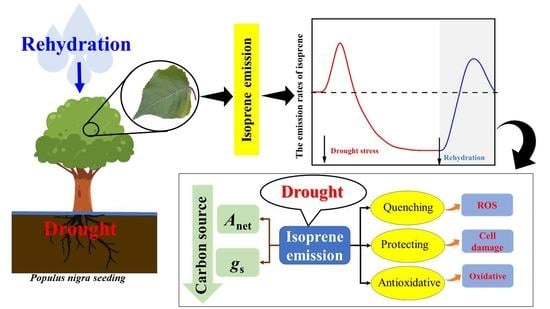
Impacts of Drought and Rehydration Cycles on Isoprene Emissions in Populus nigra Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chamber Design
2.2. Drought Stress Experiments
2.2.1. Soil Water Content
2.2.2. Isoprene Sampling and Gas Exchange
2.2.3. Quantification of Isoprene
2.3. Quality Assurance/Quality Control
3. Results and Discussion
3.1. SWC and Physiological Parameters
3.2. Isoprene Emission Rates under Drought Stress
3.3. Isoprene Emissions and Physiological Parameters during Rehydration
3.4. The Impact of Physiological Parameters on Isoprene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunier, A.; Ormeño, E.; Piga, D.; Armengaud, A.; Boissard, C.; Lathière, J.; Fernandez, C. Isoprene contribution to ozone production under climate change conditions in the French Mediterranean area. Reg. Environ. Chang. 2020, 20, 111. [Google Scholar] [CrossRef]
- Zhang-Turpeinen, H.; Kivimaenpaa, M.; Aaltonen, H.; Berninger, F.; Koster, E.; Koster, K.; Pumpanen, J. Wildfire effects on BVOC emissions from boreal forest floor on permafrost soil in Siberia. Sci. Total Environ. 2020, 711, 134851. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Zimmerman, P. A global model of natural volatile organic compound emissions. J. Geophys. Res. Atmos. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Palmer, P.I.; Marvin, M.R.; Siddans, R.; Kerridge, B.J.; Moore, D.P. Nocturnal survival of isoprene linked to formation of upper tropospheric organic aerosol. Science 2022, 375, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Butler, T.M.; Crowley, J.N.; Dillon, T.J.; Fischer, H.; Ganzeveld, L.; Harder, H.; Lawrence, M.G.; Martinez, M.; Taraborrelli, D.; et al. Atmospheric oxidation capacity sustained by a tropical forest. Nature 2008, 452, 737–740. [Google Scholar] [CrossRef]
- Fu, D.; Millet, D.B.; Wells, K.C.; Payne, V.H.; Yu, S.; Guenther, A.; Eldering, A. Direct retrieval of isoprene from satellite-based infrared measurements. Nat. Commun. 2019, 10, 3811. [Google Scholar] [CrossRef]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, M.; Yan, F.; Su, H.; Wang, S.; Liao, H.; Gao, H. Impacts of biogenic emissions from urban landscapes on summer ozone and secondary organic aerosol formation in megacities. Sci. Total Environ. 2022, 814, 152654. [Google Scholar] [CrossRef]
- Ma, M.; Gao, Y.; Ding, A.; Su, H.; Liao, H.; Wang, S.; Gao, H. Correction to “Development and Assessment of a High-Resolution Biogenic Emission Inventory from Urban Green Spaces in China”. Environ. Sci. Technol. 2022, 56, 3300–3301. [Google Scholar] [CrossRef]
- Yang, W.; Cao, J.; Wu, Y.; Kong, F.; Li, L. Review on plant terpenoid emissions worldwide and in China. Sci. Total Environ. 2021, 787, 147454. [Google Scholar] [CrossRef]
- Kammer, J.; Flaud, P.M.; Chazeaubeny, A.; Ciuraru, R.; Le Menach, K.; Geneste, E.; Villenave, E. Biogenic volatile organic compounds (BVOCs) reactivity related to new particle formation (NPF) over the Landes forest. Atmos. Res. 2020, 237, 104869. [Google Scholar] [CrossRef]
- Penuelas, J.; Staudt, M. BVOCs and global change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.R. On the essentials of drought in a changing climate. Science 2020, 368, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ji, M.; Xie, Y.; Wang, S.; He, Y.; Ran, J. Global semi-arid climate change over last 60 years. Clim. Dyn. 2016, 46, 1131–1150. [Google Scholar] [CrossRef]
- Bonn, B.; Magh, R.-K.; Rombach, J.; Kreuzwieser, J. Biogenic isoprenoid emissions under drought stress: Different responses for isoprene and terpenes. Biogeosciences 2019, 16, 4627–4645. [Google Scholar] [CrossRef]
- Barchet, G.L.; Dauwe, R.; Guy, R.D.; Schroeder, W.R.; Soolanayakanahally, R.Y.; Campbell, M.M.; Mansfield, S.D. Investigating the drought-stress response of hybrid poplar genotypes by metabolite profiling. Tree Physiol. 2014, 34, 1203–1219. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kuhn, U.; Harley, P.C.; Staudt, M.; Arneth, A.; Cescatti, A.; Peñuelas, J. Estimations of isoprenoid emission capacity from enclosure studies: Measurements, data processing, quality and standardized measurement protocols. Biogeosciences 2011, 8, 2209–2246. [Google Scholar] [CrossRef]
- Ortega, J.; Helmig, D. Approaches for quantifying reactive and low-volatility biogenic organic compound emissions by vegetation enclosure techniques—Part A. Chemosphere 2008, 72, 343–364. [Google Scholar] [CrossRef]
- Yu, H.; Blande, J.D. Diurnal variation in BVOC emission and CO2 gas exchange from above- and belowground parts of two coniferous species and their responses to elevated O3. Environ. Pollut. 2021, 278, 116830. [Google Scholar] [CrossRef]
- Yuan, X.; Feng, Z.; Shang, B.; Calatayud, V.; Paoletti, E. Ozone exposure, nitrogen addition and moderate drought dynamically interact to affect isoprene emission in poplar. Sci. Total Environ. 2020, 734, 139368. [Google Scholar] [CrossRef]
- Laothawornkitkul, J.; Taylor, J.E.; Paul, N.D.; Hewitt, C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009, 183, 27–51. [Google Scholar] [CrossRef] [PubMed]
- van Meeningen, Y.; Schurgers, G.; Rinnan, R.; Holst, T. Isoprenoid emission response to changing light conditions of English oak, European beech and Norway spruce. Biogeosciences 2017, 14, 4045–4060. [Google Scholar] [CrossRef]
- Huang, L.; McDonald-Buller, E.C.; McGaughey, G.; Kimura, Y.; Allen, D.T. Annual variability in leaf area index and isoprene and monoterpene emissions during drought years in Texas. Atmos. Environ. 2014, 92, 240–249. [Google Scholar] [CrossRef]
- Huang, L.; McGaughey, G.; McDonald-Buller, E.; Kimura, Y.; Allen, D.T. Quantifying regional, seasonal and interannual contributions of environmental factors on isoprene and monoterpene emissions estimates over eastern Texas. Atmos. Environ. 2015, 106, 120–128. [Google Scholar] [CrossRef]
- Potosnak, M.J.; LeStourgeon, L.; Pallardy, S.G.; Hosman, K.P.; Gu, L.; Karl, T.; Guenther, A.B. Observed and modeled ecosystem isoprene fluxes from an oak-dominated temperate forest and the influence of drought stress. Atmos. Environ. 2014, 84, 314–322. [Google Scholar] [CrossRef]
- Genard-Zielinski, A.-C.; Boissard, C.; Ormeño, E.; Lathière, J.; Reiter, I.M.; Wortham, H.; Fernandez, C. Seasonal variations of Quercus pubescens isoprene emissions from an in natura forest under drought stress and sensitivity to future climate change in the Mediterranean area. Biogeosciences 2018, 15, 4711–4730. [Google Scholar] [CrossRef]
- Beckett, M.; Loreto, F.; Velikova, V.; Brunetti, C.; Di Ferdinando, M.; Tattini, M.; Farrant, J.M. Photosynthetic limitations and volatile and non-volatile isoprenoids in the poikilochlorophyllous resurrection plant Xerophyta humilisduring dehydration and rehydration. Plant Cell Environ. 2012, 35, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Bourtsoukidis, E.; Kawaletz, H.; Radacki, D.; Schütz, S.; Hakola, H.; Hellén, H.; Bonn, B. Impact of flooding and drought conditions on the emission of volatile organic compounds of Quercus robur and Prunus serotina. Trees 2013, 28, 193–204. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Remmel, T.; Niinemets, Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot 2014, 100, 55–63. [Google Scholar] [CrossRef]
- Šimpraga, M.; Verbeeck, H.; Demarcke, M.; Joó, É.; Pokorska, O.; Amelynck, C.; Steppe, K. Clear link between drought stress, photosynthesis and biogenic volatile organic compounds in Fagus sylvatica L. Atmos. Environ. 2011, 45, 5254–5259. [Google Scholar] [CrossRef]
- Tani, A.; Tozaki, D.; Okumura, M.; Nozoe, S.; Hirano, T. Effect of drought stress on isoprene emission from two major Quercus species native to East Asia. Atmos. Environ. 2011, 45, 6261–6266. [Google Scholar] [CrossRef]
- Mu, Z.; Llusià, J.; Liu, D.; Ogaya, R.; Asensio, D.; Zhang, C.; Peñuelas, J. Seasonal and diurnal variations of plant isoprenoid emissions from two dominant species in Mediterranean shrubland and forest submitted to experimental drought. Atmos. Environ. 2018, 191, 105–115. [Google Scholar] [CrossRef]
- Perreca, E.; Rohwer, J.; Gonzalez-Cabanelas, D.; Loreto, F.; Schmidt, A.; Gershenzon, J.; Wright, L.P. Effect of drought on the Methylerythritol 4-Phosphate (MEP) pathway in the isoprene emitting conifer picea glauca. Front. Plant Sci. 2020, 11, 546295. [Google Scholar] [CrossRef] [PubMed]
- Seco, R.; Karl, T.; Guenther, A.; Hosman, K.P.; Pallardy, S.G.; Gu, L.; Kim, S. Ecosystem-scale volatile organic compound fluxes during an extreme drought in a broadleaf temperate forest of the Missouri Ozarks (central USA). Glob. Chang. Biol. 2015, 21, 3657–3674. [Google Scholar] [CrossRef]
- Niinemets, Ü. Mild versus severe stress and BVOCs: Thresholds, priming and consequences. Trends Plant Sci. 2010, 15, 145–153. [Google Scholar] [CrossRef]
- Otu-Larbi, F.; Bolas, C.G.; Ferracci, V.; Staniaszek, Z.; Jones, R.L.; Malhi, Y.; Ashworth, K. Modelling the effect of the 2018 summer heatwave and drought on isoprene emissions in a UK woodland. Glob. Chang. Biol. 2019, 26, 2320–2335. [Google Scholar] [CrossRef]
- Fortunati, A.; Barta, C.; Brilli, F.; Centritto, M.; Zimmer, I.; Schnitzler, J.r.-P.; Loreto, F. Isoprene emission is not temperature-dependent during and after severe drought-stress: A physiological and biochemical analysis. Plant J. 2008, 55, 687–697. [Google Scholar] [CrossRef]
- Trowbridge, A.M.; Stoy, P.C.; Adams, H.D.; Law, D.J.; Breshears, D.D.; Helmig, D.; Monson, R.K. Drought supersedes warming in determining volatile and tissue defenses of piñon pine (Pinus edulis). Environ. Res. Lett. 2019, 14, 065006. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Chaowiwat, W.; Wang, C. Climate change impact on major crop yield and water footprint under CMIP6 climate projections in repeated drought and flood areas in Thailand. Sci. Total Environ. 2022, 807 (Pt 2), 150741. [Google Scholar] [CrossRef]
- Skendzic, S.; Zovko, M.; Zivkovic, I.P.; Lesic, V.; Lemic, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Lun, X.; Lin, Y.; Chai, F.; Fan, C.; Li, H.; Liu, J. Reviews of emission of biogenic volatile organic compounds (BVOCs) in Asia. J. Environ. Sci. 2020, 95, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Ferracci, V.; Bolas, C.G.; Freshwater, R.A.; Staniaszek, Z.; King, T.; Jaars, K.; Harris, N.R.P. Continuous isoprene measurements in a UK temperate forest for a whole growing season: Effects of drought stress during the 2018 heatwave. Geophys. Res. Lett. 2020, 47, e2020GL088885. [Google Scholar] [CrossRef]
- Sun, Z.; Shen, Y.; Niinemets, U. Responses of isoprene emission and photochemical efficiency to severe drought combined with prolonged hot weather in hybrid Populus. J. Exp. Bot. 2020, 71, 7364–7381. [Google Scholar] [CrossRef] [PubMed]
- Tomiolo, S.; Metz, J.; Blackwood, C.B.; Djendouci, K.; Henneberg, L.; Muller, C.; Tielborger, K. Short-term drought and long-term climate legacy affect production of chemical defenses among plant ecotypes. Environ. Exp. Bot. 2017, 141, 124–131. [Google Scholar] [CrossRef]
- Zhang, P.; He, Y.; Feng, Y.; De La Torre, R.; Jia, H.; Tang, J.; Cubbage, F. An analysis of potential investment returns of planted forests in South China. New For. 2019, 50, 943–968. [Google Scholar] [CrossRef]
- Wells, K.C.; Millet, D.B.; Payne, V.H.; Deventer, M.J.; Bates, K.H.; de Gouw, J.A.; Graus, M.; Warneke, C.; Wisthaler, A.; Fuentes, J.D. Satellite isoprene retrievals constrain emissions and atmospheric oxidation. Nature 2020, 585, 225–233. [Google Scholar] [CrossRef]
- Schnitzler, J.P.; Louis, S.; Behnke, K.; Loivamaki, M. Poplar volatiles—Biosynthesis, regulation and (eco)physiology of isoprene and stress-induced isoprenoids. Plant Biol. 2010, 12, 302–316. [Google Scholar] [CrossRef]
- Central Government of China. Forest Cover Area from Artifcial Afforestation in the Three Northern Regions Shelter Forest Regions. Available online: http://www.gov.cn/xinwen/2018-12/24/content_5351500.htm (accessed on 20 September 2022).
- Zhang, X.; Huang, T.; Zhang, L.; Shen, Y.; Zhao, Y.; Gao, H.; Ma, J. Three-North shelter forest program contribution to long-term increasing trends of biogenic isoprene emissions in northern China. Atmos. Chem. Phys. 2016, 16, 6949–6960. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Fang, C.; Monson, R.K.; Cowling, E.B. Isoprene emission, photosynthesis, and growth in sweetgum (Liquidambar styraciflua) seedlings exposed to short- and long-term drying cycles. Tree Physiol. 1996, 16, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Brilli, F.; Barta, C.; Fortunati, A.; Lerdau, M.; Loreto, F.; Centritto, M. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007, 175, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Lupke, M.; Leuchner, M.; Steinbrecher, R.; Menzel, A. Quantification of monoterpene emission sources of a conifer species in response to experimental drought. AoB Plants 2017, 9, plx045. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, E.; Rey, A.; Greenberg, J.; Harley, P.; Grace, J.; Malhi, Y.; Guenther, A. Effect of drought on isoprene emission rates from leaves of Quercus virginiana Mill. Atmos. Environ. 2004, 38, 6149–6156. [Google Scholar] [CrossRef]
- Li, L.Y.; Guenther, A.B.; Gu, D.S.; Seco, R.; Nagalingam, S. Impact of short-term drought stress on volatile organic compounds emissions from Pinus massoniana. China Environ. Sci. 2020, 40, 3776–3780. [Google Scholar] [CrossRef]
- Parveen, S.; Rashid, M.H.; Inafuku, M.; Iwasaki, H.; Oku, H. Molecular regulatory mechanism of isoprene emission under short-term drought stress in the tropical tree Ficus septica. Tree Physiol. 2019, 39, 440–453. [Google Scholar] [CrossRef]
- Nogués, I.; Medori, M.; Calfapietra, C. Limitations of monoterpene emissions and their antioxidant role in Cistus sp. under mild and severe treatments of drought and warming. Environ. Exp. Bot. 2015, 119, 76–86. [Google Scholar] [CrossRef]
- Funk, J.L.; Mak, J.E.; Lerdau, M.T. Stress-induced changes in carbon sources for isoprene production in Populus deltoides. Plant Cell Environ. 2004, 27, 747–755. [Google Scholar] [CrossRef]
- Velikova, V.; Brunetti, C.; Tattini, M.; Doneva, D.; Ahrar, M.; Tsonev, T.; Loreto, F. Physiological significance of isoprenoids and phenylpropanoids in drought response of Arundinoideae species with contrasting habitats and metabolism. Plant Cell Environ. 2016, 39, 2185–2197. [Google Scholar] [CrossRef]
- Fall, R.; Monson, R.K. Isoprene emission rate and intercellular isoprene concentration as influenced by stomatal distribution and conductance. Plant Physiol. 1992, 100, 987–992. [Google Scholar] [CrossRef]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Guidi, L.; Ferrini, F.; Brunetti, C.; Di Ferdinando, M.; Biricolti, S.; Tattini, M. Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: An excess light stress affair? J. Plant Physiol. 2012, 169, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Brunetti, C.; Tattini, M.; Romano, A.; Biasioli, F.; Tognetti, R.; Centritto, M. Dissecting the role of isoprene and stress-related hormones (ABA and ethylene) in Populus nigra exposed to unequal root zone water stress. Tree Physiol. 2017, 37, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calcerrada, J.; Buatois, B.; Chiche, E.; Shahin, O.; Staudt, M. Leaf isoprene emission declines in Quercus pubescens seedlings experiencing drought—Any implication of soluble sugars and mitochondrial respiration? Environ. Exp. Bot. 2013, 85, 36–42. [Google Scholar] [CrossRef]
- Saunier, A.; Ormeno, E.; Wortham, H.; Temime-Roussel, B.; Lecareux, C.; Boissard, C.; Fernandez, C. Chronic drought decreases anabolic and catabolic BVOC emissions of Quercus pubescens in a Mediterranean Forest. Front. Plant Sci. 2017, 8, 71. [Google Scholar] [CrossRef]
- Centritto, M.; Brilli, F.; Fodale, R.; Loreto, F. Different sensitivity of isoprene emission, respiration and photosynthesis to high growth temperature coupled with drought stress in black poplar (Populus nigra) saplings. Tree Physiol. 2011, 31, 275–286. [Google Scholar] [CrossRef]
- Bamberger, I.; Ruehr, N.K.; Schmitt, M.; Gast, A.; Wohlfahrt, G.; Arneth, A. Isoprene emission and photosynthesis during heatwaves and drought in black locust. Biogeosciences 2017, 14, 3649–3667. [Google Scholar] [CrossRef]
- Llusia, J.; Roahtyn, S.; Yakir, D.; Rotenberg, E.; Seco, R.; Guenther, A.; Peñuelas, J. Photosynthesis, stomatal conductance and terpene emission response to water availability in dry and mesic Mediterranean forests. Trees 2015, 30, 749–759. [Google Scholar] [CrossRef]
- Ormeño, E.; Mévy, J.P.; Vila, B.; Bousquet-Mélou, A.; Greff, S.; Bonin, G.; Fernandez, C. Water deficit stress induces different monoterpene and sesquiterpene emission changes in Mediterranean species. Relationship between terpene emissions and plant water potential. Chemosphere 2007, 67, 276–284. [Google Scholar] [CrossRef]




| Plant | Location | Emission Rates | Increased Rates (%) | Reference | |
|---|---|---|---|---|---|
| Pre-Stress | Stress | ||||
| P. nigra | Lab | 15.7 nmol m−2s−1 | 24.9 nmol m−2s−1 | 58.0 | Short durations (this study) |
| P. nigra | Lab | 15.7 nmol m−2s−1 | 25.9 nmol m−2s−1 | 64.2 | Long durations (this study) |
| Quercus ilex | Prades forest, Catalonia | N.A | N.A | 68 | [32] |
| Xerophyta humilis | Lab | 1 nmol m−2s−1 | 4 nmol m−2s−1 | 300 | [27] |
| Pinus massoniana | Lab | N.A | N.A | 190 | [56] |
| Ficus septica | Lab | N.A | N.A | 160 | [57] |
| Cistus monspeliensis | Natural Reserve, Italy | 210 nmol m−2s−1 | 340 nmol m−2s−1 | 61.3 | [58] |
| Quercus pubescens | A forest in France | 78.4 μgC−1gDMh−1 | 104.8 μgC−1g DMh−1 | 33.7 | [26] |
| Populus deltoides | Lab | 37.6 nmol m−2s−1 | 48.8 nmol m−2s−1 | 37.4 | [59] |
| Plant | Drought Durations | Rehydr-ation | Emission Rates (nmol m−2s−1) | References | ||
|---|---|---|---|---|---|---|
| Rehydration | Pre-Stress | Stress | ||||
| P. nigra | 8 Days | 3 Days | 18.3 | 18.3 | 18.3 | Short durations (this study) |
| P. nigra | 17 Days | 5 Days | 17.8 | 17.8 | 17.8 | Long Durations (this study) |
| Quercus virginiana Mill. | 12Days | 4 Dys | 20.5 | 20.5 | 20.5 | [55] |
| Robinia pseudoacacia L. | N.A | N.A | Completely recover | Completely recover | Completely recover | [68] |
| Xerophyta humilis | RWC 0% | RWC 100% | 5.2 | 5.2 | 5.2 | [27] |
| Populus alba | FTSW5 | 7 Days | 24.58 | 24.58 | 24.58 | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Zhang, Y.; Zhang, H.; Ge, X.; Gu, D.; Liu, X.; Bai, J.; Ma, Z.; Tan, Y.; Zhu, F.; et al. Impacts of Drought and Rehydration Cycles on Isoprene Emissions in Populus nigra Seedlings. Int. J. Environ. Res. Public Health 2022, 19, 14528. https://doi.org/10.3390/ijerph192114528
Han Z, Zhang Y, Zhang H, Ge X, Gu D, Liu X, Bai J, Ma Z, Tan Y, Zhu F, et al. Impacts of Drought and Rehydration Cycles on Isoprene Emissions in Populus nigra Seedlings. International Journal of Environmental Research and Public Health. 2022; 19(21):14528. https://doi.org/10.3390/ijerph192114528
Chicago/Turabian StyleHan, Zhiyu, Yisheng Zhang, Houyong Zhang, Xuan Ge, Dasa Gu, Xiaohuan Liu, Jianhui Bai, Zizhen Ma, Yan Tan, Feng Zhu, and et al. 2022. "Impacts of Drought and Rehydration Cycles on Isoprene Emissions in Populus nigra Seedlings" International Journal of Environmental Research and Public Health 19, no. 21: 14528. https://doi.org/10.3390/ijerph192114528
APA StyleHan, Z., Zhang, Y., Zhang, H., Ge, X., Gu, D., Liu, X., Bai, J., Ma, Z., Tan, Y., Zhu, F., Xia, S., Du, J., Tan, Y., Shu, X., Tang, J., & Sun, Y. (2022). Impacts of Drought and Rehydration Cycles on Isoprene Emissions in Populus nigra Seedlings. International Journal of Environmental Research and Public Health, 19(21), 14528. https://doi.org/10.3390/ijerph192114528