An Evaluation of Exposure to 18 Toxic and/or Essential Trace Elements Exposure in Maternal and Cord Plasma during Pregnancy at Advanced Maternal Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Sample Collection and Laboratory Analyses
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
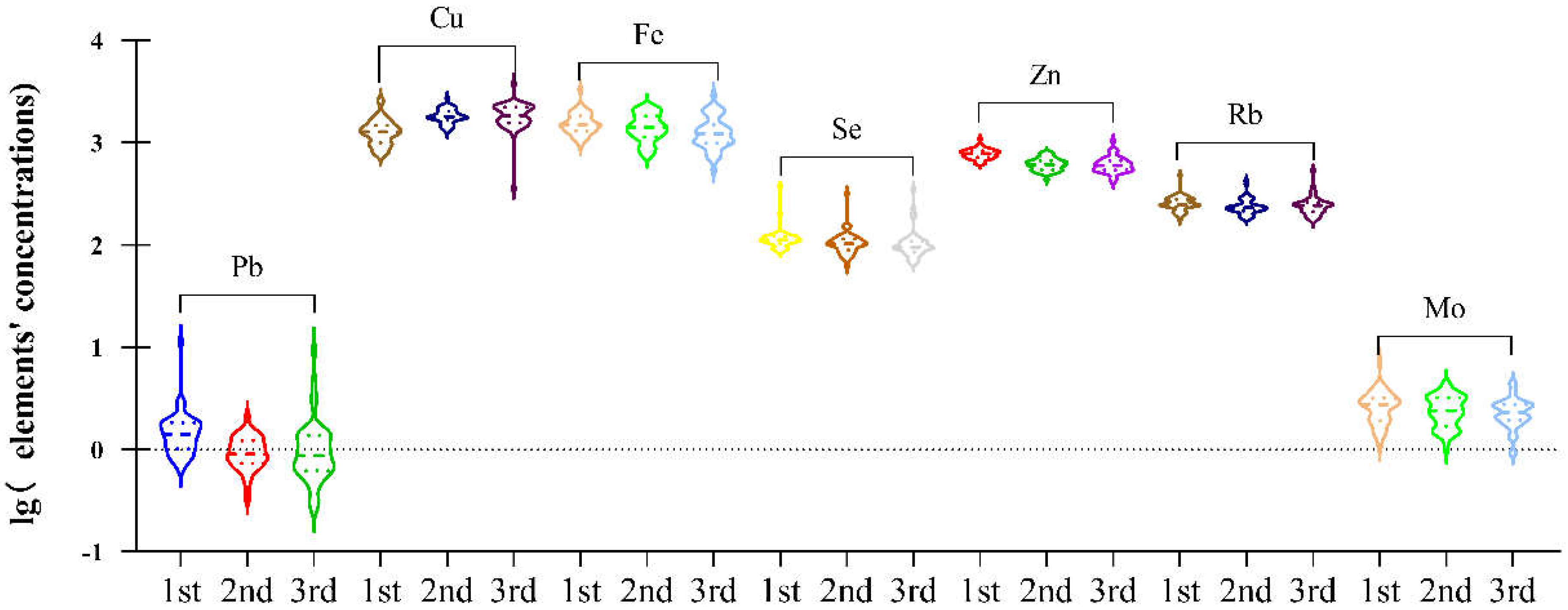
3.2. Concentrations of Elements in Maternal Plasma during Pregnancy
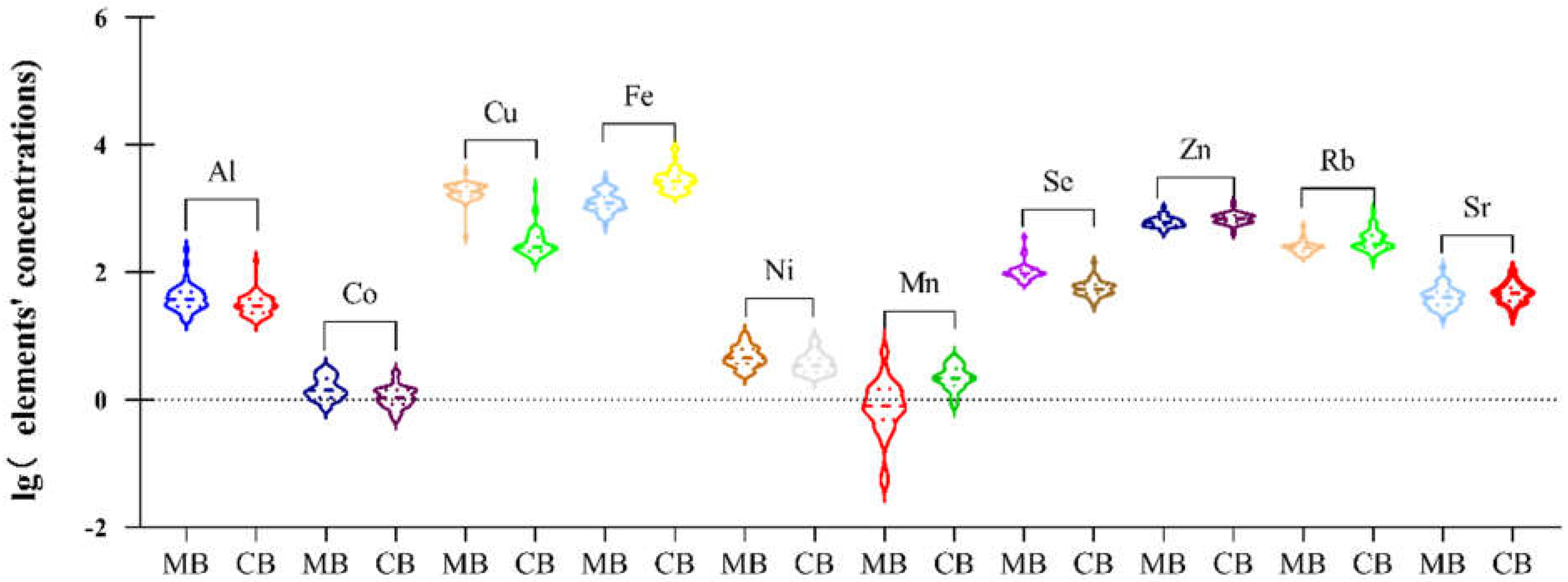
3.3. Concentrations of Elements in Cord Plasma and Maternal Plasma
3.4. Spearman Correlations between 18 Elements in Maternal Plasma at 3rd Trimester
3.5. Spearman Correlations between 18 Elements in Cord Plasma
3.6. Correlation Analysis of Trace Elements in Maternal Blood (MB) at 3rd Trimester and in Cord Blood (CB)
4. Discussion
4.1. Summary of the Study
4.2. Concentration of Trace Elements during Pregnancy
4.3. Concentration of Trace Elements in Paired Maternal and Cord Plasma
4.4. Correlation between Paired Samples of Trace Elements
4.5. Correlation between Two Element Concentrations
4.6. AMA Pregnancy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMA | advanced maternal age |
| LOD | limit of detection |
References
- Al-Saleh, I.; Shinwari, N.; Mashhour, A.; Rabah, A. Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. Int. J. Hyg. Environ. Health 2014, 217, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, W.; Wang, D.; Jin, Y.; Chen, X.; Xu, Y. The effects of prenatal exposure to low-level cadmium, lead and selenium on birth outcomes. Chemosphere 2014, 108, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Shirai, S.; Suzuki, Y.; Yoshinaga, J.; Mizumoto, Y. Maternal exposure to low-level heavy metals during pregnancy and birth size. J. Environ. Sci. Health Part A 2010, 45, 1468–1474. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [Green Version]
- King, J.C. The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. J. Nutr. 2003, 133, 1732S–1736S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fall, C.H.D.; Yajnik, C.S.; Rao, S.; Davies, A.A.; Brown, N.; Farrant, H.J.W. Micronutrients and fetal growth. J. Nutr. 2003, 133, 1747S–1756S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumbold, A.; Duley, L.; Crowther, C.A.; Haslam, R.R. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst. Rev. 2008, 2008, CD004227. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Dechend, R.; Pijnenborg, R. Learning from the placenta: Acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension 2010, 56, 1026–1034. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.; Nieboer, E.; Sandanger, T.M.; Wilsgaard, T.; Thomassen, Y.; Veyhe, A.S.; Odland, J.Ø. Changes in maternal blood concentrations of selected essential and toxic elements during and after pregnancy. J. Environ. Monit. 2011, 13, 2143–2152. [Google Scholar] [CrossRef]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Kontic-Vucinic, O.; Sulovic, N.; Radunovic, N. Micronutrients in women’s reproductive health: II. Minerals and trace elements. Int. J. Fertil Womens Med. 2006, 51, 116–124. [Google Scholar] [PubMed]
- Needham, L.L.; Grandjean, P.; Heinzow, B.; Jørgensen, P.J.; Nielsen, F.; Patterson, D.G.; Sjödin, A.; Turner, W.E.; Weihe, P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ. Sci. Technol. 2011, 45, 1121–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkowitz, G.S.; Skovron, M.L.; Lapinski, R.H.; Berkowitz, R.L. Delayed childbearing and the outcome of pregnancy. N. Engl. J. Med. 1990, 322, 659–664. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, Y.; Li, X.; Jiang, F.; Zhang, Y.; Ma, J.; Song, Y.; Ma, J.; Fu, W.; Pang, R.; et al. A Lancet Commission on 70 years of women’s reproductive, maternal, newborn, child, and adolescent health in China. Lancet 2021, 397, 2497–2536. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, L.; Jin, H.; Yu, J.; Li, X.; Fu, H.; Wen, L.; Qi, H.; Tong, C.; Saffery, R.; et al. Advanced maternal age causes premature placental senescence and malformation via dysregulated α-Klotho expression in trophoblasts. Aging Cell 2021, 20, e13417. [Google Scholar] [CrossRef]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef]
- Betts, K.S. CDC updates guidelines for children’s lead exposure. Environ. Health Perspect. 2012, 120, a268. [Google Scholar] [CrossRef] [Green Version]
- Niedzwiecki, M.M.; Eggers, S.; Joshi, A.; Dolios, G.; Cantoral, A.; Lamadrid-Figueroa, H.; Amarasiriwardena, C.; Téllez-Rojo, M.M.; Wright, R.O.; Petrick, L. Lead exposure and serum metabolite profiles in pregnant women in Mexico City. Environ. Health 2021, 20, 125. [Google Scholar] [CrossRef]
- Gulson, B.L.; Jameson, C.W.; Mahaffey, K.R.; Mizon, K.J.; Korsch, M.J.; Vimpani, G. Pregnancy increases mobilization of lead from maternal skeleton. J. Lab. Clin. Med. 1997, 130, 51–62. [Google Scholar] [CrossRef]
- Téllez-Rojo, M.M.; Hernández-Avila, M.; Lamadrid-Figueroa, H.; Smith, D.; Hernández-Cadena, L.; Mercado, A.; Aro, A.; Schwartz, J.; Hu, H. Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. Am. J. Epidemiol. 2004, 160, 668–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomaa, A.; Hu, H.; Bellinger, D.; Schwartz, J.; Tsaih, S.-W.; Gonzalez-Cossio, T.; Schnaas, L.; Peterson, K.; Aro, A.; Hernandez-Avila, M. Maternal bone lead as an independent risk factor for fetal neurotoxicity: A prospective study. Pediatrics 2002, 110, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Shinwari, N.; Mashhour, A.; Mohamed, G.E.D.; Rabah, A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int. J. Hyg. Environ. Health 2011, 214, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.; Mizon, K.; Korsch, M.; Taylor, A. Revisiting mobilisation of skeletal lead during pregnancy based on monthly sampling and cord/maternal blood lead relationships confirm placental transfer of lead. Arch. Toxicol. 2016, 90, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Martínez, M.Á.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part A. concentrations in maternal blood, urine and cord blood. Environ. Res. 2019, 177, 108599. [Google Scholar] [CrossRef]
- Mariath, A.B.; Bergamaschi, D.P.; Rondó, P.H.C.; Tanaka, A.C.D.A.; Hinnig, P.d.F.; Abbade, J.F.; Diniz, S.G. The possible role of selenium status in adverse pregnancy outcomes. Br. J. Nutr. 2011, 105, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Searle, E.; Kelly, L.; Johnsen, S.; Bodman-Smith, K.; Bath, S.C.; Mao, J.; Redman, C.W.G. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: A randomised, controlled pilot trial. Br. J. Nutr. 2014, 112, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Choi, R.; Sun, J.; Yoo, H.; Kim, S.; Cho, Y.Y.; Kim, H.J.; Kim, S.W.; Chung, J.H.; Oh, S.-Y.; Lee, S.-Y. A Prospective Study of Serum Trace Elements in Healthy Korean Pregnant Women. Nutrients 2016, 8, 749. [Google Scholar] [CrossRef] [Green Version]
- Duck, K.A.; Connor, J.R. Iron uptake and transport across physiological barriers. Biometals 2016, 29, 573–591. [Google Scholar] [CrossRef] [Green Version]
- Sangkhae, V.; Nemeth, E. Placental iron transport: The mechanism and regulatory circuits. Free Radic. Biol. Med. 2019, 133, 254–261. [Google Scholar] [CrossRef]
- Kantola, M.; Purkunen, R.; Kröger, P.; Tooming, A.; Juravskaja, J.; Pasanen, M.; Saarikoski, S.; Vartiainen, T. Accumulation of cadmium, zinc, and copper in maternal blood and developmental placental tissue: Differences between Finland, Estonia, and St. Petersburg. Environ. Res. 2000, 83, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Giroux, E.; Schechter, P.J.; Schoun, J. Diminished albumin binding of zinc in serum of pregnant women. Clin. Sci. Mol. Med. 1976, 51, 545–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, C.A.; King, J.C. Reduced serum zinc concentration during pregnancy. Obstet. Gynecol. 1983, 62, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hunt, I.F.; Murphy, N.J.; Cleaver, A.E.; Faraji, B.; Swendseid, M.E.; Coulson, A.H.; Clark, V.A.; Laine, N.; Davis, C.A.; Smith, J.C. Zinc supplementation during pregnancy: Zinc concentration of serum and hair from low-income women of Mexican descent. Am. J. Clin. Nutr. 1983, 37, 572–582. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Tamura, T.; Neggers, Y.; Copper, R.L.; Johnston, K.E.; DuBard, M.B.; Hauth, J.C. The effect of zinc supplementation on pregnancy outcome. JAMA 1995, 274, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Krebs, N.F.; Jacobs, M.A.; Favier, A.; Guyette, L.; Ikle, D.N. Zinc nutritional status during pregnancy: A longitudinal study. Am. J. Clin. Nutr. 1983, 37, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Sorenson, J.C.; Pollard, E.L.; Kirby, J.K.; Audhya, T. Evidence-Based Recommendations for an Optimal Prenatal Supplement for Women in the U.S., Part Two: Minerals. Nutrients 2021, 13, 1849. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Guan, Q.; Xu, L.; Zhao, S.; Duan, J.; Wang, Y.; Xia, Y.; Xu, Q. Exposure to multiple trace elements and miscarriage during early pregnancy: A mixtures approach. Environ. Int. 2022, 162, 107161. [Google Scholar] [CrossRef]
- Liang, C.-M.; Wu, X.-Y.; Huang, K.; Yan, S.-Q.; Li, Z.-J.; Xia, X.; Pan, W.-J.; Sheng, J.; Tao, Y.-R.; Xiang, H.-Y.; et al. Trace element profiles in pregnant women’s sera and umbilical cord sera and influencing factors: Repeated measurements. Chemosphere 2019, 218, 869–878. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Piao, J.; Mao, D.; Li, Y.; Li, W.; Yang, L.; Yang, X. Reference Values of 14 Serum Trace Elements for Pregnant Chinese Women: A Cross-Sectional Study in the China Nutrition and Health Survey 2010–2012. Nutrients 2017, 9, 309. [Google Scholar] [CrossRef]
- Osman, K.; Akesson, A.; Berglund, M.; Bremme, K.; Schütz, A.; Ask, K.; Vahter, M. Toxic and essential elements in placentas of Swedish women. Clin. Biochem. 2000, 33, 131–138. [Google Scholar] [CrossRef]
- Liu, M.; Wang, D.; Wang, C.; Yin, S.; Pi, X.; Li, Z.; Wang, L.; Liu, J.; Yin, C.; Jin, L.; et al. High concentrations of aluminum in maternal serum and placental tissue are associated with increased risk for fetal neural tube defects. Chemosphere 2021, 284, 131387. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimou, B.; Albatineh, A.N.; Salihu, H.M.; Gasana, J. Ambient PM2.5 Aluminum and Elemental Carbon and Placental Abruption Morbidity. J. Occup. Environ. Med. 2017, 59, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Ebisu, K.; Bell, M.L. Airborne PM2.5 chemical components and low birth weight in the northeastern and mid-Atlantic regions of the United States. Environ. Health Perspect. 2012, 120, 1746–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Lin, Y.; Tian, X.; Li, J.; Chen, X.; Yang, J.; Li, X.; Deng, Y.; Li, N.; Liang, J.; et al. Association between maternal aluminum exposure and the risk of congenital heart defects in offspring. Birth Defects Res. A Clin. Mol. Teratol. 2016, 106. [Google Scholar] [CrossRef]
- Hokin, B.; Adams, M.; Ashton, J.; Louie, H. Comparison of the dietary cobalt intake in three different Australian diets. Asia Pac. J. Clin. Nutr. 2004, 13, 289–291. [Google Scholar]
- Liang, C.; Wang, J.; Xia, X.; Wang, Q.; Li, Z.; Tao, R.; Tao, Y.; Xiang, H.; Tong, S.; Tao, F. Serum cobalt status during pregnancy and the risks of pregnancy-induced hypertension syndrome: A prospective birth cohort study. J. Trace Elem. Med. Biol. 2018, 46, 39–45. [Google Scholar] [CrossRef]
- Li, Z.-J.; Liang, C.-M.; Xia, X.; Huang, K.; Yan, S.-Q.; Tao, R.-W.; Pan, W.-J.; Sheng, J.; Tao, Y.-R.; Xiang, H.-Y.; et al. Association between maternal and umbilical cord serum cobalt concentration during pregnancy and the risk of preterm birth: The Ma’anshan birth cohort (MABC) study. Chemosphere 2019, 218, 487–492. [Google Scholar] [CrossRef]
- Rudge, C.V.; Röllin, H.B.; Nogueira, C.M.; Thomassen, Y.; Rudge, M.C.; Odland, J.Ø. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J. Environ. Monit. 2009, 11, 1322–1330. [Google Scholar] [CrossRef] [Green Version]
- Kopp, R.S.; Kumbartski, M.; Harth, V.; Brüning, T.; Käfferlein, H.U. Partition of metals in the maternal/fetal unit and lead-associated decreases of fetal iron and manganese: An observational biomonitoring approach. Arch. Toxicol. 2012, 86, 1571–1581. [Google Scholar] [CrossRef]
- Schramel, P.; Lill, G.; Hasse, S.; Klose, B.J. Mineral- and trace element concentrations in human breast milk, placenta, maternal blood, and the blood of the newborn. Biol. Trace Elem. Res. 1988, 16, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Krachler, M.; Rossipal, E.; Micetic-Turk, D. Trace element transfer from the mother to the newborn—Investigations on triplets of colostrum, maternal and umbilical cord sera. Eur. J. Clin. Nutr. 1999, 53, 486–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, A. Whole blood manganese levels in pregnancy and the neonate. Nutrition 1999, 15, 731–734. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional aspects of soy formula. Acta Paediatr. Suppl. 1994, 402, 105–108. [Google Scholar] [CrossRef]
- Zota, A.R.; Ettinger, A.S.; Bouchard, M.; Amarasiriwardena, C.J.; Schwartz, J.; Hu, H.; Wright, R.O. Maternal blood manganese levels and infant birth weight. Epidemiology 2009, 20, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Than, N.G.; Romero, R.; Tarca, A.L.; Draghici, S.; Erez, O.; Chaiworapongsa, T.; Kim, Y.M.; Kim, S.K.; Vaisbuch, E.; Tromp, G. Mitochondrial manganese superoxide dismutase mRNA expression in human chorioamniotic membranes and its association with labor, inflammation, and infection. J. Matern. Fetal Neona 2009, 22, 1000–1013. [Google Scholar] [CrossRef] [Green Version]
- Nandakumaran, M.; Al-Sannan, B.; Al-Sarraf, H.; Al-Shammari, M. Maternal-fetal transport kinetics of manganese in perfused human placental lobule in vitro. J. Matern Fetal. Neona 2016, 29, 274–278. [Google Scholar] [CrossRef]
- Baeyens, W.; Vrijens, J.; Gao, Y.; Croes, K.; Schoeters, G.; Den Hond, E.; Sioen, I.; Bruckers, L.; Nawrot, T.; Nelen, V.; et al. Trace metals in blood and urine of newborn/mother pairs, adolescents and adults of the Flemish population (2007–2011). Int. J. Hyg. Environ. Health 2014, 217, 878–890. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu. Rev. Plant. Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [Green Version]
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic life and Human Health. Int. J. Environ. Res. Public. Health 2020, 17, 2204. [Google Scholar] [CrossRef] [Green Version]
- Arbuckle, T.E.; Liang, C.L.; Morisset, A.S.; Fisher, M.; Weiler, H.; Cirtiu, C.M.; Legrand, M.; Davis, K.; Ettinger, A.S.; Fraser, W.D.; et al. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The mirec study. Chemosphere 2016, 163, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Callan, A.C.; Hinwood, A.L.; Ramalingam, M.; Boyce, M.; Heyworth, J.; McCafferty, P.; Odland, J.Ø. Maternal exposure to metals—Concentrations and predictors of exposure. Environ. Res. 2013, 126, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.S.; Arbuckle, T.E.; Fisher, M.; Liang, C.L.; Davis, K.; Cirtiu, C.M.; Bélanger, P.; LeBlanc, A.; Fraser, W.D.; MIREC Study Group. Arsenic levels among pregnant women and newborns in canada: Results from the maternal-infant research on environmental chemicals (mirec) cohort. Environ. Res. 2017, 153, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Esquinas, E.; Pérez-Gómez, B.; Fernández-Navarro, P.; Fernández, M.A.; de Paz, C.; Pérez-Meixeira, A.M.; Gil, E.; Iriso, A.; Sanz, J.C.; Astray, J.; et al. Lead, mercury and cadmium in umbilical cord blood and its association with parental epidemiological variables and birth factors. BMC Public Health 2013, 13, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-H.; Weng, K.-P.; Lin, C.-C.; Wang, C.-C.; Lee, C.T.-C.; Ger, L.-P.; Wu, M.-T. Maternal and umbilical cord blood levels of mercury, manganese, iron, and copper in southern Taiwan: A cross-sectional study. J. Chin. Med Assoc. 2017, 80, 442–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igra, A.M.; Harari, F.; Lu, Y.; Casimiro, E.; Vahter, M. Boron exposure through drinking water during pregnancy and birth size. Environ. Int. 2016, 95, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Liu, J.; Ye, B.; Ren, A. Concentrations of selected heavy metals in maternal blood and associated factors in rural areas in Shanxi Province, China. Environ. Int. 2014, 66, 157–164. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Chung, J.-Y.; An, H.S.; Park, S.Y.; Kim, B.-G.; Bae, J.W.; Han, M.; Cho, Y.J.; Hong, Y.-S. Biomonitoring of Lead, Cadmium, Total Mercury, and Methylmercury Levels in Maternal Blood and in Umbilical Cord Blood at Birth in South Korea. Int. J. Environ. Res. Public Health 2015, 12, 13482–13493. [Google Scholar] [CrossRef]
- Li, A.; Zhuang, T.; Shi, J.; Liang, Y.; Song, M. Heavy metals in maternal and cord blood in Beijing and their efficiency of placental transfer. J. Environ. Sci. 2019, 80, 99–106. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, H.; Zheng, T.; Zhang, W.; Yang, C.; Yu, L.; Sun, X.; Xia, W.; Xu, S.; Li, Y. Exposure to metal mixtures and hypertensive disorders of pregnancy: A nested case-control study in China. Environ. Pollut. 2022, 306. [Google Scholar] [CrossRef]
- Röllin, H.B.; Nogueira, C.; Olutola, B.; Channa, K.; Odland, J. Prenatal Exposure to Aluminum and Status of Selected Essential Trace Elements in Rural South African Women at Delivery. Int. J. Environ. Res. Public Health 2018, 15, 1494. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, T.; Saphier, M.; Mashiach, Y.; Paz-Tal, O.; Saphier, O. Elements in maternal blood and amniotic fluid determined by ICP-MS. J. Matern. Neonatal Med. 2014, 28, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, B.; Arıca, E.; Söylemezoğlu, T. Assessing reference levels of nickel and chromium in cord blood, maternal blood and placenta specimens from Ankara, Turkey. J. Turk. Gynecol. Assoc. 2021, 22, 187–195. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Weisskopf, M.; Williams, P.L.; Parsons, P.J.; Palmer, C.D.; Louis, G.M.B.; James-Todd, T. A Prospective Study of Early Pregnancy Essential Metal(loid)s and Glucose Levels Late in the Second Trimester. J. Clin. Endocrinol. Metab. 2019, 104, 4295–4303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, R.; Cai, X.; Xiao, R.; Yu, H. Trace elements profiles of maternal blood, umbilical cord blood, and placenta in Beijing, China. J. Matern. Neonatal Med. 2017, 32, 1755–1761. [Google Scholar] [CrossRef]
- Taylor, C.M.; Golding, J.; Emond, A.M. Lead, cadmium and mercury levels in pregnancy: The need for international consensus on levels of concern. J. Dev. Orig. Health Dis. 2014, 5, 16–30. [Google Scholar]




| Variables | Mean ± SD/n (%) |
|---|---|
| Age (years) | 37.2 ± 1.9 |
| Birth weight (g) | 3226.0 ± 474.9 |
| Birth length (cm) | 49.3 ± 2.3 |
| Ethnic group | |
| Han | 43 (89.6%) |
| Others | 5 (10.4%) |
| Education level | |
| Secondary school | 2 (4.2%) |
| Undergraduate | 24 (50%) |
| Graduate or higher | 22 (45.8%) |
| Occupation | |
| Worker/business/services | 26 (54.2%) |
| Public official | 15 (31.3%) |
| Others | 5 (13.2%) |
| None | 2 (5.3%) |
| Gravidity | |
| 0 | 12 (25.0%) |
| ≥1 | 36 (75.0%) |
| Parity | |
| 0 | 24 (50.0%) |
| ≥1 | 24 (50.0%) |
| Elements | LOD | 1st Trimester a | 2nd Trimester a | 3rd Trimester a | p Value b |
|---|---|---|---|---|---|
| Toxic elements | |||||
| Al | 0.1 | 37.24 (31.02–46.38) | 35.73 (32.8–43.01) | 37.33 (28.83–48.8) | 0.763 |
| As | 0.05 | 0.42 (0.22–0.74) | 0.46 (0.28–0.81) | 0.46 (0.25–1.04) | 0.483 |
| Cd | 0.003 | 1.27 (0.98–1.44) | 1.02 (0.64–1.23) | 0.97 (0.65–1.29) | 0.133 |
| Hg | 0.007 | 0.92 (0.49–1.19) | 0.82 (0.3–1.09) | 0.73 (0.27–1.22) | 0.979 |
| Pb | 0.012 | 1.4 (1.03–1.82) | 0.9 (0.74–1.21) | 0.87 (0.63–1.37) | <0.001 |
| Sb | 0.003 | 3.78 (2.76–5.62) | 3.47 (2.96–3.97) | 3.69 (3.34–4.39) | 0.920 |
| Essential trace elements | |||||
| B | 0.005 | 21.39 (16.52–26.84) | 20.14 (16.16–23.22) | 23.32 (16.04–29.67) | 0.248 |
| Co | 0.001 | 1.41 (1.03–1.83) | 1.41 (1.03–1.83) | 1.39 (1.11–2.1) | 0.717 |
| Cr | 0.002 | 0.61 (0.42–1.13) | 0.68 (0.51–1.07) | 0.66 (0.5–1.07) | 0.524 |
| Cu | 0.03 | 1272.96 (1011.26–1477.93) | 1796.51 (1666.11–2021.09) | 1844.87 (1558.37–2219.37) | <0.001 |
| Fe | 0.9 | 1500.96 (1302.29–1759.41) | 1413.25 (1154.64–1812.04) | 1225.48 (989.72–1487.56) | 0.011 |
| Ni | 0.005 | 4.26 (3.34–5.31) | 4.02 (3.39–4.91) | 4.51 (3.68–6.15) | 0.305 |
| Mn | 0.008 | 0.66 (0.26–1.15) | 0.95 (0.69–1.22) | 0.79 (0.5–1.43) | 0.281 |
| Se | 0.05 | 111.3 (104.12–120.88) | 102.65 (89.58–114.11) | 95.28 (86.14–108.22) | <0.001 |
| Zn | 0.06 | 781.89 (720.24–826.77) | 612.57 (547.82–666.14) | 597.27 (541.48–667.52) | <0.001 |
| Rb | 0.00396 | 248.26 (235.97–279.1) | 230.95 (215.43–254.81) | 242.01 (211.81–259.06) | 0.002 |
| Sr | 0.01 | 34.54 (29.03–40.28) | 33.37 (27.17–40.81) | 40.07 (30.64–48.07) | 0.071 |
| Mo | 0.002 | 2.75 (1.92–3.19) | 2.4 (1.78–3.2) | 2.31 (1.94–2.76) | 0.043 |
| Elements | LOD | >LOD (%) | 3rd Trimester a | Delivery a | p Value b |
|---|---|---|---|---|---|
| Toxic elements | |||||
| Al | 0.1 | 100% | 37.33 (28.83–48.8) | 29.51 (22.85–37.26) | 0.004 |
| As | 0.05 | 91.7% | 0.46 (0.25–1.04) | 0.43 (0.26–0.76) | 0.089 |
| Cd | 0.003 | 100% | 0.97 (0.65–1.29) | 0.93 (0.71–1.17) | 0.806 |
| Hg | 0.007 | 97.9% | 0.73 (0.27–1.22) | 0.82 (0.63–1.13) | 0.659 |
| Pb | 0.012 | 100% | 0.87 (0.63–1.37) | 0.78 (0.63–1.05) | 0.412 |
| Sb | 0.003 | 100% | 3.69 (3.34–4.39) | 4.08 (3.08–4.63) | 0.552 |
| Essential trace elements | |||||
| B | 0.005 | 100% | 23.32 (16.04–29.67) | 21.71 (15.35–27.67) | 0.644 |
| Co | 0.001 | 100% | 1.39 (1.11–2.1) | 1.09 (0.85–1.41) | <0.001 |
| Cr | 0.002 | 100% | 0.66 (0.5–1.07) | 0.56 (0.35–0.94) | 0.200 |
| Cu | 0.03 | 100% | 1844.87 (1558.37–2219.37) | 249.98 (214.09–351.26) | <0.001 |
| Fe | 0.9 | 100% | 1225.48 (989.72–1487.56) | 2663.86 (2139.78–3249.15) | <0.001 |
| Ni | 0.005 | 100% | 4.51 (3.68–6.15) | 3.41 (2.76–4.39) | 0.007 |
| Mn | 0.008 | 91.7% | 0.79 (0.5–1.43) | 2.17 (1.74–3) | <0.001 |
| Se | 0.05 | 100% | 95.28 (86.14–108.22) | 53.57 (46.42–63.09) | <0.001 |
| Zn | 0.06 | 100% | 597.27 (541.48–667.52) | 686.61 (608.64–775.12) | <0.001 |
| Rb | 0.00396 | 100% | 242.01 (211.81–259.06) | 276.28 (246.35–378.05) | <0.001 |
| Sr | 0.01 | 100% | 40.07 (30.64–48.07) | 46.85 (35.24–56.22) | 0.021 |
| Mo | 0.002 | 100% | 2.31 (1.94–2.76) | 2.14 (1.82–2.6) | 0.126 |
| Elements | r | p Value a |
|---|---|---|
| Toxic elements | ||
| Al | 0.023 | 0.878 |
| As | 0.383 | 0.007 |
| Cd | 0.184 | 0.211 |
| Hg | −0.137 | 0.352 |
| Pb | −0.042 | 0.777 |
| Sb | −0.056 | 0.703 |
| Essential trace elements | ||
| B | 0.176 | 0.231 |
| Co | −0.014 | 0.926 |
| Cr | −0.069 | 0.640 |
| Cu | 0.143 | 0.333 |
| Fe | 0.043 | 0.771 |
| Ni | −0.087 | 0.557 |
| Mn | −0.016 | 0.913 |
| Se | 0.194 | 0.187 |
| Zn | 0.171 | 0.245 |
| Rb | 0.263 | 0.071 |
| Sr | 0.444 | 0.002 |
| Mo | 0.416 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, T.; Jia, X.; Shi, H.; Gong, X.; Ma, J.; Gan, Z.; Yu, Z.; Li, Z.; Wei, Y. An Evaluation of Exposure to 18 Toxic and/or Essential Trace Elements Exposure in Maternal and Cord Plasma during Pregnancy at Advanced Maternal Age. Int. J. Environ. Res. Public Health 2022, 19, 14485. https://doi.org/10.3390/ijerph192114485
Gu T, Jia X, Shi H, Gong X, Ma J, Gan Z, Yu Z, Li Z, Wei Y. An Evaluation of Exposure to 18 Toxic and/or Essential Trace Elements Exposure in Maternal and Cord Plasma during Pregnancy at Advanced Maternal Age. International Journal of Environmental Research and Public Health. 2022; 19(21):14485. https://doi.org/10.3390/ijerph192114485
Chicago/Turabian StyleGu, Tingfei, Xiaoqian Jia, Huifeng Shi, Xiaoli Gong, Jinxi Ma, Zhihang Gan, Zhixin Yu, Zhiwen Li, and Yuan Wei. 2022. "An Evaluation of Exposure to 18 Toxic and/or Essential Trace Elements Exposure in Maternal and Cord Plasma during Pregnancy at Advanced Maternal Age" International Journal of Environmental Research and Public Health 19, no. 21: 14485. https://doi.org/10.3390/ijerph192114485






