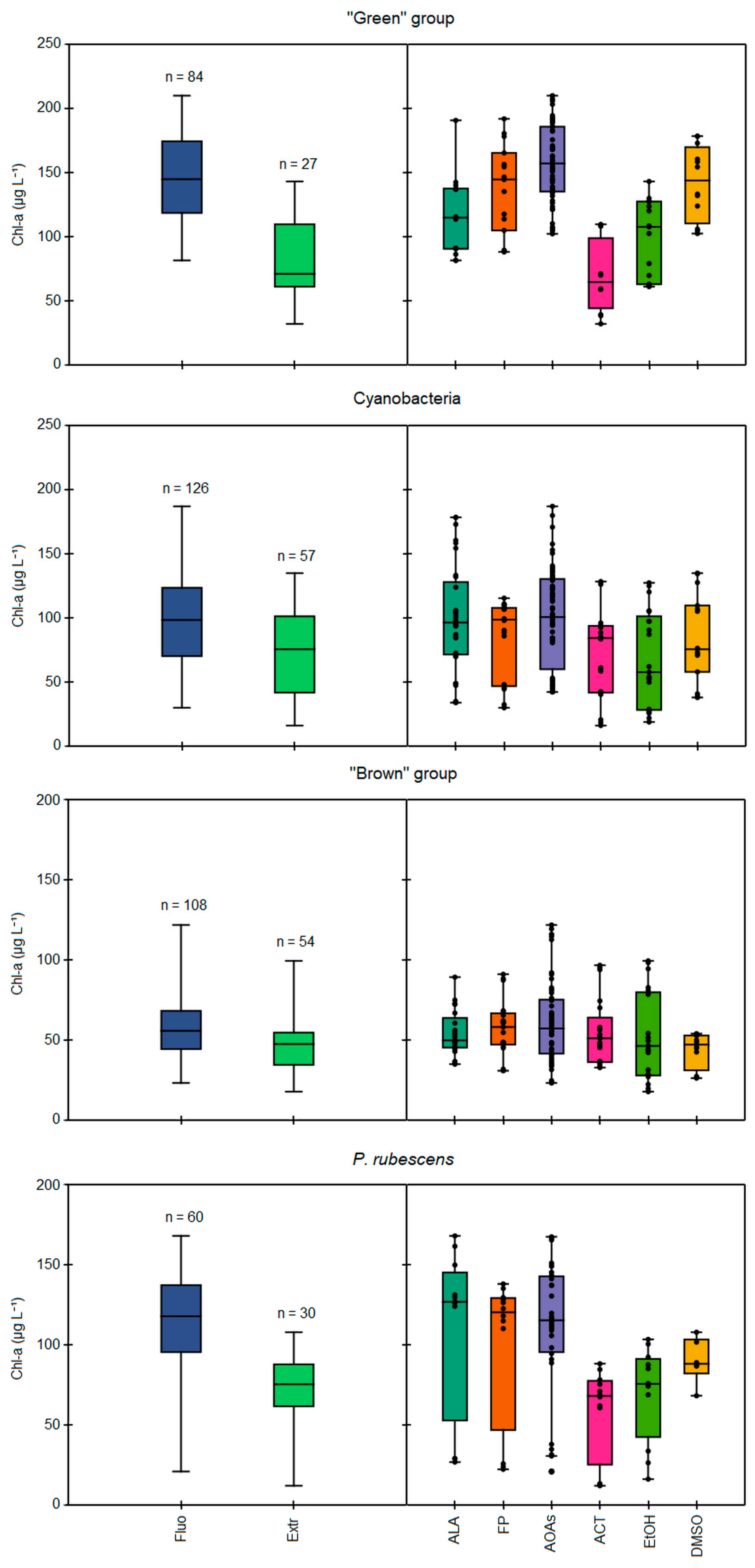
3.2. Comparison between In Vivo Fluorescence and Extraction Approaches
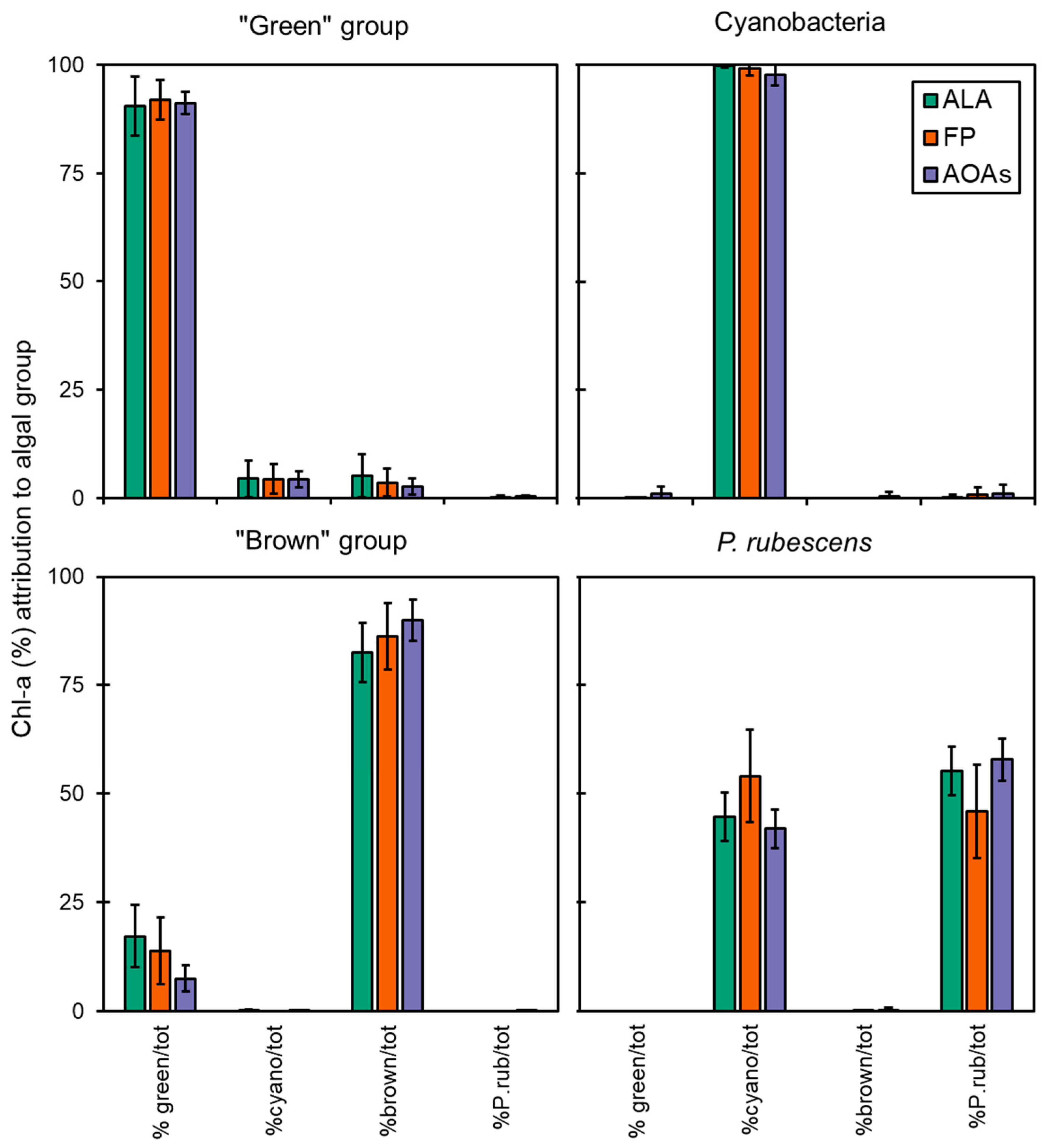
3.3. Algal Group Assignment and Performance of Spectrofluorometers
Specific Detection of P. rubescens by Probes
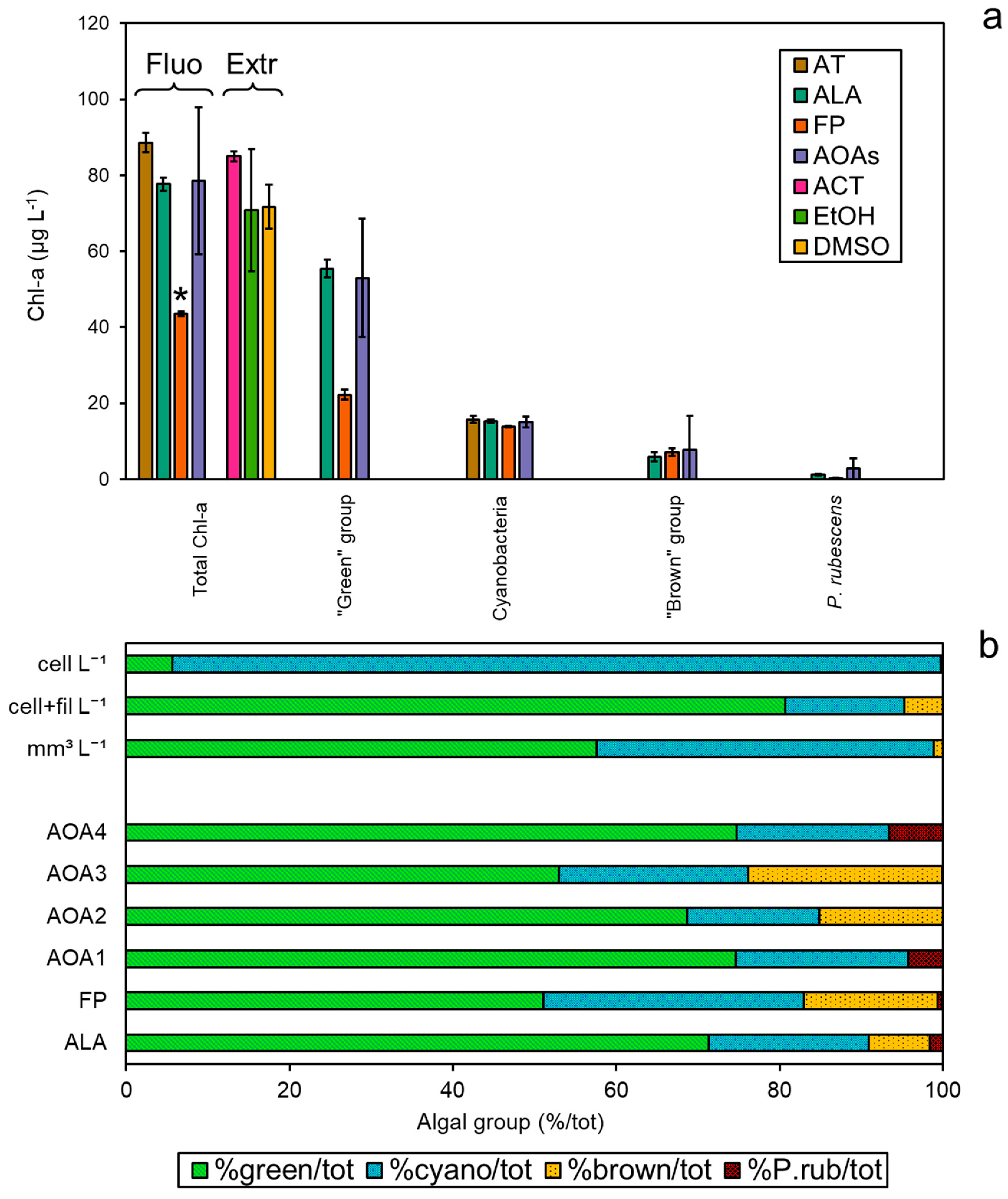
3.4. Comparison among Methodologies Using Freshwater Sample
4. Discussion
5. Conclusions
- Discrepancies in Chl-a determination among the two approaches were observed; however, the use of strong solvents, i.e., dimethyl sulfoxide, can improve pigment extraction, and the choice of probe model can lead to more accurate results.
- The correct algal group assignment by the spectrofluorometers was achieved, especially for Cyanobacteria, suggesting their use as reliable supportive tools in drinking water monitoring.
- The misattributions observed were low and consistent; thus, the recalibration of the probes with fresh cultures of local algal and cyanobacterial strains should be encouraged to optimize their detection.
- The intercalibration approach applied here was revealed to be useful for improving the use and performance of in vivo fluorescence tools for the monitoring of freshwater phytoplankton and Cyanobacteria, and to gain a better understanding of their correct functioning by non-trained staff, ultimately ensuring an improvement of drinking water management aimed at preventing environmental and public health issues related to the presence of harmful cyanobacteria.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Humpage, A.; Cunliffe, D. Exposure to Cyanotoxins. Understanding It and Short-Term Interventions to Prevent It. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA; on behalf of the World Health Organization: Geneva, Switzerland, 2021; pp. 295–332. [Google Scholar]
- Jochimsen, E.M.; Carmichael, W.W.; An, J.; Cardo, D.M.; Cookson, S.T.; Holmes, C.E.M.; Antunes, M.B.; de Melo Filho, D.A.; Lyra, T.M.; Barreto, V.S.T.; et al. Liver Failure and Death after Exposure to Microcystins at a Hemodialysis Center in Brazil. N. Engl. J. Med. 1998, 338, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Vuorio, K.; Lepistö, L.; Holopainen, A.L. Intercalibrations of Freshwater Phytoplankton Analyses. Boreal Environ. Res. 2007, 12, 561–569. [Google Scholar]
- Gregor, J.; Maršálek, B. Freshwater Phytoplankton Quantification by Chlorophyll a: A Comparative Study of in Vitro, in Vivo and in Situ Methods. Water Res. 2004, 38, 517–522. [Google Scholar] [CrossRef]
- Hawkins, P.R.; Holliday, J.; Kathuria, A.; Bowling, L. Change in Cyanobacterial Biovolume Due to Preservation by Lugol’s Iodine. Harmful Algae 2005, 4, 1033–1043. [Google Scholar] [CrossRef]
- Izydorczyk, K.; Carpentier, C.; Mrówczyński, J.; Wagenvoort, A.; Jurczak, T.; Tarczyńska, M. Establishment of an Alert Level Framework for Cyanobacteria in Drinking Water Resources by Using the Algae Online Analyser for Monitoring Cyanobacterial Chlorophyll A. Water Res. 2009, 43, 989–996. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef]
- Mackey, M.D.; Mackey, D.J.; Higgins, H.W.; Wright, S.W. CHEMTAX—A Program for Estimating Class Abundances from Chemical Markers: Application to HPLC Measurements of Phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 265–283. [Google Scholar] [CrossRef]
- Horváth, H.; Kovács, A.W.; Riddick, C.; Présing, M. Extraction Methods for Phycocyanin Determination in Freshwater Filamentous Cyanobacteria and Their Application in a Shallow Lake. Eur. J. Phycol. 2013, 48, 278–286. [Google Scholar] [CrossRef]
- Catherine, A.; Escoffier, N.; Belhocine, A.; Nasri, A.B.; Hamlaoui, S.; Yéprémian, C.; Bernard, C.; Troussellier, M. On the Use of the FluoroProbe®, a Phytoplankton Quantification Method Based on Fluorescence Excitation Spectra for Large-Scale Surveys of Lakes and Reservoirs. Water Res. 2012, 46, 1771–1784. [Google Scholar] [CrossRef]
- Gregor, J.; Geriš, R.; Maršálek, B.; Heteša, J.; Marvan, P. In Situ Quantification of Phytoplankton in Reservoirs Using a Submersible Spectrofluorometer. Hydrobiologia 2005, 548, 141–151. [Google Scholar] [CrossRef]
- Leboulanger, C.; Dorigo, U.; Jacquet, S.; Le Berre, B.; Paolini, G.; Humbert, J.F. Application of a Submersible Spectrofluorometer for Rapid Monitoring of Freshwater Cyanobacterial Blooms: A Case Study. Aquat. Microb. Ecol. 2002, 30, 83–89. [Google Scholar] [CrossRef]
- Echenique-Subiabre, I.; Dalle, C.; Duval, C.; Heath, M.W.; Couté, A.; Wood, S.A.; Humbert, J.F.; Quiblier, C. Application of a Spectrofluorimetric Tool (Bbe BenthoTorch) for Monitoring Potentially Toxic Benthic Cyanobacteria in Rivers. Water Res. 2016, 101, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Rosero-López, D.; Walter, M.T.; Flecker, A.S.; Ontaneda, D.F.; Dangles, O. Standardization of Instantaneous Fluoroprobe Measurements of Benthic Algal Biomass and Composition in Streams. Ecol. Indic. 2021, 121, 107185. [Google Scholar] [CrossRef]
- Bertone, E.; Burford, M.A.; Hamilton, D.P. Fluorescence Probes for Real-Time Remote Cyanobacteria Monitoring: A Review of Challenges and Opportunities. Water Res. 2018, 141, 152–162. [Google Scholar] [CrossRef]
- McBride, C.G.; Rose, K.C. Automated High-Frequency Monitoring and Research. In Lake Restoration Handbook; Hamilton, D.P., Collier, K.J., Quinn, J.M., Howard-Williams, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 419–461. [Google Scholar]
- Nguyen, T.; Roddick, F.A.; Fan, L. Impact of Green Algae on the Measurement of Microcystis aeruginosa Populations in Lagoon-Treated Wastewater with an Algae Online Analyser. Environ. Technol. 2015, 36, 556–565. [Google Scholar] [CrossRef]
- Rolland, A.; Rimet, F.; Jacquet, S. A 2-Year Survey of Phytoplankton in the Marne Reservoir (France): A Case Study to Validate the Use of an in Situ Spectrofluorometer by Comparison with Algal Taxonomy and Chlorophyll a Measurements. Knowl. Manag. Aquat. Ecosyst. 2010, 42, 1–19. [Google Scholar] [CrossRef]
- Kring, S.A.; Figary, S.E.; Boyer, G.L.; Watson, S.B.; Twiss, M.R. Rapid in Situ Measures of Phytoplankton Communities Using the Bbe FluoroProbe: Evaluation of Spectral Calibration, Instrument Intercompatibility, and Performance Range. Can. J. Fish. Aquat. Sci. 2014, 71, 1087–1095. [Google Scholar] [CrossRef]
- Zamyadi, A.; McQuaid, N.; Dorner, S.; Bird, D.F.; Burch, M.; Baker, P.; Hobson, P.; Prévost, M. Cyanobacterial Detection Using in Vivo Fluorescence Probes: Managing Interferences for Improved Decision-Making. J. Am. Water Works Assoc. 2012, 104, 466–479. [Google Scholar] [CrossRef]
- Silva, T.; Giani, A.; Figueredo, C.; Viana, P.; Khac, V.T.; Lemaire, B.J.; Tassin, B.; Nascimento, N.; Vinçon-Leite, B. Comparison of Cyanobacteria Monitoring Methods in a Tropical Reservoir by In Vivo and In Situ Spectrofluorometry. Ecol. Eng. 2016, 97, 79–87. [Google Scholar] [CrossRef][Green Version]
- Zamyadi, A.; Choo, F.; Newcombe, G.; Stuetz, R.; Henderson, R.K. A Review of Monitoring Technologies for Real-Time Management of Cyanobacteria: Recent Advances and Future Direction. TrAC Trends Anal. Chem. 2016, 85, 83–96. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and Nitrogen Removal Capacity of Desmodesmus communis and of a Natural Microalgae Consortium in a Batch Culture System in View of Urban Wastewater Treatment: Part I. Water Res. 2013, 47, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.P. The Influence of the Mineral Composition of the Medium on the Growth of Planktonic Algae: Part I. Methods and Culture Media. J. Ecol. 1942, 30, 284–325. [Google Scholar] [CrossRef]
- Cramer, M.; Myers, J. Growth and Photosynthetic Characteristics of Euglena Gracilis. Arch. Mikrobiol. 1952, 17, 384–402. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and Properties of Unicellular Blue-Green Algae (Order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, J.; De Ville, M.; Day, J.; Turner, M. Culture Collection of Algae & Protozoa: Catalogue of Strains; CCAP: Dunbeg, Oban, UK, 1995. [Google Scholar]
- Beakes, G.W.; Canter, H.M.; Jaworski, G.H.M. Zoospore Ultrastructure of Zygorhizidium affluens and Z. planktonicum, Two Chytrids Parasitizing the Diatom Asterionella formosa. Can. J. Bot. 1988, 66, 1054–1067. [Google Scholar] [CrossRef]
- Rousso, B.Z.; Bertone, E.; Stewart, R.A.; Rinke, K.; Hamilton, D.P. Light-Induced Fluorescence Quenching Leads to Errors in Sensor Measurements of Phytoplankton Chlorophyll and Phycocyanin. Water Res. 2021, 198, 117133. [Google Scholar] [CrossRef]
- Ritchie, R.J. Universal Chlorophyll Equations for Estimating Chlorophylls a, b, c, and d and Total Chlorophylls in Natural Assemblages of Photosynthetic Organisms Using Acetone, Methanol, or Ethanol Solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- ISO 10260:1992; Water Quality: Measurement of Biochemical Parameters: Spectrometric Determination of the Chlorophyll-a Concentration. International Organization for Standardization: Geneva, Switzerland, 1992.
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Utermöhl, H. Neue Wege in Der Quantitativen Erfassung Des Plankton (Mit Besonderer Berücksichtigung Des Ultraplanktons.). Int. Ver. Theor. Angew. Limnol. Verh. 1931, 5, 567–596. [Google Scholar] [CrossRef]
- Napiórkowska-Krzebietke, A.; Kobos, J. Assessment of the Cell Biovolume of Phytoplankton Widespread in Coastal and Inland Water Bodies. Water Res. 2016, 104, 532–546. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Escoffier, N.; Bernard, C.; Hamlaoui, S.; Groleau, A.; Catherine, A. Quantifying Phytoplankton Communities Using Spectral Fluorescence: The Effects of Species Composition and Physiological State. J. Plankton Res. 2015, 37, 233–247. [Google Scholar] [CrossRef]
- Simon, D.; Helliwell, S. Extraction and Quantification of Chlorophyll a from Freshwater Green Algae. Water Res. 1998, 32, 2220–2223. [Google Scholar] [CrossRef]
- Shoaf, W.T.; Lium, B.W. Improved Extraction of Chlorophyll a and b from Algae Using Dimethyl Sulfoxide. Limnol. Oceanogr. 1976, 21, 926–928. [Google Scholar] [CrossRef]
- Schumann, R.; Häubner, N.; Klausch, S.; Karsten, U. Chlorophyll Extraction Methods for the Quantification of Green Microalgae Colonizing Building Facades. Int. Biodeterior. Biodegrad. 2005, 55, 213–222. [Google Scholar] [CrossRef]
- Sartory, D.P.; Grobbelaar, J.U. Extraction of Chlorophyll a from Freshwater Phytoplankton for Spectrophotometric Analysis. Hydrobiologia 1984, 114, 177–187. [Google Scholar] [CrossRef]
- Wasmund, N.; Topp, I.; Schories, D. Optimising the Storage and Extraction of Chlorophyll Samples. Oceanologia 2006, 48, 125–144. [Google Scholar]
- Castle, S.C.; Morrison, C.D.; Barger, N.N. Extraction of Chlorophyll a from Biological Soil Crusts: A Comparison of Solvents for Spectrophotometric Determination. Soil Biol. Biochem. 2011, 43, 853–856. [Google Scholar] [CrossRef]
- Qin, H.; Li, S.; Li, D. An Improved Method for Determining Phytoplankton Chlorophyll a Concentration without Filtration. Hydrobiologia 2013, 707, 81–95. [Google Scholar] [CrossRef]
- Baudelet, P.H.; Ricochon, G.; Linder, M.; Muniglia, L. A New Insight into Cell Walls of Chlorophyta. Algal Res. 2017, 25, 333–371. [Google Scholar] [CrossRef]
- Richardson, T.L.; Lawrenz, E.; Pinckney, J.L.; Guajardo, R.C.; Walker, E.A.; Paerl, H.W.; MacIntyre, H.L. Spectral Fluorometric Characterization of Phytoplankton Community Composition Using the Algae Online Analyser®. Water Res. 2010, 44, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Goldman, E.A.; Smith, E.M.; Richardson, T.L. Estimation of Chromophoric Dissolved Organic Matter (CDOM) and Photosynthetic Activity of Estuarine Phytoplankton Using a Multiple-Fixed-Wavelength Spectral Fluorometer. Water Res. 2013, 47, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Tan, L.; Wang, J. Combined Toxicities of Copper Nanoparticles with Carbon Nanotubes on Marine Microalgae Skeletonema Costatum. Environ. Sci. Pollut. Res. 2018, 25, 13127–13133. [Google Scholar] [CrossRef]
- Techer, D.; Fontaine, P.; Personne, A.; Viot, S.; Thomas, M. Allelopathic Potential and Ecotoxicity Evaluation of Gallic and Nonanoic Acids to Prevent Cyanobacterial Growth in Lentic Systems: A Preliminary Mesocosm Study. Sci. Total Environ. 2016, 547, 157–165. [Google Scholar] [CrossRef]
- Senar, O.E.; Creed, I.F.; Strandberg, U.; Arts, M.T. Browning Reduces the Availability—But Not the Transfer—Of Essential Fatty Acids in Temperate Lakes. Freshw. Biol. 2019, 64, 2107–2119. [Google Scholar] [CrossRef]
- Trommer, G.; Poxleitner, M.; Stibor, H. Responses of Lake Phytoplankton Communities to Changing Inorganic Nitrogen Supply Forms. Aquat. Sci. 2020, 82, 22. [Google Scholar] [CrossRef]
- Wilken, S.; Soares, M.; Urrutia-Cordero, P.; Ratcovich, J.; Ekvall, M.K.; Van Donk, E.; Hansson, L.A. Primary Producers or Consumers? Increasing Phytoplankton Bacterivory along a Gradient of Lake Warming and Browning. Limnol. Oceanogr. 2018, 63, S142–S155. [Google Scholar] [CrossRef]
- Li, J.; Parkefelt, L.; Persson, K.M.; Pekar, H. Improving Cyanobacteria and Cyanotoxin Monitoring in Surface Waters for Drinking Water Supply. J. Water Secur. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Meyns, S.; Illi, R.; Ribi, B. Comparison of Chlorophyll-a Analysis by HPLC and Spectrophotometry: Where Do the Differences Come From? Arch. Hydrobiol. 1994, 132, 129–139. [Google Scholar] [CrossRef]
- Hartmann, A.; Horn, H.; Röske, I.; Röske, K. Comparison of Fluorometric and Microscopical Quantification of Phytoplankton in a Drinking Water Reservoir by a One-Season Monitoring Program. Aquat. Sci. 2019, 81, 19. [Google Scholar] [CrossRef]
- Choo, F.; Zamyadi, A.; Stuetz, R.M.; Newcombe, G.; Newton, K.; Henderson, R.K. Enhanced Real-Time Cyanobacterial Fluorescence Monitoring through Chlorophyll-a Interference Compensation Corrections. Water Res. 2019, 148, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, H.L.; Lawrenz, E.; Richardson, T.L. Taxonomic Discrimination of Phytoplankton by Spectral Fluorescence. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Suggett, D., Prášil, O., Borowitzka, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 129–169. ISBN 9789048192687. [Google Scholar]
- Joh, G.; Choi, Y.S.; Shin, J.K.; Lee, J. Problematic Algae in the Sedimentation and Filtration Process of Water Treatment Plants. J. Water Supply Res. Technol. AQUA 2011, 60, 219–230. [Google Scholar] [CrossRef]
- Violi, J.P.; Facey, J.A.; Mitrovic, S.M.; Colville, A.; Rodgers, K.J. Production of β-Methylamino-L-Alanine (BMAA) and Its Isomers by Freshwater Diatoms. Toxins 2019, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.W.; Howell, E.T.; Watson, S.B.; Smith, R.E.H. Improved Estimates of Phytoplankton Community Composition Based on in Situ Spectral Fluorescence: Use of Ordination and Field-Derived Norm Spectra for the Bbe FluoroProbe. Can. J. Fish. Aquat. Sci. 2016, 73, 1472–1482. [Google Scholar] [CrossRef]
- Bowling, L.; Ryan, D.; Holliday, J.; Honeyman, G. Evaluation of in Situ Fluorometry to Determine Cyanobacterial Abundance in the Murray and Lower Darling Rivers, Australia. River Res. Appl. 2013, 29, 1059–1071. [Google Scholar] [CrossRef]
- Bertone, E.; Chuang, A.; Burford, M.A.; Hamilton, D.P. In-Situ Fluorescence Monitoring of Cyanobacteria: Laboratory-Based Quantification of Species-Specific Measurement Accuracy. Harmful Algae 2019, 87, 101625. [Google Scholar] [CrossRef]
| Algal Group | Strain Name | Strain Details | Supplier or Ref. |
|---|---|---|---|
| “Green” group | Euglena gracilis | SAG 1224-5/25 | SAG |
| Chlorella vulgaris (“C”) | CCAP 211/52 | CCAP | |
| Chlorella vulgaris (“K”) | K-1801 | NIVA-CCA | |
| Desmodesmus communis | Locally isolated (artificial freshwater pond, Forlì-Cesena, Italy) | [24] | |
| Cyanobacteria | Planktothrix rubescens | CCAP 1459/22 | CCAP |
| Planktothrix agardhii | CCAP 1459/16 | CCAP | |
| Microcystis aeruginosa | CCAP 1450/10 | CCAP | |
| cf. Dolichospermum sp. | Locally isolated (Reno River, Ravenna, Italy) | This study | |
| cf. Anabaena sp. | Locally isolated (Reno River, Ravenna, Italy) | This study | |
| “Brown” group | Stephanodiscus hantzschii | CCAP 1079/4 | CCAP |
| Fragilaria crotonensis | CCAP 1029/20 | CCAP | |
| cf. Cyclotella sp. | Locally isolated (Reno River, Ravenna, Italy) | This study |
| Algal Group | Fluo/Extr | ALA/Extr | FP/Extr | AOAs/Extr | Fluo/ACT | Fluo/EtOH | Fluo/DMSO |
|---|---|---|---|---|---|---|---|
| “Green” group | 1.44 ± 0.45 | 1.27 ± 0.52 | 1.44 ± 0.42 | 1.61 ± 0.44 | 2.04 ± 0.62 | 1.46 ± 0.50 | 1.07 ± 0.34 |
| Cyanobacteria | 1.25 ± 0.19 | 1.13 ± 0.25 | 1.25 ± 0.19 | 1.36 ± 0.21 | 1.21 ± 0.16 | 1.54 ± 0.43 | 1.12 ± 0.21 |
| “Brown” group | 1.23 ± 0.27 | 1.20 ± 0.33 | 1.22 ± 0.35 | 1.27 ± 0.20 | 1.18 ± 0.16 | 1.53 ± 0.72 | 1.20 ± 0.22 |
| P. rubescens | 1.58 ± 0.12 | 1.66 ± 0.16 | 1.48 ± 0.17 | 1.58 ± 0.22 | 1.88 ± 0.22 | 1.42 ± 0.25 | 1.50 ± 0.17 |
| Target Algal Group | ALA (Er%) | FP (Er%) | AOAs (Er%) | AT (Er%) |
|---|---|---|---|---|
| “Green” group | 6.4 ± 4.3 | 8.1 ± 4.6 | 8.0 ± 3.7 | - |
| Cyanobacteria | 0.2 ± 0.5 | 0.9 ± 1.6 | 3.1 ± 3.6 | 1.0 ± 1.4 |
| “Brown” group | 16.1 ± 7.7 | 13.8 ± 7.6 | 10.7 ± 3.1 | - |
| P. rubescens | 44.7 ± 5.6 | 54.1 ± 10.7 | 42.5 ± 5.2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonazzi, M.; Pezzolesi, L.; Guerrini, F.; Vanucci, S.; Graziani, G.; Vasumini, I.; Pandolfi, A.; Servadei, I.; Pistocchi, R. Improvement of In Vivo Fluorescence Tools for Fast Monitoring of Freshwater Phytoplankton and Potentially Harmful Cyanobacteria. Int. J. Environ. Res. Public Health 2022, 19, 14075. https://doi.org/10.3390/ijerph192114075
Simonazzi M, Pezzolesi L, Guerrini F, Vanucci S, Graziani G, Vasumini I, Pandolfi A, Servadei I, Pistocchi R. Improvement of In Vivo Fluorescence Tools for Fast Monitoring of Freshwater Phytoplankton and Potentially Harmful Cyanobacteria. International Journal of Environmental Research and Public Health. 2022; 19(21):14075. https://doi.org/10.3390/ijerph192114075
Chicago/Turabian StyleSimonazzi, Mara, Laura Pezzolesi, Franca Guerrini, Silvana Vanucci, Giancarlo Graziani, Ivo Vasumini, Andrea Pandolfi, Irene Servadei, and Rossella Pistocchi. 2022. "Improvement of In Vivo Fluorescence Tools for Fast Monitoring of Freshwater Phytoplankton and Potentially Harmful Cyanobacteria" International Journal of Environmental Research and Public Health 19, no. 21: 14075. https://doi.org/10.3390/ijerph192114075
APA StyleSimonazzi, M., Pezzolesi, L., Guerrini, F., Vanucci, S., Graziani, G., Vasumini, I., Pandolfi, A., Servadei, I., & Pistocchi, R. (2022). Improvement of In Vivo Fluorescence Tools for Fast Monitoring of Freshwater Phytoplankton and Potentially Harmful Cyanobacteria. International Journal of Environmental Research and Public Health, 19(21), 14075. https://doi.org/10.3390/ijerph192114075






