Tracing Sulfate Source and Transformation in the Groundwater of the Linhuan Coal Mining Area, Huaibei Coalfield, China
Abstract
:1. Introduction
2. Materials and Methods
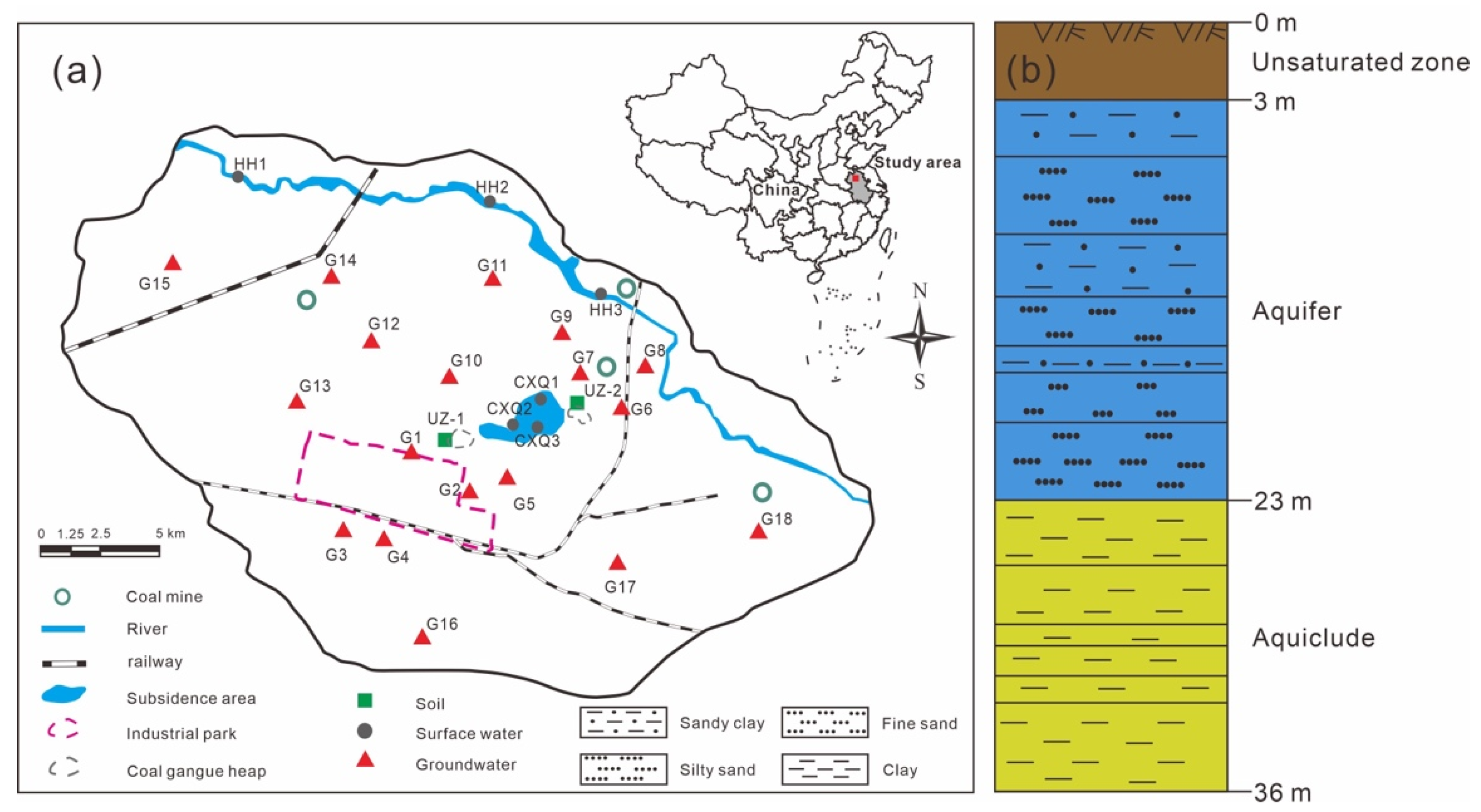
2.1. Study Sites
2.2. Sampling and Sample Preparation
2.3. Hydrochemical and Isotopic Analyses
3. Results and Discussion
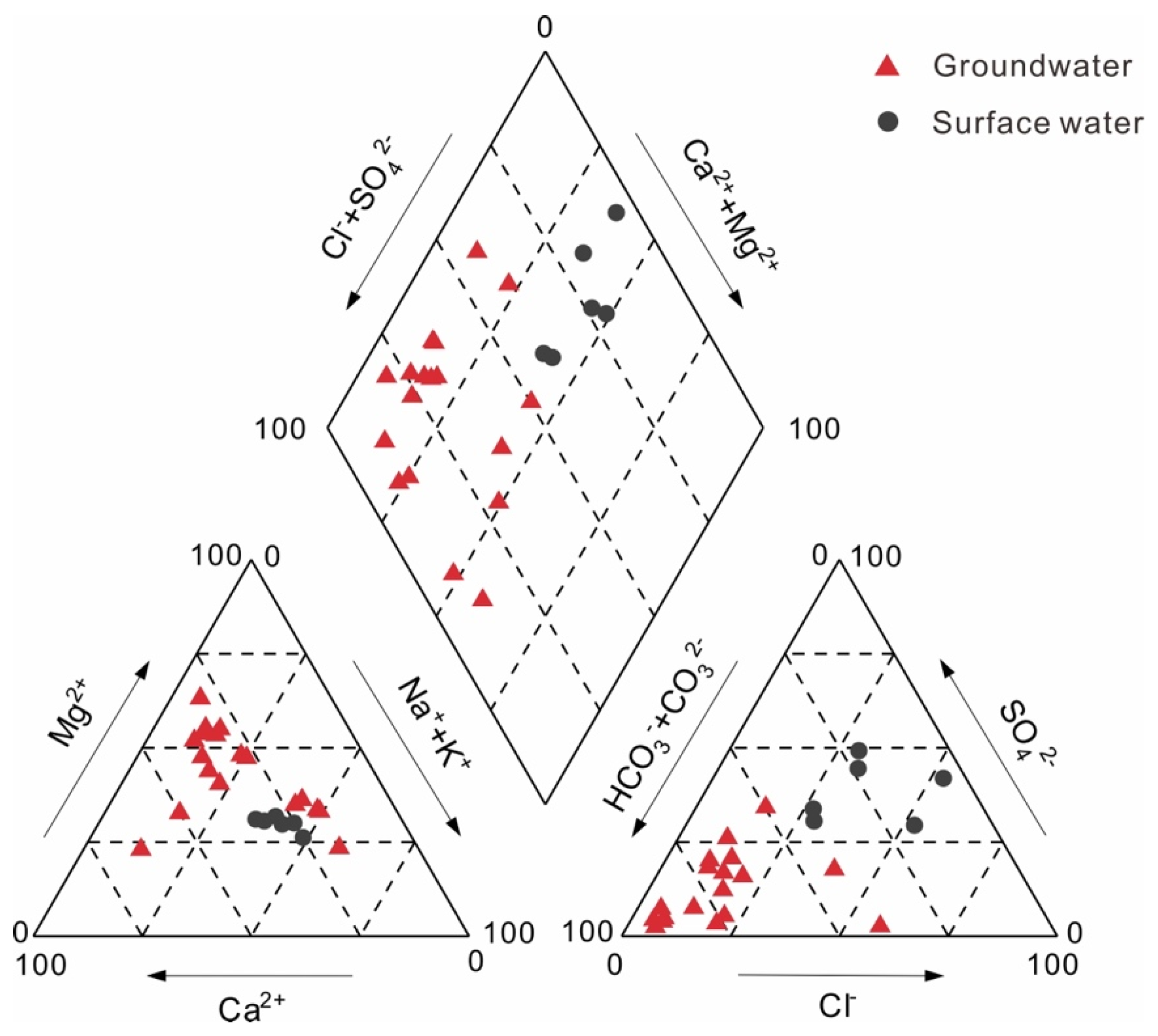
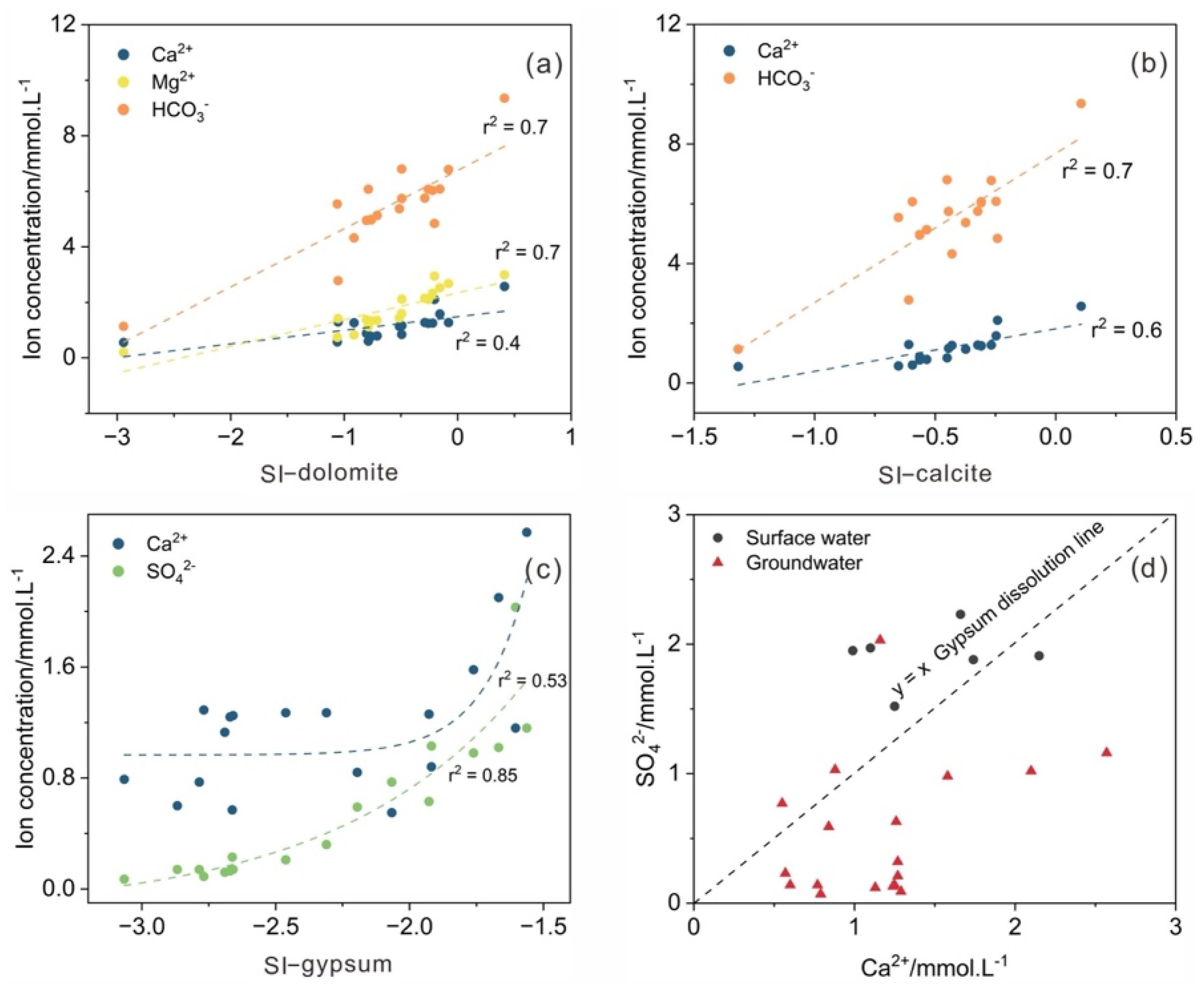
3.1. Hydrogeochemical Processes
3.2. Sulfate Distributions in the Surface Water, UZ and Groundwater
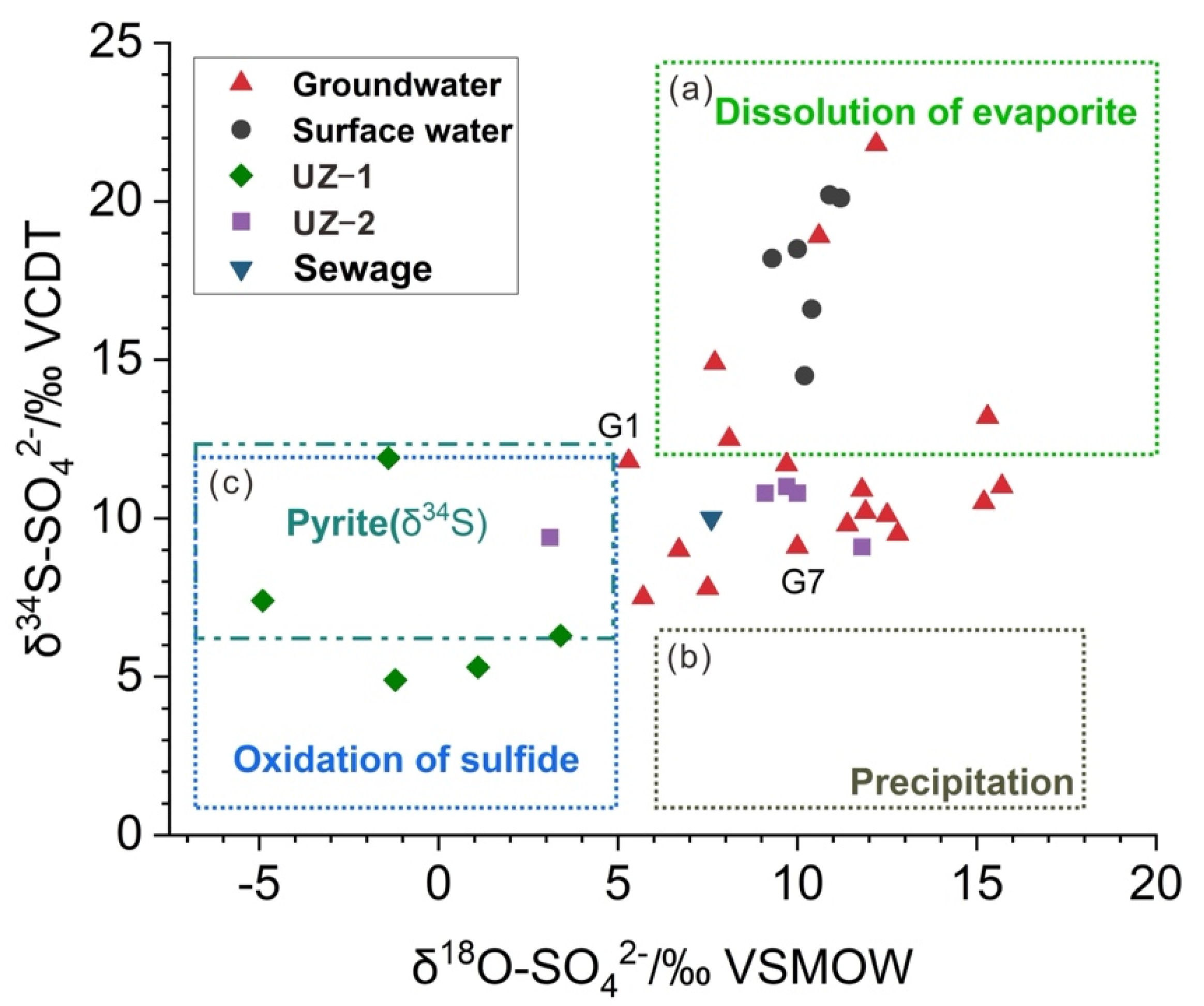
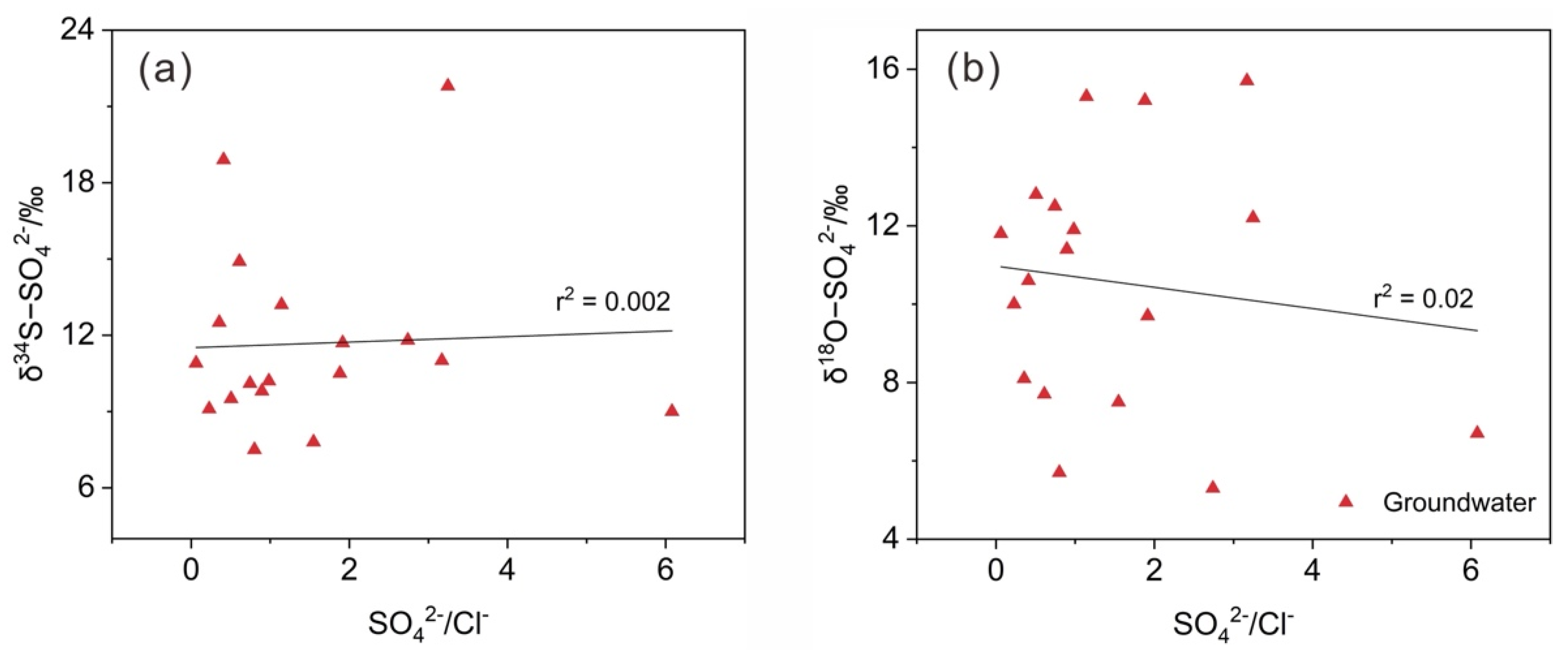
3.3. Sulfate Sources in the Groundwater
3.4. Sulfate Sources in the UZ
3.5. Biogeochemical Processes of Sulfate
4. Conclusions
- (1)
- The results of sulfate isotopes showed that SO42− in the shallow groundwater was mainly derived from the natural dissolution of evaporite, oxidation of sulfide and sewage.
- (2)
- The oxidation of sulfide was sulfate source to groundwater near the gangue heaps. The groundwater sulfate near the gangue heaps and industrial park compound contamination area was mainly affected by oxidation of sulfide and industrial and domestic sewage.
- (3)
- During the process, BSR did not affect the sulfate isotopic composition of groundwater.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.J.; Wang, J.Y.; He, H.T.; Mishra, V.; Li, Y.H.; Wang, J.X.; Zhao, C.L. Geochemistry of Carboniferous coals from the Laoyaogou mine, Ningwu coalfield, Shanxi Province, northern China: Emphasis on the enrichment of valuable elements. Fuel 2020, 279, 118414. [Google Scholar] [CrossRef]
- Fernando, W.A.M.; Ilankoon, I.M.S.K.; Syed, T.H.; Yellishetty, M. Challenges and opportunities in the removal of sulphate ions in contaminated mine water: A review. Miner. Eng. 2018, 117, 74–90. [Google Scholar] [CrossRef]
- Galhardi, J.A.; Bonotto, D.M. Hydrogeochemical features of surface water and groundwater contaminated with acid mine drainage (AMD) in coal mining areas: A case study in southern Brazil. Environ. Sci. Pollut. Res. 2016, 23, 18911–18927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Wen, S.M.; Wang, H.; Feng, Q.C. Sulfidization regulation of cuprite by pre-oxidation using sodium hypochlorite as an oxidant. Int. J. Min. Sci. Technol. 2021, 31, 1117–1128. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, S.M.; Feng, Q.C.; Wang, H. Enhanced sulfidization of azurite surfaces by ammonium phosphate and its effect on flotation. Int. J. Miner. Metall. Mater. 2022, 29, 1150–1160. [Google Scholar] [CrossRef]
- Relph, K.E.; Stevenson, E.I.; Turchyn, A.V.; Antler, G.; Bickle, M.J.; Baronas, J.J.; Darby, S.E.; Parsons, D.R.; Tipper, E.T. Partitioning riverine sulfate sources using oxygen and sulfur isotopes: Implications for carbon budgets of large rivers. Earth Planet Sci. Lett. 2021, 567, 116957. [Google Scholar] [CrossRef]
- Liu, J.K.; Han, G.L. Major ions and δ34S (SO4) in Jiulongjiang River water: Investigating the relationships between natural chemical weathering and human perturbations. Sci. Total Environ. 2020, 724, 138208. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.D.; Zhao, Z.Q.; Liu, C.Q. Using dual isotopic data to track the sources and behaviors of dissolved sulfate in the western North China plain. Appl. Geochem. 2015, 52, 43–56. [Google Scholar] [CrossRef]
- Kim, D.M.; Oh, Y.S.; Lee, J.S. δ34S and δ18O of sulfates and Zn/Cd ratios reveal the cause of soil and groundwater contamination in metalliferous mining areas. J. Geochem. Explor. 2020, 209, 106437. [Google Scholar] [CrossRef]
- Killingsworth, B.A.; Bao, H.M. Significant Human Impact on the Flux and delta S-34 of Sulfate from the Largest River in North America. Environ. Sci. Technol. 2015, 49, 4851–4860. [Google Scholar] [CrossRef]
- Gammons, C.H.; Brown, A.; Poulson, S.R.; Henderson, T.H. Using stable isotopes (S, O) of sulfate to track local contamination of the Madison karst aquifer, Montana, from abandoned coal mine drainage. Appl. Geochem. 2013, 31, 228–238. [Google Scholar] [CrossRef]
- Willacker, J.J.; Eagles-Smith, C.A.; Ackerman, J.T. Mercury bioaccumulation in estuarine fishes: Novel insights from sulfur stable isotopes. Environ. Sci. Technol. 2017, 51, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Ma, Z.M.; Xu, X.J. Research on the mechanism of sulfate pollution of groundwater in Jiaozuo area. Appl. Mech. Mater. 2014, 665, 436–439. [Google Scholar] [CrossRef]
- Sun, J.; Takahashi, Y.; Strosnider, W.H.J.; Kogure, T.; Wu, P.; Cao, X.X. Tracing and quantifying contributions of end members to karst water at a coalfield in southwest China. Chemosphere 2019, 234, 777–788. [Google Scholar] [CrossRef]
- Geurts, J.J.M.; Sarneel, J.M.; Willers, B.J.C.; Roelofs, J.G.M.; Verhoeven, J.T.A.; Lamers, L.P.M. Interacting effects of sulphate pollution, sulphide toxicity and eutrophication on vegetation development in fens: A mesocosm experiment. Environ. Pollut. 2009, 157, 2072–2081. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.L.; Su, J.; Yue, F.J.; Zhong, J.; Chen, S. Oxidation of pyrite and reducing nitrogen fertilizer enhanced the carbon cycle by driving terrestrial chemical weathering. Sci. Total Environ. 2021, 768, 144343. [Google Scholar] [CrossRef]
- Zhang, H.T.; Xu, G.Q.; Zhan, H.B.; Chen, X.Q.; Liu, M.C.; Wang, M.H. Identification of hydrogeochemical processes and transport paths of a multi-aquifer system in closed mining regions. J. Hydrol. 2020, 589, 125344. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Jiang, Y.J.; Yuan, D.X.; Cui, J.; Li, Y.; Yang, J.; Cao, M. Source and flux of anthropogenically enhanced dissolved inorganic carbon: A comparative study of urban and forest karst catchments in Southwest China. Sci. Total Environ. 2020, 725, 138255. [Google Scholar] [CrossRef]
- Cao, X.X.; Wu, P.; Zhou, S.Q.; Sun, J.; Han, Z.W. Tracing the origin and geochemical processes of dissolved sulphate in a karst-dominated wetland catchment using stable isotope indicators. J. Hydrol. 2018, 562, 210–222. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.W.; Hou, X.W.; Lin, M.L.; Ren, X.X.; Li, J.; Zhang, M.; Zheng, J. Multi-isotopes and hydrochemistry combined to reveal the major factors affecting Carboniferous groundwater evolution in the Huaibei coalfield, North China. Sci. Total Environ. 2021, 791, 148420. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, L.G.; Dong, X.L.; Jiang, C.L.; Wei, X.P. Sources and mixing of sulfate contamination in the water environment of a typical coal mining city, China: Evidence from stable isotope characteristics. Environ. Geochem. Health 2020, 42, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Banks, D.; Boyce, A.J.; Burnside, N.M.; Janson, E.; Roqueñi Gutierrez, N. On the common occurrence of sulphate with elevated δ34S in European mine waters: Sulphides, evaporites or seawater? Int. J. Coal Geol. 2020, 232, 103619. [Google Scholar] [CrossRef]
- Spalding, R.F.; Hirsh, A.J.; Exner, M.E.; Little, N.A.; Kloppenborg, K.L. Applicability of the dual isotopes δ15N and δ18O to identify nitrate in groundwater beneath irrigated cropland. J. Contam. Hydrol. 2019, 220, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Seibert, S.; Descourvieres, C.; Skrzypek, G.; Deng, H.L.; Prommer, H. Model-based analysis of δ34S signatures to trace sedimentary pyrite oxidation during managed aquifer recharge in a heterogeneous aquifer. J. Hydrol. 2017, 548, 368–381. [Google Scholar] [CrossRef]
- Zhou, J.W.; Zhang, Y.P.; Zhou, A.G.; Liu, C.F.; Cai, H.S.; Liu, Y.D. Application of hydrochemistry and stable isotopes (δ34S, δ18O and δ37Cl) to trace natural and anthropogenic influences on the quality of groundwater in the piedmont region, Shijiazhuang, China. Appl. Geochem. 2016, 71, 63–72. [Google Scholar] [CrossRef]
- Moya, C.E.; Raiber, M.; Taulis, M.; Cox, M.E. Using environmental isotopes and dissolved methane concentrations to constrain hydrochemical processes and inter-aquifer mixing in the Galilee and Eromanga Basins, Great Artesian Basin, Australia. J. Hydrol. 2016, 539, 304–318. [Google Scholar] [CrossRef]
- Mangalo, M.; Meckenstock, R.U.; Stichler, W.; Einsiedl, F. Stable isotope fractionation during bacterial sulfate reduction is controlled by reoxidation of intermediates. Geochim. Cosmochim. Acta 2007, 71, 4161–4171. [Google Scholar] [CrossRef]
- Tuttle, M.L.W.; Breit, G.N.; Cozzarelli, I.M. Processes affecting δ34S and δ18O values of dissolved sulfate in alluvium along the Canadian River, central Oklahoma, USA. Chem. Geol. 2009, 265, 455–467. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Galuszka, A.; Dolęgowska, S. Stable isotope geochemistry of acid mine drainage from the Wisniowka area (south-central Poland). Appl. Geochem. 2018, 95, 45–56. [Google Scholar] [CrossRef]
- Feng, J.L.; Chen, F.; Hu, H.P. Isotopic study of the source and cycle of sulfur in the Yamdrok Tso basin, Southern Tibet, China. Appl. Geochem. 2017, 85, 61–72. [Google Scholar] [CrossRef]
- Pu, J.B.; Yuan, D.X.; Zhang, C.; Zhao, H.P. Hydrogeochemistry and possible sulfate sources in karst groundwater in Chongqing, China. Environ. Earth Sci. 2013, 68, 159–168. [Google Scholar] [CrossRef]
- Lu, Y.W.; Li, H.J.; Si, B.C.; Li, M. Chloride tracer of the loess unsaturated zone under sub-humid region: A potential proxy recording high-resolution hydroclimate. Sci. Total Environ. 2020, 710, 135511. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.E.C.; Edmunds, W.M. Unsaturated zone hydrostratigraphies: A novel archive of past climates in dryland continental regions. Earth Sci. Rev. 2016, 157, 121–144. [Google Scholar] [CrossRef]
- Lin, H. Earth’s Critical Zone and hydropedology: Concepts, characteristics, and advances. Hydrol. Earth Syst. Sci. 2010, 14, 25–45. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Huang, T.M.; Zhang, F.; Li, Z.B.; Ma, B.Q.; Li, Y.M.; Pang, Z.H. Origin of sulfate in the unsaturated zone and groundwater of a loess aquifer. Hydrol. Process 2021, 35, 14166. [Google Scholar] [CrossRef]
- Ma, B.Q.; Huang, T.M.; Li, J.; Li, Z.B.; Long, Y.; Zhang, F.; Pang, Z.H. Tracing nitrate source and transformation in a semiarid loess aquifer with the thick unsaturated zone. Catena 2021, 198, 105045. [Google Scholar] [CrossRef]
- Huang, T.M.; Ma, B.Q.; Pang, Z.H.; Li, Z.; Li, Z.B.; Long, Y. How does precipitation recharge groundwater in loess aquifers? Evidence from multiple environmental tracers. J. Hydrol. 2020, 583, 124532. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Wang, H.W.; Lu, C. Tracing sulfate origin and transformation in an area with multiple sources of pollution in northern China by using environmental isotopes and Bayesian isotope mixing model. Environ. Pollut. 2020, 265, 115105. [Google Scholar] [CrossRef]
- Chen, L.W.; Xie, W.P.; Feng, X.Q.; Zhang, N.Q.; Yin, X.X. Formation of hydrochemical composition and spatio-temporal evolution mechanism under mining-induced disturbance in the Linhuan coal-mining area. Arab. J. Geosci. 2017, 10, 57. [Google Scholar] [CrossRef]
- Zheng, L.G.; Chen, X.; Dong, X.L.; Wei, X.P.; Jiang, C.L.; Tang, Q. Using δ34S-SO4 and δ18O-SO4 to trace the sources of sulfate in different types of surface water from the Linhuan coal-mining subsidence area of Huaibei, China. Ecotox. Environ. Saf. 2019, 181, 231–240. [Google Scholar] [CrossRef]
- Li, Q.G.; Ju, Y.W.; Lu, W.Q.; Wang, G.C.; Neupane, B.; Sun, Y. Water-rock interaction and methanogenesis in formation water in the southeast Huaibei coalfield, China. Mar. Petrol. Geol. 2016, 77, 435–447. [Google Scholar] [CrossRef]
- Yang, C.F.; Lu, G.N.; Xie, Y.Y.; Guo, L.; Chen, M.Q.; Ge, L.Y.; Dang, Z. Sulfate migration and transformation characteristics in paddy soil profile affected by acid mine drainage. Environ. Res. 2021, 200, 111732. [Google Scholar] [CrossRef] [PubMed]
- Rinder, T.; Dietzel, M.; Stammeier, J.A.; Leis, A.; Bedoya-González, D.; Hilberg, S. Geochemistry of coal mine drainage, groundwater, and brines from the Ibbenbüren mine, Germany: A coupled elemental-isotopic approach. Appl. Geochem. 2020, 121, 104693. [Google Scholar] [CrossRef]
- Sahoo, S.; Khaoash, S. Impact assessment of coal mining on groundwater chemistry and its quality from Brajrajnagar coal mining area using indexing models. J. Geochem. Explor. 2020, 215, 106559. [Google Scholar] [CrossRef]
- Chen, S.; Gui, H.R. Chemical characteristics of river water in Huaibei Coalfield, China and its significance. Earth. Environ. 2016, 44, 414–421. (In Chinese) [Google Scholar] [CrossRef]
- Yang, S.; Wang, Q.X.; Zhao, J.; Wang, Z.C.; Xu, K.; Zhang, X.X.; Wei, X.P.; Dong, X.L.; Zheng, L.G. Distribution characteristics of soil sulfate and its relationship with environmental factors in Linhuan mining area. J. Anhui Agric. Univ. 2021, 48, 968–974. (In Chinese) [Google Scholar] [CrossRef]
- Li, S.L.; Xu, S.; Wang, T.J.; Yue, F.J.; Peng, T.; Zhong, J.; Wang, L.C.; Chen, J.A.; Wang, S.J.; Chen, X.; et al. Effects of agricultural activities coupled with karst structures on riverine biogeochemical cycles and environmental quality in the karst region. Agric. Ecosyst. Environ. 2020, 303, 107120. [Google Scholar] [CrossRef]
- Jambrina-Enríquez, M.; Recio, C.; Armenteros, I. Biogeochemical characterization of a Mediterranean shallow lake using stable isotopes: Laguna del Cristo (NW Iberian Peninsula). Environ. Earth. Sci. 2018, 77, 49. [Google Scholar] [CrossRef]
- Lamban, L.J.; Jodar, J.; Custodio, E.; Soler, A.; Sapriza, G.; Soto, R. Isotopic and hydrogeochemical characterization of high-altitude karst aquifers in complex geological settings. The Ordesa and Monte Perdido National Park (Northern Spain) case study. Sci. Total Environ. 2015, 506, 466–479. [Google Scholar] [CrossRef] [Green Version]
- Ren, K.; Pan, X.D.; Zeng, J.; Jiao, Y.J. Distribution and source identification of dissolved sulfate by dual isotopes in waters of the Babu subterranean river basin, SW China. J. Radioanal. Nucl. Chem. 2017, 312, 317–328. [Google Scholar] [CrossRef]
- Kaown, D.; Koh, D.C.; Mayer, B.; Lee, K.K. Identification of nitrate and sulfate sources in groundwater using dual stable isotope approaches for an agricultural area with different land use (Chuncheon, mid-eastern Korea). Agric. Ecosyst. Environ. 2009, 132, 223–231. [Google Scholar] [CrossRef]
- Hosono, T.; Delinom, R.; Nakano, T.; Kagabu, M.; Shimada, J. Evolution model of δ34S and δ18O in dissolved sulfate in volcanic fan aquifers from recharge to coastal zone and through the Jakarta urban area, Indonesia. Sci. Total Environ. 2011, 409, 2541–2554. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gan, Y.; Zhou, A.; Liu, Y. Relationship between water discharge and sulfate sources of the Yangtze River inferred from seasonal variations of sulfur and oxygen isotopic compositions. J. Geochem. Explor. 2015, 153, 30–39. [Google Scholar] [CrossRef]
- Hosono, T.; Wang, C.H.; Umezawa, Y.; Nakano, T.; Onodera, S.; Nagata, T.; Yoshimizu, C.; Tayasu, I.; Taniguchi, M. Multiple isotope (H, O, N, S and Sr) approach elucidates complex pollution causes in the shallow groundwaters of the Taipei urban area. J. Hydrol. 2011, 397, 23–36. [Google Scholar] [CrossRef]
- Einsiedl, F.; Mayer, B. Sources and processes affecting sulfate in a karstic groundwater system of the Franconian Alb, southern Germany. Environ. Sci. Technol. 2005, 39, 7118–7125. [Google Scholar] [CrossRef]
- Li, X.; Gan, Y.; Zhou, A.; Liu, Y.; Wang, D. Hydrological controls on the sources of dissolved sulfate in the Heihe River, a large inland river in the arid north-western China, inferred from S and O isotopes. Appl. Geochem. 2013, 35, 99–109. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q. Research advances in identifying sulfate contamination sources of water environment by using stable isotopes. Int. J. Environ. Res. Publ. Health 2019, 16, 1914. [Google Scholar] [CrossRef] [Green Version]
- Otero, N.; Soler, A.; Canals, A. Controls of δ34S and δ18O in dissolved sulphate: Learning from a detailed survey in the Llobregat River (Spain). Appl. Geochem. 2008, 23, 1166–1185. [Google Scholar] [CrossRef]
- Bottrell, S.; Tellam, J.; Bartlett, R.; Hughes, A. Isotopic composition of sulfate as a tracer of natural and anthropogenic influences on groundwater geochemistry in an urban sandstone aquifer, Birmingham, UK. Appl. Geochem. 2008, 23, 2382–2394. [Google Scholar] [CrossRef]
- Samborska, K.; Halas, S.; Bottrell, S.H. Sources and impact of sulphate on groundwaters of Triassic carbonate aquifers, Upper Silesia, Poland. J. Hydrol. 2013, 486, 136–150. [Google Scholar] [CrossRef]
- Gunn, J.; Bottrell, S.H.; Lowe, D.J.; Worthington, S.R.H. Deep groundwater flow and geochemical processes in limestone aquifers: Evidence from thermal waters in Derbyshire, England, UK. Hydrogeol. J. 2006, 14, 868–881. [Google Scholar] [CrossRef]
- Schulte, P.; van Geldern, R.; Freitag, H.; Karim, A.; Negrel, P.; Petelet-Giraud, E.; Probst, A.; Probst, J.L.; Telmer, K.; Veizer, J.; et al. Applications of stable water and carbon isotopes in watershed research: Weathering, carbon cycling, and water balances. Earth Sci. Rev. 2011, 109, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Gammons, C.H.; Duaime, T.E.; Parker, S.R.; Poulson, S.R.; Kennelly, P. Geochemistry and stable isotope investigation of acid mine drainage associated with abandoned coal mines in central Montana, USA. Chem. Geol. 2010, 269, 100–112. [Google Scholar] [CrossRef]
- Sun, J.; Kobayashi, T.; Strosnider, W.H.J.; Wu, P. Stable sulfur and oxygen isotopes as geochemical tracers of sulfate in karst waters. J. Hydrol. 2017, 551, 245–252. [Google Scholar] [CrossRef]
- Dold, B.; Spangenberg, J.E. Sulfur speciation and stable isotope trends of water-soluble sulfates in mine tailings profiles. Environ. Sci. Technol. 2005, 39, 5650–5656. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Yun, S.T.; Yoon, S.; Mayer, B. Signature of oxygen and sulfur isotopes of sulfate in ground and surface water reflecting enhanced sulfide oxidation in mine areas. Appl. Geochem. 2019, 100, 143–151. [Google Scholar] [CrossRef]
- Jiang, C.L.; Zhao, Q.; Zheng, L.G.; Chen, X.; Li, C.; Ren, M.X. Distribution, source and health risk assessment based on the Monte Carlo method of heavy metals in shallow groundwater in an area affected by mining activities, China. Ecotox. Environ. Saf. 2021, 224, 112679. [Google Scholar] [CrossRef]
- Kong, L.J.; Jiang, C.L.; Zheng, L.G.; Cheng, H.; Ren, M.X.; Min, F.H.; Fang, L.B. Characters of hydrochemistry and their influenced factors of different waters in the Linhuan coal minina subsidence area of Huaibei City. J. Lake Sci. 2017, 29, 1158–1167. (In Chinese) [Google Scholar] [CrossRef] [Green Version]
- Antler, G.; Turchyn, A.; Rennie, V.; Herut, B.; Sivan, O. Coupled sulfur and oxygen isotope insight into bacterial sulfate reduction in the natural environment. Geochim. Cosmochim. Acta 2013, 118, 98–117. [Google Scholar] [CrossRef] [Green Version]
- Brunner, B.; Bernasconi, S.M. A revised isotope fractionation model for dissimilatory sulfate reduction in sulfate reducing bacteria. Geochim. Cosmochim. Acta 2005, 69, 4759–4771. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, C. A preliminary study on sulfate reduction bacteria behaviors in groundwater by sulfur and carbon isotopes: A case study in Jiaozuo City, China. Ecotoxicology 2014, 23, 2014–2024. [Google Scholar] [CrossRef]
- Jin, L.; Siegel, D.I.; Lautz, L.K.; Mitchell, M.J.; Dahms, D.E.; Mayer, B. Calcite precipitation driven by the common ion effect during groundwater-surface-water mixing: A potentially common process in streams with geologic settings containing gypsum. Geol. Soc. Am. Bull. 2010, 122, 1027–1038. [Google Scholar] [CrossRef]
- Knoller, K.; Fauville, A.; Mayer, B.; Strauch, G.; Friese, K.; Veizer, J. Sulfur cycling in an acid mining lake and its vicinity in Lusatia, Germany. Chem. Geol. 2004, 204, 303–323. [Google Scholar] [CrossRef]
- Aharon, P.; Fu, B. Sulfur and oxygen isotopes of coeval sulfate-sulfide in pore fluids of cold seep sediments with sharp redox gradients. Chem. Geol. 2003, 195, 201–218. [Google Scholar] [CrossRef]
- Christensen, T.H.; Bjerg, P.L.; Banwart, S.A.; Jakobsen, R.; Heron, G.; Albrechtsen, H.J. Characterization of redox conditions in groundwater contaminant plumes. J. Contam. Hydrol. 2000, 45, 165–241. [Google Scholar] [CrossRef]


| Type | pH | Ec | DO | K+ | Na+ | Ca2+ | Mg2+ | Fe | Cl− | SO42− | HCO3− | TDS | SI−c * | SI−d * | SI−g * | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| us/cm | mg/L | |||||||||||||||
| Min | 7.64 | 1238.0 | 9.21 | 2.83 | 79.01 | 39.45 | 24.71 | 0.18 | 83.05 | 145.87 | 33.53 | 475.58 | −1.30 | −2.48 | −1.64 | |
| Max | 7.86 | 1928.0 | 11.26 | 13.37 | 108.41 | 85.82 | 47.88 | 23.30 | 239.08 | 213.68 | 304.43 | 735.35 | −0.33 | −0.48 | −1.40 | |
| Surface water | Mean | 7.74 | 1582.67 | 10.54 | 5.84 | 92.08 | 59.21 | 36.86 | 6.20 | 138.01 | 183.54 | 154.01 | 592.55 | −0.78 | −1.40 | −1.52 |
| SD | 0.07 | 297.40 | 0.76 | 3.45 | 10.01 | 16.17 | 8.36 | 8.89 | 59.34 | 19.94 | 86.09 | 96.42 | 0.32 | 0.65 | 0.10 | |
| CV | 0.01 | 0.19 | 0.07 | 0.59 | 0.11 | 0.27 | 0.23 | 1.43 | 0.43 | 0.11 | 0.56 | 0.16 | −0.41 | −0.46 | −0.07 | |
| Min | 7.76 | 590.00 | 1.90 | 0.31 | 4.17 | 22.14 | 4.80 | 0.02 | 8.95 | 6.61 | 68.93 | 93.04 | −1.32 | −2.94 | −3.07 | |
| Max | 8.31 | 1604.00 | 7.52 | 2.05 | 235.75 | 102.73 | 71.79 | 31.18 | 159.83 | 195.13 | 570.35 | 701.62 | 0.11 | 0.41 | −1.56 | |
| Groundwater | Mean | 7.94 | 887.94 | 4.35 | 0.71 | 44.46 | 46.94 | 41.55 | 3.08 | 47.69 | 52.33 | 331.12 | 399.24 | −0.45 | −0.63 | −2.31 |
| SD | 0.14 | 292.68 | 1.73 | 0.53 | 31.82 | 20.20 | 18.35 | 8.48 | 41.59 | 49.76 | 100.15 | 141.73 | 0.28 | 0.67 | 0.47 | |
| CV | 0.02 | 0.33 | 0.40 | 0.74 | 0.72 | 0.43 | 0.44 | 2.75 | 0.87 | 1.00 | 0.30 | 0.36 | −0.61 | −1.07 | −0.20 |
| Type | Sample No./Depth (m) | δ34S−SO42− (‰) | δ18O−SO42− (‰) | SO42− (mg/L) |
|---|---|---|---|---|
| HH1 | 14.5 | 10.2 | 213.7 | |
| HH2 | 18.5 | 10 | 180.8 | |
| HH3 | 18.2 | 9.3 | 183.8 | |
| Surface water | CXQ1 | 16.6 | 10.4 | 145.9 |
| CXQ2 | 20.1 | 11.2 | 189.4 | |
| CXQ3 | 20.2 | 10.9 | 187.7 | |
| Mean ± SD | 18.0 ± 2.0 | 10.3 ± 0.6 | 183.5 ± 19.9 | |
| CV | 0.1 | 0.1 | 0.1 | |
| 0–40 | 7.4 | −1.2 | 18.3 | |
| 40–80 | 4.9 | −4.9 | 26.4 | |
| 80–120 | 5.3 | −1.4 | 30.5 | |
| UZ−1 | 120–160 | 6.3 | 3.4 | 44.4 |
| 160–200 | 11.9 | 1.1 | 57.4 | |
| Mean ± SD | 7.2 ± 2.5 | −0.6 ± 2.8 | 35.4 ± 13.9 | |
| CV | 0.4 | −4.6 | 0.4 | |
| 0–40 | 10.8 | 9.1 | 54.7 | |
| 40–80 | 9.4 | 3.1 | 71.2 | |
| 80–120 | 9.1 | 11.8 | 65.1 | |
| UZ−2 | 120–160 | 11.0 | 9.7 | 76.5 |
| 160–200 | 10.8 | 10.0 | 80.8 | |
| Mean ± SD | 10.2 ± 0.8 | 8.7 ± 3.0 | 69.6 ± 9.1 | |
| CV | 0.1 | 0.3 | 0.4 | |
| G1 | 11.8 | 5.3 | 60.0 | |
| G2 | 11 | 15.7 | 98.9 | |
| G3 | 14.9 | 7.7 | 97.7 | |
| G4 | 9 | 6.7 | 13.2 | |
| G5 | 10.2 | 11.9 | 56.7 | |
| G6 | 21.8 | 12.2 | 195.1 | |
| G7 | 9.1 | 10.0 | 94.3 | |
| G8 | 12.5 | 8.1 | 20.3 | |
| G9 | 11.7 | 9.7 | 12.8 | |
| Groundwater | G10 | 10.1 | 12.5 | 11.73 |
| G11 | 18.9 | 10.6 | 74.2 | |
| G12 | 9.8 | 11.4 | 13.0 | |
| G13 | 7.8 | 7.5 | 111.5 | |
| G14 | 10.9 | 11.8 | 9.0 | |
| G15 | 7.5 | 5.7 | 30.7 | |
| G16 | 13.2 | 15.3 | 13.9 | |
| G17 | 9.5 | 12.8 | 6.6 | |
| G18 | 10.5 | 15.2 | 22.4 | |
| Mean ± SD | 11.7 ± 3.6 | 10.6 ± 3.1 | 52 ± 49.8 | |
| CV | 0.3 | 0.3 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Jiang, C.; Li, C.; Zheng, L. Tracing Sulfate Source and Transformation in the Groundwater of the Linhuan Coal Mining Area, Huaibei Coalfield, China. Int. J. Environ. Res. Public Health 2022, 19, 14434. https://doi.org/10.3390/ijerph192114434
Cheng L, Jiang C, Li C, Zheng L. Tracing Sulfate Source and Transformation in the Groundwater of the Linhuan Coal Mining Area, Huaibei Coalfield, China. International Journal of Environmental Research and Public Health. 2022; 19(21):14434. https://doi.org/10.3390/ijerph192114434
Chicago/Turabian StyleCheng, Lili, Chunlu Jiang, Chang Li, and Liugen Zheng. 2022. "Tracing Sulfate Source and Transformation in the Groundwater of the Linhuan Coal Mining Area, Huaibei Coalfield, China" International Journal of Environmental Research and Public Health 19, no. 21: 14434. https://doi.org/10.3390/ijerph192114434






