Abstract
Breast cancer is the most commonly occurring cancer in women, and it is a major cause of cancer death around the world. With the development of diagnostic methods and improvements in treatment methods, the incidence rate of breast cancer and the number of breast cancer survivors continue to simultaneously increase. We used national registry database to analyze the features that affect employment and return to work among breast cancer survivors. A total of 23,220 employees, who were newly diagnosed with breast cancer were recruited based on the Labor Insurance Database (LID), the Taiwan Cancer Registry (TCR), and National Health Insurance Research Database (NHIRD) during the period 2004–2015. The correlations between return to work (RTW) and independent confounding factors were examined using Cox proportional hazards model. Survival probability was analyzed using the Kaplan–Meir method. After adjusting for confounding variables, cancer stage, chemotherapy and higher income were significantly negatively correlated with RTW. Among breast cancer survivors, RTW was found to be related to a lower risk of all-cause mortality in both the unadjusted and fully adjusted model. Patients who had RTW exhibited better survival in all stages. Work-, disease- and treatment-related factors influenced RTW among employees with breast cancer. RTW was associated with better breast cancer survival. Our study demonstrates the impact of RTW and the associated factors on breast cancer survivorship.
1. Introduction
Breast cancer is the most commonly occurring cancer in women. Furthermore, it is a major cause of cancer death and has a high incidence rate around the world [1]. Studies show that the majority of white people with breast cancer are in their 60s, whereas the majority of nonwhite people with breast cancer are in their 40s in the US, meaning that they are categorized as being of mature working age [2]. Undoubtedly, this is a bigger issue as regards the US labor nonwhite force. Fortunately, improvements in breast cancer screening, such as mammography techniques, and treatments including less aggressive surgery, adjuvant chemotherapy, and hormonal therapy, have reduced the recurrence rates, mortality rates, and increased the survival rates in all patients with breast cancer [3,4,5]. According to the present study, breast cancer patients currently have a 5-year survival range from 79% to 93% in the European Union [6]. As the number of breast cancer survivors has increased, employment has become a big concern [7]. Breast cancer survivors face many challenges. There are both associated with the physical side effects of treatment, and with quality-of-life, psychological, social, and financial issues [8,9,10,11].
The ability to return to work (RTW) is a confirmation of the social or family status of cancer survivors and an indication that the cancer has been cured [12]. There are many studies discussing RTW in breast cancer survivors and the literature suggests that the majority of cancer survivors can return to work after treatment [13,14]. Previous studies showed multiple factors influencing RTW for breast cancer patients, such as socio-demographic characteristics, work-related factors, disease and treatment-related factors [13]. Moreover, the altitude of employers also had a pivotal role in successful return to work in breast cancer survivors [13]. Therefore, this is important to study the relationship between cancer survivors, RTW, and mortality.
In one study, 80% of breast cancer patients went on sick leave after diagnosis, but only 56% came back to work after finishing treatment [15]. In another review, researchers found that 43% to 93% of breast cancer patients could RTW within 1 year of diagnosis [16]. In a focus group study by Tamminga et al., breast cancer survivors who were 2 years post-diagnosis reported physical impairments related to the treatment as a barrier for their RTW [17]. Schmidt ME and her colleagues found that persistent tiredness and cognitive issues were correlated with no RTW [18]. Economic problems also play an important role among breast cancer survivors [9]. De Boer et al. reported that unemployment status is associated with cancer survivorship [19]. Lauzier et al. demonstrated wage losses and associated financial stress among breast cancer patients [20]. Drolet et al. showed that breast cancer patients took 6 months of sick leave on average [21]. Hence, being employed and RTW may lighten the financial burden and improve quality of life [22]. RTW also represents a return to normal life and leads to an improved social role [23].
To date, most studies exploring changes in employment status in cancer survivors have relatively short follow-up period [24]. There are few studies that focus on long-term employment status among breast cancer survivors [24]. Moreover, prior research mainly focused on Western populations [7]. This study’s objective was to analyze the effects of socio-economic factors, treatment factors, and disease-related factors among breast cancer survivors on RTW and mortality by conducting a national registry-based cohort study. We hope that this study highlights the benefits of returning to work for breast cancer survivors in Asian populations.
2. Materials and Methods
2.1. Study Population
We established a retrospective cohort study by recruiting employees based on the Labor Insurance Database (LID), the Taiwan Cancer Registry (TCR), and National Health Insurance Research Database (NHIRD) during the period 2004–2015 in Taiwan. Through links with the LID, TCR, and NHIRD using personal identification numbers, we obtained work-related data, including employment information, employee’s industry, monthly income, and company scale. Primary diagnosis of breast cancer is coded by the International Classification of Diseases for Oncology–3rd edition (ICD-O-3). In this cohort study, we enrolled 23,220 participants who were first diagnosed with breast cancer (ICD-O-3 C50) during the study period (2004–2010) and had a minimum follow-up time of 2 years from cancer diagnosis. The tracking period was from January 2004 to December 2015. We excluded participants who were younger than 20, who had two or more separate cancers, including breast cancer, and who were out of work at baseline. All protocols were executed by the Institutional Review Board (IRB) of Tri-Service General Hospital (TSGH).
2.2. Covariates
Demographic data including age, employment information, employee’s industry, monthly income and company scale were obtained from the LID. We classified monthly income into USD ≤ 960, USD > 960~1273 and USD > 1273. We classified company scale into company closed, small (less than five people), medium (less than 200 people) and large (more than 200 people). Clinical comorbidities were collected from the NHIRD according to the International Classification of Diseases, 9th Revision, Clinical Modification codes. Clinical comorbidities included disorders of lipid metabolism, cerebrovascular diseases, chronic pulmonary diseases, peptic ulcer diseases, renal diseases, liver diseases, psychoses, and depression. The International Classification of Diseases, 9th Revision, Clinical Modification codes for clinical comorbidities mentioned above is listed in Supplementary Table S1. The pathological breast cancer stage and treatment types for breast cancer, such as surgical management, radiation therapy, and chemotherapy were also collected from the NHIRD. Covariates were assessed at the time of 2 years after cancer diagnosis.
2.3. Outcome Assessment
The primary outcome of our study was RTW 2 years after breast cancer diagnosis. RTW was selected as the primary outcome because RTW of breast cancer patients was regarded as an important part of recovery. Every enrolled worker was followed from the date of primary diagnosis of breast cancer to the end of the follow-up or to death. The secondary outcome was the all-cause mortality within the follow-up period.
2.4. Statistical Analysis
We utilized the SAS (software) package (SAS 9.3, SAS Institute Inc., Cary, North Carolina) to perform our data analyses. Two-sided p values < 0.05 were considered to be statistically significant. Continuous variables are indicated as the means and standard deviations (SD); categorical variables are indicated as frequencies and percentages. The chi-squared test was used for testing relationships between categorical variables and the Wilcoxon rank-sum test or independent t-test was used for continuous variables. Employment status or RTW was evaluated 2 years after breast cancer diagnosis. Survival time was followed from the first breast cancer diagnosis to the date of death between 1 January 2004 and 31 December 2015. Univariate and multivariate analyses using Cox proportional hazard models were performed to assess the effect of covariates on RTW. The correlation between RTW and all-cause mortality among breast cancer patients was also investigated by the multivariate Cox proportional hazards model. Age, gender, pathological breast cancer stage, received treatment, monthly income, employee’s industry, and company scale were included in the fully adjusted model. Survival probability was analyzed using the Kaplan–Meir method.
3. Results
3.1. Characteristics of the Study Population
A total of 16,083 newly diagnosed female breast cancer patients were enrolled and analyzed in the study. The mean age of the participants was 48.2 ± 7.8 years, and more than half of patients (62.8%) had a monthly income range below USD 960. From all the cases, 5880 were stage 2 (36.5%), followed by stage 1 (32.7%), stage 3 (15.6%), stage 0 (13%) and stage 4 (2%). In addition, 93.9% of women underwent surgical treatment and 42.7% received chemotherapy. The main industry category was manufacturing (31.7%). Other covariates, including comorbidities before cancer diagnosis and company scale, are listed in Table 1. We divided the study population into an RTW group and non-RTW group. The RTW group included patients who were continuing employment and those who were re-employed. The non-RTW group included patients who were unemployed or did not RTW. In general, the data show that the non-RTW group had a higher prevalence of suffering comorbidities and chemotherapy treatment, had a higher income, worked in small- to medium-sized companies, and exhibited a higher pathology stage.

Table 1.
Characteristics of the study population.
3.2. Univariate Analysis of Independent Factors Associated with RTW in Cox Proportional Hazards Models
Table 2 presents the factors associated with RTW in the 2-year univariate analysis using Cox proportional hazards models. Early-stage cancer was related to a higher likelihood of RTW. On the contrary, factors including age, receiving chemotherapy or hormone replacement therapy, a monthly income over USD 1273, working in small- to medium-sized company were associated with a lower likelihood of RTW. Several industry categories, including the wholesale and retail trade (wholesale), information and communication (information), financial and insurance activities (financial activity), professional, scientific and technical activities (technical activates), public administration and defense (public administration) and human health and social work activities (human health) were correlated with a lower likelihood of RTW.

Table 2.
Univariate analysis of RTW in Cox proportional hazards models.
3.3. Multivariate Analysis of Independent Factors Associated with RTW in Cox Proportional Hazards Models
Table 3 presents variables associated with RTW in the 2-year multivariate analysis using Cox proportional hazards models. After adjusting for other variables, Table 3 shows almost the same outcomes as Table 2, except for the industry categories. The association between industry categories and RTW was not statistically significant after adjusting for confounding factors. Cancer stage was inversely associated with likelihood of RTW. Patients who received chemotherapy were less likely to RTW. Patients with a higher income (a monthly income above USD1273) were less likely to RTW. Patients working in small- to medium-sized company were less likely to RTW.

Table 3.
Multivariate analysis of RTW in Cox proportional hazards models.
3.4. Influence of RTW on Survival Outcomes
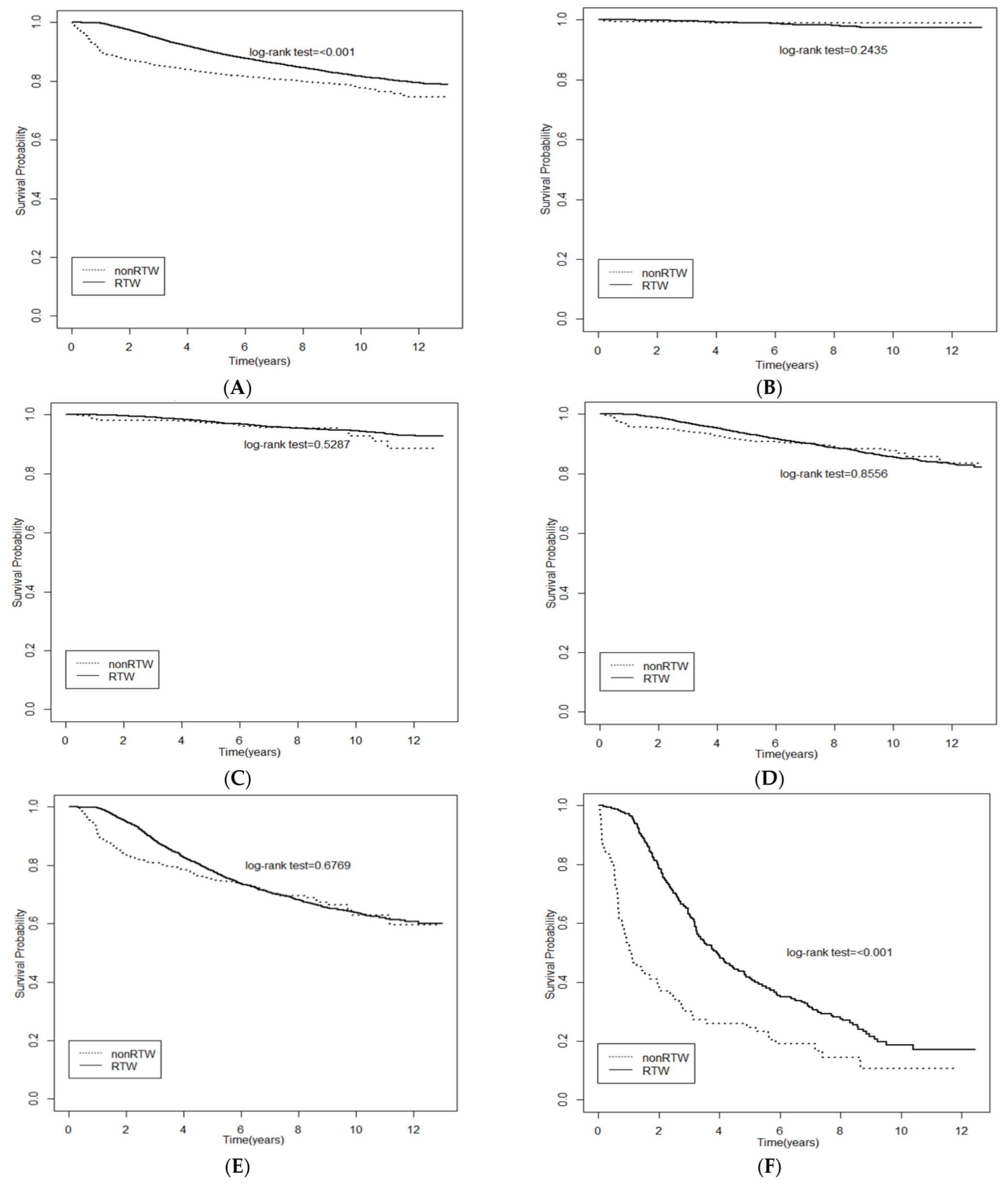
Figure 1 shows the significant differences of survival probability between the RTW group and non-RTW group using Kaplan–Meier curves. A better survival probability was found in the RTW group than the non-RTW group in participants with stage 2 (Figure 1D), stage 3 (Figure 1E), and stage 4 cancer (Figure 1F). Table 4 presents the relationship between the independent variates and all-cause mortality in the multivariate Cox proportional hazard models. RTW was found to be related to a lower risk of all-cause mortality among breast cancer patients in the fully adjusted model (HR = 0.585; 95% CI, 0.526 to 0.649).
Figure 1.
Kaplan-Meier (KM) curves showed survival probability of RTW for all (A) and stage 0–4 (B–F) breast cancer employers. (A) All stage; (B) Stage 0; (C) Stage 1; (D) Stage 2; (E) Stage 3; (F) Stage 4.

Table 4.
Multivariate analysis of relationship between independent variables and all-cause mortality of breast cancer patients.
4. Discussion
We conducted a retrospective cohort study to evaluate the factors that affect employment status in newly diagnosed female breast cancer patients. We analyzed 23,220 newly diagnosed female breast cancer patients from 2004 to 2010 and tracked whether these patients returned to work or not from 2004 to 2015. We demonstrated that early-stage breast cancer was positively associated with RTW, and higher income and receiving chemotherapy were negatively correlated with RTW. RTW was also found to be associated with better survival among breast cancer patients.
Multiple factors were identified in previous research related to RTW, such as education, ethnicity, chemotherapy, heavy physical work, poor health, fatigue, depression and emotional distress [16]; however, few studies include factors such as comorbidities, industry category, with a follow-up of more than 5 years in Asian populations [25,26]. The results of these studies showed that socio-demographic, disease-related, and work-related factors are important elements in RTW. Socio-demographic factors, for the example patient’s age, educational level, ethnicity, and marital status, were correlated with RTW [25,27]. A retrospective cohort study from Canada noted that older breast cancer patients were less likely to return to work [27]. A low educational level was found to be associated with unemployment [14]. Due to limitations related to our database, age was the only socio-demographic factor highlighted in this study. Age was significantly associated as a negative factor with RTW in the univariate (HR = 0.996; 95% CI, 0.994~0.998) and multivariate (HR = 0.995; 95% CI, 0.993~0.997) regression models. Psychological factors, including worry, depression, and frustration were found to affect RTW [28]. An observational study by Wolvers et al. revealed self-efficacy was correlated with earlier RTW [29].
Disease-related factors, such as stage of breast cancer, surgery, chemotherapy, radiotherapy, post-treatment side effects (fatigue, pain, nausea, vomiting, arm morbidity, and cognitive dysfunction), and multiple co-morbidities were reported to affect RTW [13,25,30,31]. Fantoni et al. showed that chemotherapy and radiotherapy were correlated with both limited and delayed RTW [31]. In a health insurance database study, the results demonstrated that chemotherapy was related with long-term disability, stopping working, and retirement in female breast cancer patients [32]. Our study was consistent with these studies. The cancer stage was another important factor that was found to be inversely related with work status and employment in previous studies [25,33]. In a French prospective study, advanced stage was correlated with unemployment [34]. A similar pattern was noted in our study.
Work-related factors, such as job type, workplace accommodation or discrimination and average salary were associated with RTW [13,25,35]. Although there are few work-related factors, such as industry category, income, and the company size in our database, we found different industry categories with different patterns of RTW in the univariate analysis. For example, industries including wholesale and retail trade (wholesale), financial and insurance activities (financial activity), scientific and technical activities (technical activates) and public administration and defense (public administration) were associated with a lower likelihood of RTW as follow-up years increased. On the other hands, industries such as professional, information and communication (information) and human health and social work activities (human health) were correlated with a higher likelihood of RTW as follow-up years increased. The results may be similar to those reported in prior research regarding manual work [36] and heavy lifting being a barrier to RTW [16].
Interestingly, higher income and work in small- or medium-sized company had a lower rate of RTW compared with a lower income and large-sized company in our study. Previous studies demonstrated that a low income was correlated with unemployment, as compared with a higher income [25,27,36,37]. Moreover, employers’ understanding of breast cancer diagnosis, treatment, and recovery is related to RTW [13]. Another cohort of 131 cancer survivors reported that socio-demographic, health-, and work-related factors influenced RTW, including company size [38]. This inconsistency may be due to the limitations of our database, including the lack of workplace accommodation, discrimination or work adjustments data, or important factors obscured by company size.
Prognostic or survival factors for breast cancer including age, cancer stage, tumor receptor status, tumor size, lymph node involvement, and histologic type, are well established [39,40]. Studies examining a variety of comorbidity related risk factors associated with breast cancer survival have been published in recent years. Obesity was correlated with poorer survival among breast cancer patients [41]. Diabetes mellitus was related with a higher risk of mortality in breast cancer survivors [42]. In addition to the common risk factors mentioned above, our study also found that RTW was negatively associated with all-cause mortality (HR = 0.585; 95% CI, 0.526 to 0.649) in a fully adjusted model. This finding should be studied more carefully to explore the possible factors related to the disease, treatment, and the workplace.
Taiwanese National Health Insurance has a coverage rate of nearly 99.9% [43]. Comprehensive content including cancer treatment costs, is provided by the Taiwanese National Health Insurance system [44]. Moreover, the Taiwan labor insurance offers 50% of the average daily insurance salary for compensation of lost wages during hospitalization. This may account for the major difference in social welfare between breast cancer survivors from Taiwan and in other countries. Cultural differences including self-stigma and gender stereotyping might affect willingness to RTW for Asian cancer patients [45,46]. However, as a result of the limitations of our database, future studies are required to address these issues.
There are several limitations in our study. Firstly, there was a lack of information about socio-demographic and work-related factor, such as family support, educational level, ethnicity, marital status, work accommodation, and discrimination, which may represent confounding factors affecting RTW, in the study database. Second, there was no available data regarding quality of life. We did not access the relationship between quality of life and RTW among breast cancer survivors. Third, the generalizability of our findings is limited because (1) our study participants were only recruited from Taiwan and (2) this study was conducted in Taiwan, which has a distinctive public welfare system.
5. Conclusions
The present study demonstrated that RTW was associated with better breast cancer survival. Cancer stage was inversely correlated with likelihood of RTW. Chemotherapy and working in a small- or medium-sized company had a negative effect on RTW. The results revealed the impact of RTW and associated factors on breast cancer survivorship. Further research is required to access other confounding variables, such as work accommodation, work discrimination, quality of life and social support.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph192114418/s1, Table S1: The ICD-9-CM codes for clinical comorbidities.
Author Contributions
Z.-Y.Y. contributed to the design of the study, responsible for data-analysis decisions, responsible for the management and retrieval of data, decided on data-collection methods and initial data analysis and interpretation, contributed to initial data analysis and interpretation, and drafted the initial article. W.-T.W., C.-H.L. and C.-L.H. decided on the data-collection methods and initial data analysis and interpretation. W.-L.C. and C.-C.W. conceptualized and designed the study, contributed to the design of the study, supervised all aspects of the study, responsible for data-analysis decisions, decided on data-collection methods and initial data analysis and interpretation, responsible for the management and retrieval of data, contributed to initial data analysis and interpretation, drafted the initial article, critically reviewed and revised the article, and approved the final version for submission. All authors meet the International Committee of Medical Journal Editors criteria for authorship. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Institute of Labor, Occupational Safety, and Health (ILOSH) and the Ministry of Labor (ILOSH107-M301) in Taiwan.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Tri-Service General Hospital (TSGH) (IRB No 1-107-05-129).
Informed Consent Statement
Subject informed consent was waived in this study because our data were collected from the database of the Taiwan Cancer Registry (TCR), Labor Insurance Database (LID) and National Health Insurance Research Database (NHIRD). Those databases were provided under de-identification status. We can only link the data by a unique encryption identity number. All of the procedures were approved by the Institutional Review Board (IRB) of Tri-Service General Hospital, Taiwan (IRB No. 1-107-05-129).
Data Availability Statement
The data underlying this study are from the Labor Insurance Database (LID) and National Health Insurance Research Database (NHIRD). The LID and NHIRD is not free to public access, and therefore, interested researchers can obtain the data through formal application to the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan. (https://dep.mohw.gov.tw/DOS/np-2497-113.html). Last accessed date: 29 June 2020.
Acknowledgments
We gratefully acknowledge the contribution of the Saou-Hsing Liou and Ya-Yuan Hsu to this paper.
Conflicts of Interest
The authors declared that they had no competing interests.
References
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, S.M.; Oseni, T.O.; Bababekov, Y.J.; Hung, Y.C.; Chang, D.C. Race/Ethnicity and Age Distribution of Breast Cancer Diagnosis in the United States. JAMA Surg. 2018, 153, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [CrossRef]
- Berry, D.A.; Cronin, K.A.; Plevritis, S.K.; Fryback, D.G.; Clarke, L.; Zelen, M.; Mandelblatt, J.S.; Yakovlev, A.Y.; Habbema, J.D.; Feuer, E.J. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 2005, 353, 1784–1792. [Google Scholar] [CrossRef]
- Winer, E.; Gralow, J.; Diller, L.; Karlan, B.; Loehrer, P.; Pierce, L.; Demetri, G.; Ganz, P.; Kramer, B.; Kris, M.; et al. Clinical cancer advances 2008: Major research advances in cancer treatment, prevention, and screening—A report from the American Society of Clinical Oncology. J. Clin. Oncol. 2009, 27, 812–826. [Google Scholar] [CrossRef]
- Dafni, U.; Tsourti, Z.; Alatsathianos, I. Breast Cancer Statistics in the European Union: Incidence and Survival across European Countries. Breast Care 2019, 14, 344–353. [Google Scholar] [CrossRef]
- Butow, P.; Laidsaar-Powell, R.; Konings, S.; Lim, C.Y.S.; Koczwara, B. Return to work after a cancer diagnosis: A meta-review of reviews and a meta-synthesis of recent qualitative studies. J. Cancer Surviv. 2020, 14, 114–134. [Google Scholar] [CrossRef]
- Mols, F.; Vingerhoets, A.J.J.M.; Coebergh, J.W.; van de Poll-Franse, L.V. Quality of life among long-term breast cancer survivors: A systematic review. Eur. J. Cancer 2005, 41, 2613–2619. [Google Scholar] [CrossRef]
- Azzani, M.; Roslani, A.C.; Su, T.T. The perceived cancer-related financial hardship among patients and their families: A systematic review. Support Care Cancer 2015, 23, 889–898. [Google Scholar] [CrossRef]
- Baucom, D.H.; Porter, L.S.; Kirby, J.S.; Gremore, T.M.; Keefe, F.J. Psychosocial issues confronting young women with breast cancer. Breast Dis. 2005, 23, 103–113. [Google Scholar] [CrossRef]
- Ewertz, M.; Jensen, A.B. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011, 50, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Stergiou-Kita, M.; Grigorovich, A.; Tseung, V.; Milosevic, E.; Hebert, D.; Phan, S.; Jones, J. Qualitative meta-synthesis of survivors’ work experiences and the development of strategies to facilitate return to work. J. Cancer Surviv. 2014, 8, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Bouknight, R.R.; Bradley, C.J.; Luo, Z. Correlates of return to work for breast cancer survivors. J. Clin. Oncol. 2006, 24, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Taskila, T.; Lindbohm, M.L. Factors affecting cancer survivors’ employment and work ability. Acta Oncol. 2007, 46, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Molina Villaverde, R.; Feliu Batlle, J.; Villalba Yllan, A.; Jimenez Gordo, A.M.; Redondo Sanchez, A.; San Jose Valiente, B.; Gonzalez Baron, M. Employment in a cohort of breast cancer patients. Occup. Med. 2008, 58, 509–511. [Google Scholar] [CrossRef]
- Islam, T.; Dahlui, M.; Majid, H.A.; Nahar, A.M.; Mohd Taib, N.A.; Su, T.T. Factors associated with return to work of breast cancer survivors: A systematic review. BMC Public Health 2014, 14 (Suppl. 3), S8. [Google Scholar] [CrossRef]
- Tamminga, S.J.; de Boer, A.G.; Verbeek, J.H.; Frings-Dresen, M.H. Breast cancer survivors’ views of factors that influence the return-to-work process--a qualitative study. Scand. J. Work Environ. Health 2012, 38, 144–154. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Scherer, S.; Wiskemann, J.; Steindorf, K. Return to work after breast cancer: The role of treatment-related side effects and potential impact on quality of life. Eur. J. Cancer Care 2019, 28, e13051. [Google Scholar] [CrossRef]
- de Boer, A.G.; Taskila, T.; Ojajarvi, A.; van Dijk, F.J.; Verbeek, J.H. Cancer survivors and unemployment: A meta-analysis and meta-regression. JAMA 2009, 301, 753–762. [Google Scholar] [CrossRef]
- Lauzier, S.; Maunsell, E.; Drolet, M.; Coyle, D.; Hebert-Croteau, N.; Brisson, J.; Masse, B.; Abdous, B.; Robidoux, A.; Robert, J. Wage losses in the year after breast cancer: Extent and determinants among Canadian women. J. Natl. Cancer Inst. 2008, 100, 321–332. [Google Scholar] [CrossRef]
- Drolet, M.; Maunsell, E.; Mondor, M.; Brisson, C.; Brisson, J.; Mâsse, B.; Deschênes, L. Work absence after breast cancer diagnosis: A population-based study. CMAJ 2005, 173, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Lundh, M.H.; Lampic, C.; Nordin, K.; Ahlgren, J.; Bergkvist, L.; Lambe, M.; Berglund, A.; Johansson, B. Changes in health-related quality of life by occupational status among women diagnosed with breast cancer—A population-based cohort study. Psycho-Oncology 2013, 22, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Waddell, G.; Burton, A.K. Is Work Good for Your Health and Wellbeing? OHRD: Belfast, UK, 2006; pp. 30–31. [Google Scholar]
- Paltrinieri, S.; Fugazzaro, S.; Bertozzi, L.; Bassi, M.C.; Pellegrini, M.; Vicentini, M.; Mazzini, E.; Costi, S. Return to work in European Cancer survivors: A systematic review. Support. Care Cancer 2018, 26, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Cho, J.; Shin, D.W.; Park, B.W.; Ahn, S.H.; Noh, D.Y.; Nam, S.J.; Lee, E.S.; Yun, Y.H. Impact of breast cancer diagnosis and treatment on work-related life and factors affecting them. Breast Cancer Res. Treat. 2009, 116, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Haruyama, Y.; Takahashi, M.; Nishiura, C.; Kojimahara, N.; Yamaguchi, N. Returning to work after sick leave due to cancer: A 365-day cohort study of Japanese cancer survivors. J. Cancer Surviv. 2016, 10, 320–329. [Google Scholar] [CrossRef]
- Drolet, M.; Maunsell, E.; Brisson, J.; Brisson, C.; Masse, B.; Deschenes, L. Not working 3 years after breast cancer: Predictors in a population-based study. J. Clin. Oncol. 2005, 23, 8305–8312. [Google Scholar] [CrossRef]
- Campagna, M.; Loscerbo, R.; Pilia, I.; Meloni, F. Return to Work of Breast Cancer Survivors: Perspectives and Challenges for Occupational Physicians. Cancers 2020, 12, 355. [Google Scholar] [CrossRef]
- Wolvers, M.D.J.; Leensen, M.C.J.; Groeneveld, I.F.; Frings-Dresen, M.H.W.; De Boer, A. Predictors for earlier return to work of cancer patients. J. Cancer Surviv. 2018, 12, 169–177. [Google Scholar] [CrossRef]
- Decker, G.M.; DeMeyer, E.S.; Kisko, D.L. Measuring the maintenance of daily life activities using the functional living index-emesis (FLIE) in patients receiving moderately emetogenic chemotherapy. J. Support Oncol. 2006, 4, 35–41, 52. [Google Scholar]
- Fantoni, S.Q.; Peugniez, C.; Duhamel, A.; Skrzypczak, J.; Frimat, P.; Leroyer, A. Factors related to return to work by women with breast cancer in northern France. J. Occup. Rehabil. 2010, 20, 49–58. [Google Scholar] [CrossRef]
- Hassett, M.J.; O’Malley, A.J.; Keating, N.L. Factors influencing changes in employment among women with newly diagnosed breast cancer. Cancer 2009, 115, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, A.; Fornander, T.; Olsson, M.; Nystedt, M.; Johansson, H.; Rutqvist, L.E. Factors associated with return to work after breast cancer treatment. Acta Oncol. 2007, 46, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Arfi, A.; Baffert, S.; Soilly, A.L.; Huchon, C.; Reyal, F.; Asselain, B.; Neffati, S.; Rouzier, R.; Héquet, D. Determinants of return at work of breast cancer patients: Results from the OPTISOINS01 French prospective study. BMJ Open 2018, 8, e020276. [Google Scholar] [PubMed]
- Johnsson, A.; Fornander, T.; Rutqvist, L.E.; Vaez, M.; Alexanderson, K.; Olsson, M. Predictors of return to work ten months after primary breast cancer surgery. Acta Oncol. 2009, 48, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Blinder, V.S.; Patil, S.; Thind, A.; Diamant, A.; Hudis, C.A.; Basch, E.; Maly, R.C. Return to work in low-income Latina and non-Latina white breast cancer survivors: A 3-year longitudinal study. Cancer 2012, 118, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, K.; Jensen, A.J.; Rugulies, R.; Christensen, J.; Bidstrup, P.E.; Johansen, C.; Huitfeldt Madsen, I.E.; Dalton, S.O. Self-reported work ability in long-term breast cancer survivors. A population-based questionnaire study in Denmark. Acta Oncol. 2013, 52, 423–429. [Google Scholar] [CrossRef]
- van Muijen, P.; Duijts, S.F.; van der Beek, A.J.; Anema, J.R. Prognostic factors of work disability in sick-listed cancer survivors. J. Cancer Surviv. 2013, 7, 582–591. [Google Scholar] [CrossRef]
- Voduc, K.D.; Cheang, M.C.; Tyldesley, S.; Gelmon, K.; Nielsen, T.O.; Kennecke, H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010, 28, 1684–1691. [Google Scholar] [CrossRef]
- Abedi, G.; Janbabai, G.; Moosazadeh, M.; Farshidi, F.; Amiri, M.; Khosravi, A. Survival Rate of Breast Cancer in Iran: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2016, 17, 4615–4621. [Google Scholar]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef]
- Peairs, K.S.; Barone, B.B.; Snyder, C.F.; Yeh, H.-C.; Stein, K.B.; Derr, R.L.; Brancati, F.L.; Wolff, A.C. Diabetes Mellitus and Breast Cancer Outcomes: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2011, 29, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.Y. Achieving and Sustaining Universal Health Coverage: Fiscal Reform of the National Health Insurance in Taiwan. Appl. Health Econ. Health Policy 2017, 15, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.C.; Lai, W.W.; Chen, H.H.; Lee, J.C.; Lin, Y.J.; Hsiao, J.R.; Cheng, Y.M.; Shan, Y.S.; Su, W.C.; Wang, J.D. Cost effectiveness of cancer treatment in Taiwan. J. Formos. Med. Assoc. 2016, 115, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.L.; Mayer, D.K.; Chou, F.H.; Hsiao, K.Y. The Experience of Cancer Stigma in Taiwan: A Qualitative Study of Female Cancer Patients. Arch. Psychiatr. Nurs. 2016, 30, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Chatters, L.; Kao, T.S.; Saint-Arnault, D.; Northouse, L. Factors Affecting Quality of Life for Korean American Cancer Survivors: An Integrative Review. Oncol. Nurs. Forum 2016, 43, E132–E142. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).