Abstract
Both type II and Z schemes can explain the charge transfer behavior of the heterojunction structure well, but the type of heterojunction structure formed between bismuth vanadium oxide and carbon nitride still has not been clarified. Herein, we rationally prepared bismuth vanadium oxide with {010} and {012} facets predominantly and carbon nitride as a decoration to construct a core-shell structure with bismuth vanadium oxide wrapped in carbon nitride to ensure the same photocatalytic reaction interface. Through energy band establishment and radical species investigation, both {010} and {012} facets dominated bismuth vanadium oxide/carbon nitride composites exhibit the type II heterojunction structures rather than the Z-scheme heterojunctions. Furthermore, to investigate the effect of type II heterojunction, the photocatalytic tetracycline degradations were performed, finding that {010} facets dominated bismuth vanadium oxide/carbon nitride composite demonstrated the higher degradation efficiency than that of {012} facets, due to the higher conduction band energy. Additionally, through the free radical trapping experiments and intermediate detection of degradation products, the superoxide radical was proven to be the main active radical to decompose the tetracycline molecules. Therein, the tetracycline molecules were degraded to water and carbon dioxide by dihydroxylation-demethylation-ring opening reactions. This work investigates the effect of crystal planes on heterojunction types through two different exposed crystal planes of bismuth vanadate oxide, which can provide some basic research and theoretical support for the progressive and controlled synthesis of photocatalysts with heterojunction structures.
1. Introduction
Tetracycline (TC) is known as the second most widely used organic compound of broad-spectrum antibiotics in the world and is also applied extensively in medical and farming applications [1,2]. TC and their derivatives are stable in the environment, which tends to damage the ecosystem and affect human health, even increasing drug resistance in humans as well as in plants and animals [3]. In recent years, the abuse of TC has led to frequent detection in wastewater emissions, surfaces and groundwater, and even in soil environments [4,5,6,7,8]. Therefore, it is urgent to find an efficient and green way to solve the problem of TC pollution. Photocatalytic semiconductor technology, with the utilization of solar energy, has high catalytic oxidation activity and is environmentally friendly, which has also proven to be a very viable strategy and has attracted the attention of researchers worldwide [9,10,11,12,13].
Many semiconductor materials such as titanium dioxide [14], zinc oxide [15], cuprous oxide [16], bismuth vanadium oxide (BiVO4) [17], and carbon nitride (g-C3N4) [18,19] have been successfully used in the photocatalytic degradation of organic pollutants. Among these materials, it has been proven that BiVO4-based platforms with different exposed crystalline planes were able to drive electrons and holes to distinct dominant crystal faces due to their variant surface energy levels [20]. Such advantages will greatly facilitate the separation of electrons and holes, thus availably improving the performance of photocatalytic semiconductors. Nevertheless, limited by the local distance of transmission, photo-generated electrons and holes excited by light irradiation on individual semiconductors still tend to combine with each other [21]. In this case, through constructing heterojunction constructions between BiVO4 and other semiconductors, the photogenerated carriers can be effectively separated by band matching [22,23,24].
However, for the configurations of BiVO4 heterojunction complex photocatalysts, there are many controversies. For example, Wang et al. [25] prepared BiVO4/g-C3N4 heterojunction composites, following the type II model of electron transfer, demonstrating increased photocatalytic degradation performance on TC. However, Li et al. [26,27,28] suggested that the electron transfer in BiVO4/g-C3N4 heterojunctions followed a Z- scheme model. The reason why the controversy is emerging is that, whether following the type II or Z-scheme heterojunction structure, both the carrier separation behavior and photocatalytic performance improvement can be well explained. However, for the sake of scientific rigor, the accurate and actual heterojunction-type structure should be figured out. Since the exposed crystal facets of semiconductors can affect their energy band constructures [29,30,31], which may further affect the heterojunction establishments, thus the different facets of exposed BiVO4/g-C3N4 composites should be investigated.
In this paper, the separately dominant {010} and {012} facets exposed BiVO4 crystals are synthesized and combined with g-C3N4 to form heterojunction structures ({010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4). Due to the flat and ordered crystal facets, the g-C3N4 sheets could be tightly packed around crystals of {010}BiVO4 and {012}BiVO4, impeding the direct contact of reactants by {010}BiVO4 and {012}BiVO4. This simplifies the carrier flow investigation and influence analysis of the TC degradation. Thus, through the establishment of energy band, the changes in the type of photogenerated radicals and the degradation performances of TC, both {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 composites proven to be the type II heterojunction structures. Additionally, the {010}BiVO4 with a more negative conduction band than {012}BiVO4 can facilitate a higher fabrication amount of superoxide radical (O2•−). In the BiVO4/g-C3N4 system, the O2•− radical exhibits a much higher efficiency than the hydroxyl radical (•OH) for TC degradation. This work is excepted to settle the contradictory phenomena on the heterojunction structures of BiVO4/g-C3N4 composites.
2. Experimental
2.1. Materials
All the chemical materials and reagents are not purified in any further way. Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, 98%), ammo-nium vanadate (NH4VO3, 99.0%), bismuth oxide (Bi2O3), and vanadium oxide (V2O5) were purchased from Innochem. Moreover, tetracycline (C22H24N2O8), sodium dodecylbenzene sulfonate (SDBS), urea (CH4N2O, 99.0%), isopropyl alcohol (C3H8O,99.5%), Sodium sulfate anhydrous (Na2SO4, 99.0%), p-Benzoquinone (C6H4O2), and Triethanolamine (C6H15NO3) were purchased from Macklin. Deionized water with a resistivity of 18.25 MΩ cm−1 was used in all experiments.
2.2. Synthesis of the g-C3N4
At a rate of 0.5 °C/min, 10 g urea in alumina crucible (with cover) was heated to 550 °C in air atmosphere and kept at this temperature for 3 h. Afterward, it was cooled naturally to room temperature, and the yellowish powder was then collected.
2.3. Preparations of {012}BiVO4 and {012}BiVO4/g-C3N4
For the synthesis of {012}BiVO4, 2 mmol bismuth nitrate, 200 mg SDBS, 1.25 M nitric acid solution, and 2 mmol ammonium vanadate were added to 100 mL of deionized water and stirred for 30 min. The solution was then transferred to a polytetrafluoroethylene hydrothermal reactor and placed in an oven at 150 °C for 12 h. After completion of the reaction, the reactor was naturally cooled to room temperature, and the product was collected by filtration and washed several times with deionized water.
For the preparation of {012}BiVO4/g-C3N4, the prepared g-C3N4 was added to the precursor solution after the stirring process, and the other steps were the same as above.
2.4. Preparation of {010}BiVO4 and {010}BiVO4/g-C3N4
For the synthesis of {010}BiVO4, 2.5 mmol of bismuth oxide and vanadium oxide were added to 25 mL of nitric acid solution (0.5 M) and subsequently stirred on a magnetic stirrer for 96 h. Then the product was collected by filtration and washed several times with deionized water.
For the preparation of {010}BiVO4/g-C3N4, the prepared {010}BiVO4 was dispersed into an ethanol solution, followed by the addition of g-C3N4/ethanol solution (20 mg/mL) and stirred at room temperature for 24 h. The subsequent steps were then carried out for the preparation of {010}BiVO4.
2.5. Structure and Characterization of Materials
The crystal phase and purity of the catalyst were determined by X-ray diffractive apparatus (XRD, Palytical X’Pert Powder) of Panaco. The microstructure and elements of the powder samples were analyzed by field emission scanning electron microscopy (SEM, JSM-7001F) and energy dispersive X-ray spectroscopy (EDX, JSM-701F). The structural characteristics of the samples were observed through a transmission electron microscope (TEM, JSM-2100F) made by Hitachi. The surface element composition and existence state of the synthesized materials were analyzed by X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific K-Alpha). The chemical bonds and groups in the samples were analyzed and determined by Fourier infrared spectroscopy (FT-IR, Thermo 6700) from Thermo Field Company. Chemical bonds and groups in the samples were analyzed. The hydroxyl radical and superoxide radical of the samples were analyzed by electron paramagnetic resonance (EPR, A300) spectrometer of Brook Corporation. The comparative area of the samples was measured by the BET tester (BET, ASAP2460) of Mack Company. The intermediate products of the photocatalytic degradation of TC were tested by waters single quadrupole liquid mass spectrometry (LC-MS, ZQ2000). The optical absorption properties of the synthesized materials were characterized by UV-Vis diffuse reflectance spectroscopy (UV-VIS DRS, U-3900) from Hitachi, which scanning range was 200–800 nm, and BaSO4 powder was used as a reference.
2.6. Photocatalytic Activity Experiment
100 mL of TC aqueous solution with a concentration of 50 mg L−1 was measured and introduced into a 250 mL reactor, and 20 mg of the sample was then added. After evenly dispersed, the sample was stirred in the dark for 30 min. During this period, 3.5 mL of the above solution was taken out every 15 min. After being filtered by a microporous membrane, the clear solution was utilized as analyte, and the adsorption rate of TC was measured using Agilent’s UV/Vis spectrophotometer (Carry 60). After the adsorption and desorption phases were balanced, the above solution was placed under a 300W xenon lamp and illuminated for 60 min. During this period, 3.5 mL of the above solution was taken out every 15 min and filtered, and the concentration of TC was measured by ultraviolet/visible spectrophotometer, and the light absorption intensity at 357 nm was recorded. The degradation rate of TC was calculated by Formula (1).
where is the absorbance of the TC when adsorption equilibrium is reached, and C is the absorbance of the TC measured by timed sampling.
2.7. Photoelectrochemical Tests
The electrochemical workstation (CHI920D) was used for photoelectric test. Ag/AgCl was employed as reference electrode, platinum electrode as counter electrode, and sodium sulfate (Na2SO4) with 1 M electrolyte as electrolyte. Preparation of working electrode: 1 mg sample was dispersed in 1 mL deionized water, and ultrasound was carried out for 20 min. 100 μL of the dispersion was then measured with a pipette gun and dropped onto the cleaned FTO conductive glass. The photochemical and electrochemical properties of the samples were tested after natural air drying.
2.8. Photocatalytic Trapping Agent Experiment
Isopropyl alcohol (IPA; 0.1M), triethanolamine (TEOA; 0.1 M), and benzoquinone (BQ; 0.2 mM) were added into TC solution (100 mL; 50 mg/L) mixed with 20 mg catalyst, after adsorption equilibrium, the illumination was then performed. During this period, 3.5 mL of the above solution was taken out every 15 min, and its absorbance at 357 nm was recorded with an ultraviolet/visible spectrophotometer. Finally, the degradation rate was calculated according to Formula (1).
3. Results and Discussion
3.1. Characteristics
Photocatalysts of {010}BiVO4 and {012}BiVO4 were separately prepared through the wet chemistry method and hydrothermal method, according to the previous reports [17,32]. g-C3N4 was prepared by a direct calculation method as described in the experimental section. The different loading ratios between {010}BiVO4 and g-C3N4 were via the physical hybrid approaches by long-time stirring operations; meanwhile, the {012}BiVO4/g-C3N4 composites were fabricated by adding different amounts of the prepared g-C3N4 into the precursor solutions of {012}BiVO4. After the synthesis, the architectures and morphologies of {010}BiVO4, {012}BiVO4, g-C3N4, {010}BiVO4/g-C3N4, and {012}BiVO4/g-C3N4 were investigated by SEM and TEM. The pure {010}BiVO4 sample displays a sheet shape with a length of ca. 1 μm (Figure 1a), while the {012}BiVO4 depicts a near-octahedral shape with a size of about 5 μm (Figure 1b). The morphology of g-C3N4 exhibits a sheet structure, as shown in Figure 1c,d. To verify the successful loading of g-C3N4 onto BiVO4, further SEM tests of {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 were performed. Since the {010}BiVO4/0.2g-C3N4 and {012}BiVO4/0.1g-C3N4 equipped the highest TC degradation performances (discussed in the section of photocatalytic performance) compared to other {010}BiVO4/xg-C3N4 and {012}BiVO4/xg-C3N4 composites, the {010}BiVO4/0.2g-C3N4 and {012}BiVO4/0.1g-C3N4 were taken as instances to investigate the morphologies and structures. Therein, the x values from {010}BiVO4/xg-C3N4 and {012}BiVO4/xg-C3N4 represent the molar ratio of g-C3N4 to BiVO4. As shown in Figure 1e–h, the two hybrids of {010}BiVO4/0.2g-C3N4 and {012}BiVO4/0.1g-C3N4 retained their flake or octahedral shapes unchanged and were almost in the same size. Magnifying the image of {010}BiVO4/0.2g-C3N4 based on TEM tests (Figure 1i), a nanosheet shell of g-C3N4 is observed to be evenly covered on the surface of {010}BiVO4. For {012}BiVO4/g-C3N4, g-C3N4 is also found to be homogeneously distributed on the surface of {012}BiVO4 with a tiny nanosheet structure (Figure 1h), indicating that {012}BiVO4 is completely wrapped in a g-C3N4 sheet. Further chemical element distribution analyses (Figure 1k,l) indicate that the g-C3N4 (elements of C and N) compound covers completely and uniformly on the surfaces of {010}BiVO4 and {012}BiVO4 (elements of Bi, V, and O) manifesting the successful combinations between g-C3N4 and {010}BiVO4 or {012}BiVO4.
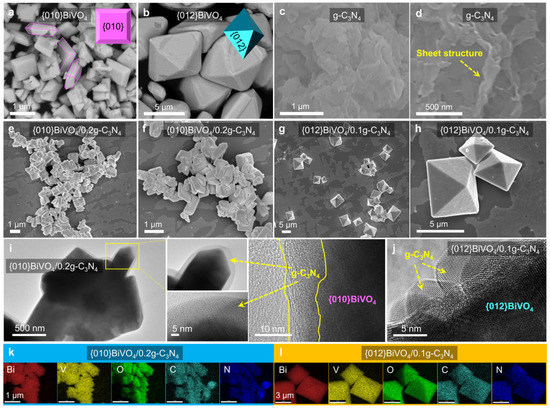
Figure 1.
(a) SEM images of {010}BiVO4, (b) {012}BiVO4, (c,d) g-C3N4, (e,f) {010}BiVO4/0.2g-C3N4, and (g,h) {012}BiVO4/0.1g-C3N4. (i) TEM images of {010}BiVO4/0.2g-C3N4 and (j) {012}BiVO4/0.1g-C3N4. (k) SEM mapping images of {010}BiVO4/0.2g-C3N4 and (l) {012}BiVO4/0.1g-C3N4.
The successful fabrications of {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 can also be verified by XRD patterns and FTIR spectra. As shown in Figure 2a, it is found that the {010}BiVO4 and {012}BiVO4 exhibited strong diffraction peaks at about 2θ = 29.0°, 30.7°, 34.7°, 35.4°, 40.0°, 42.5°, 47.0°, 50.1°, 53.4°, 58.5°, and 59.7°, which corresponds to the (112), (004), (200), (020), (121), (015), (204), (220), (116), (312), and (026) crystal facets of the monoclinic BiVO4 (PDF#75-1867), respectively. At the same time, no other diffraction peak is involved indicating a pure crystal phase of BiVO4 [33]. After integration with g-C3N4, it is observed that no crystal destruction happened on both {010}BiVO4 and {012}BiVO4. No obvious g-C3N4 diffraction peaks appeared in both {010}BiVO4/0.2g-C3N4 and {012}BiVO4/0.1g-C3N4 samples, which may be ascribed to the small loading amount of g-C3N4 [34,35]. The structural composition of {010}BiVO4/0.2g-C3N4 and {012}BiVO4/0.1g-C3N4 were explored by FTIR spectra. As illustrated in Figure 2b, {010}BiVO4 appears the vibrational peaks at 470 and 612 cm−1, which should be attributed to the symmetric bending vibration of VO43− and the stretching vibration of Bi-O, respectively [36,37]. While for {012}BiVO4, it is observed that the peak wave numbers of symmetric bending vibration of VO43− and the stretching vibration of Bi-O decrease to 452.22 and 561.22 cm−1, respectively. The different vibrations of {010}BiVO4 and {012}BiVO4 may be attributed to the diversely exposed crystal planes. Additionally, the vibrational peaks of g-C3N4 from the {010}BiVO4/0.2g-C3N4 and {012}BiVO4/0.1g-C3N4 samples can also be detected at 1231, 1309, and 1399 cm−1, which are attributed to the C-N aromatic ring vibrations [30]. These confirm the successful integration between BiVO4 and g-C3N4.
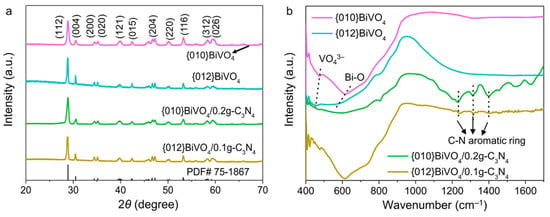
Figure 2.
(a) XRD patterns and (b) FTIR spectra of {010}BiVO4, {012}BiVO4, {010}BiVO4/0.2g-C3N4, and {012}BiVO4/0.1g-C3N4.
The surface chemical compositions and electron states of {010}BiVO4/0.2g-C3N4 and {012}BiVO4/0.1g-C3N4 were analyzed by XPS. The survey XPS spectra (Figure 3a) demonstrate that the elements of Bi, V, and O existed in all of {010}BiVO4, {010}BiVO4/0.2g-C3N4, {012}BiVO4, and {012}BiVO4/0.1g-C3N4 samples, indicating the successful fabrications of BiVO4. The N peaks in the {010}BiVO4/0.2g-C3N4 and {012}BiVO4/0.1g-C3N4 were also detected at a binding energy of about 400 eV after loading g-C3N4, suggesting that g-C3N4 has been successfully loaded on both {010}BiVO4 and {012}BiVO4 surfaces. Moreover, the high-resolution XPS patterns of Bi 4f on {010}BiVO4, {010}BiVO4/0.2g-C3N4, {012}BiVO4, and {012}BiVO4/0.1g-C3N4 has been investigated. As shown in Figure 3b, peaks at 159.3 and 164.6 eV correspond to Bi3+ 4f7/2 and Bi3+ 4f5/2 [38], respectively, which implies that element Bi is present in {010}BiVO4 at +3 valence. While after introducing the g-C3N4 substrate, the locations of Bi3+ 4f7/2 and Bi3+ 4f5/2 shift to 159.1 and 164.4 eV, respectively. Similar results also have happened between {012}BiVO4 and {012}BiVO4/0.1g-C3N4, where the locations of Bi3+ 4f7/2 and Bi3+ 4f5/2 change from 159.1 and 164.4 eV to 159.0 and 164.3 eV, respectively. These indicate that the electrons transfer from BiVO4 to g-C3N4. The same result can also be reflected on the high-resolution XPS spectra of V 2p, as shown in Figure S1a. Furthermore, The high-resolution XPS spectra of O 1s were illustrated with two or three peaks of deconvolution in Figure S1b. Peaks at about 530.0 and 532.0 eV are assigned to the lattice O2- and adsorbed O2- molecules, respectively [39,40]. Interestingly, for {010}BiVO4, a characteristic peak of adsorbed O2- was observed at about 531.4 eV, which can be attributed to the adsorbed -OH [41]. Instead, for {012}BiVO4, the characteristic peak of adsorbed O2- is at about 532.5 eV that originates from the C=O adsorption [37]. These different properties can ascribe to the diversely exposed crystal planes, which are in accordance with the analysis of FTIR. Furthermore, after introducing g-C3N4 substrate, the binding energy of lattice O2- decreased in both {010}BiVO4/0.2g-C3N4 and {012}BiVO4/0.1g-C3N4, indicating the electrons transfer from BiVO4 to g-C3N4 and corresponding to results of XPS spectra of Bi 4f and V 2p.
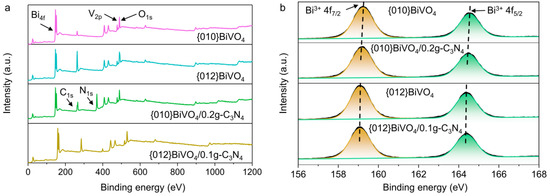
Figure 3.
(a) Survey XPS spectra and (b) high-resolution XPS spectra of Bi 4f on {010}BiVO4, {010}BiVO4/0.2g-C3N4, {012}BiVO4, and {012}BiVO4/0.1g-C3N4.
3.2. Construction of Energy Band Structure
To judge the heterojunction structures of {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4, the energy band structures with alignments of {010}BiVO4, {012}BiVO4, and g-C3N4 should be tested and established. UV-diffuse absorption spectra of g-C3N4, {010}BiVO4, {012}BiVO4, {010}BiVO4/0.2g-C3N4, and {012}BiVO4/0.1g-C3N4 samples were first studied (Figure S2) and demonstrated decently visible absorptions maximum to 550 nm facilitating the subsequent visible light degradation performances. Based on the tauc equation, the bandgap values of {010}BiVO4, {012}BiVO4, and g-C3N4 were calculated to be 2.41, 2.39, and 2.80 eV (Figure 4a), which were transformed from the corresponding UV-diffuse absorption spectrum. The conduction band potentials of {010}BiVO4, {012}BiVO4, and g-C3N4 were calculated by electrochemical experiments. As shown in Figure 4b, positive tangents in Mott–Schottky plots of {010}BiVO4, {012}BiVO4, and g-C3N4 were observed, which reveals the n-type semiconductor characteristics of these prepared samples [42,43]. For n-type semiconductors, the conduction band (ECB) is considered very close to the flat band [44,45]. Hence, by calculating the intercept of the tangent line in Mott–Schottky plots, the conduction band energy potentials of {010}BiVO4, {012}BiVO4, and g-C3N4 were estimated as −0.45, −0.24 and −0.07 V vs. NHE at pH = 0, respectively. Since the bandgap energy has been confirmed by UV-diffuse absorption spectrum, through Formula (2), the valence band (EVB) potentials of {010}BiVO4, {012}BiVO4, and g-C3N4 were calculated to be 1.96, 2.15, and 2.73 V vs. NHE at pH = 0, respectively.
Eg = EVB − ECB
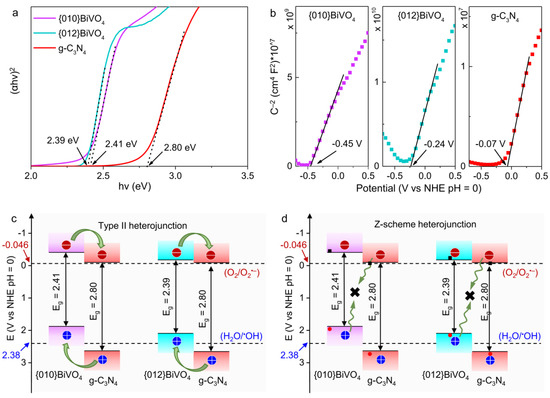
Figure 4.
(a) Tauc curves of {010}BiVO4, {012}BiVO4, and g-C3N4 for bandgap tests. (b) Mott–Schottky plots of {010}BiVO4, {012}BiVO4, and g-C3N4 for conduction band energy tests. (c) The band structures of {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 with type II heterojunction. (d) The band structures of {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 with Z-scheme heterojunction.
Matching the band energy of {010}BiVO4 and {012}BiVO4 with a g-C3N4 display that two types of heterojunctions can be formed for {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4: type II heterojunction (Figure 4c) and Z-scheme heterojunction (Figure 4d). For type II heterojunction, under light irradiation, the photoinduced electrons from the conduction bands of {010}BiVO4 and {012}BiVO4 can flow to g-C3N4 with lower conduction band energy. Moreover, the photoinduced holes from the valence bands of g-C3N4 can inject into {010}BiVO4 and {012}BiVO4 with higher valence band energy potentials. Thus, theoretically, the photoelectrons accumulated on the conduction band of g-C3N4 and the photoholes on the valence bands of {010}BiVO4 and {012}BiVO4 can separately engage in the reduction and oxidation reactions. However, considering that both {010}BiVO4 and {012}BiVO4 have been wrapped by g-C3N4 sheets, merely the photoelectrons from the conduction band of g-C3N4 can participate in the anticipated reduction reaction. At the same time, the photoholes can only be trapped in {010}BiVO4 and {012}BiVO4 crystals. For Z-scheme heterojunction, under light irradiation, the photoinduced holes from the valence bands of {010}BiVO4 and {012}BiVO4 will integrate and dissipate with the photoinduced electrons from the conduction bands of g-C3N4, leaving the photoelectrons on the conduction bands of {010}BiVO4 and {012}BiVO4 as well as the photoholes on the valence band of g-C3N4. Similarly, given the morphologies of {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4, only the photoholes on the valence band of g-C3N4 can engage in the desirable oxidation reaction. Based on the above analyses of carrier transfer, the actual heterojunctions of {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4, either type II heterojunction or Z-scheme heterojunction, can be figured out by radical tests and photodegradation performances interpretations.
3.3. Radical Species Analyses
To figure out the heterojunction types of {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4, the radical species have been analyzed. According to the previous reports [46,47], the •OH radical generation potential from H2O oxidation is 2.38 V vs. NHE at pH = 0 (Equation (3)), and O2 can be reduced to O2•− radical by photoelectrons at −0.046 V vs. NHE at pH = 0 (Equation (4)).
H2O + h+ → •OH + H+ E = 2.38 V vs. NHE at pH = 0
O2 + e− → O2•− E = −0.046 vs. NHE at pH = 0
Therefore, based on the energy band structures, for {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 with type II heterojunction (Figure 4c), the O2•− should be the main radical species, since the photoelectrons of {010}BiVO4 and {012}BiVO4 will flow into the g-C3N4 shell for O2 reduction while the photoholes of g-C3N4 will be trapped in the inner core of {010}BiVO4 and {012}BiVO4. On the contrary, for Z-scheme heterojunction (Figure 4d), the •OH should be the dominant radical species because the photoholes of g-C3N4 can oxidize H2O into •OH, but the photoelectrons are arrested by inner {010}BiVO4 and {012}BiVO4.
To reveal the practical radical species fabrications, the EPR spectra were carried out for g-C3N4, {010}BiVO4/0.2g-C3N4 and {012}BiVO4/0.1g-C3N4 systems under aerobic conditions. Both •OH (Figure 5a) and O2•− radicals (Figure 5b) were generated using g-C3N4 as a photocatalyst, which is reasonable as the valence band and conduction band energy potentials of g-C3N4 are more positive and negative than H2O oxidation and O2 reduction potentials. After the combination of {010}BiVO4 or {012}BiVO4 with g-C3N4, the concentrations of •OH radical decreased considerably, while the concentration promotions of O2•− radical were observed. The change trends of •OH and O2•− radical conform to the type II heterojunction. Therein, the photoelectrons from {010}BiVO4 or {012}BiVO4 transfer into g-C3N4, elevating the O2 reduction for O2•− radical fabrication. However, the photoholes of g-C3N4 migrate to the wrapped {010}BiVO4 or {012}BiVO4 inhibiting the H2O oxidation into •OH radical. Therefore, both heterojunctions of {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 have proven to be type II heterojunctions.
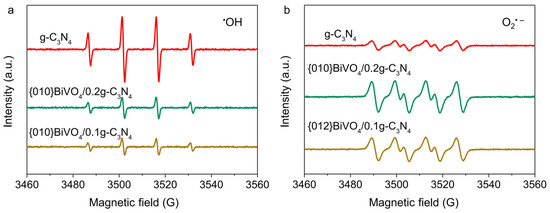
Figure 5.
(a) EPR spectra of •OH and (b) O2•− radicals for g-C3N4, {010}BiVO4/0.2g-C3N4, and {012}BiVO4/0.1g-C3N4.
3.4. Photocatalytic Performances
The type II heterojunctions of {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 can also be proven by their photocatalytic performances. The photocatalytic performances of {010}BiVO4, {010}BiVO4/g-C3N4, {012}BiVO4, and {012}BiVO4/g-C3N4 were investigated by degradations of TC under visible light irradiation. Compared with pure {010}BiVO4 and {012}BiVO4, the additions of g-C3N4 evidently promote the degradation performances of TC molecules. As shown in Figure 6a, after 60 min of light exposure, a maximum of 56% of TC was degraded by {010}BiVO4/0.2g-C3N4, while only 26% was degraded by {012}BiVO4/0.1g-C3N4. These different degradation performances can be explained by the type II heterogeneous structure, as the {010}BiVO4 equips a higher conduction band than {012}BiVO4 driving more photoelectrons into g-C3N4 and promoting a larger amount of O2•− radicals for TC degradation. Moreover, the Z-scheme heterojunction cannot interpret the performance variation. Because, if it is the Z-scheme heterojunction, equal photoholes should remain on the valence band of g-C3N4, producing the same amount of •OH radical and resulting in the same TC degradation performances between {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4. Thus, the Z-scheme heterojunction is repelled. Subsequently, as shown in Figure 6a, the further loading of g-C3N4 led to the reduced degradation performances on both {010}BiVO4/0.3g-C3N4 and {012}BiVO4/0.3g-C3N4, which may be attributed to the thick g-C3N4 shells inhibiting the light absorption of BiVO4. Thus no sufficient photoelectrons were produced and transferred from BiVO4 to g-C3N4 to guarantee the high degradation performances. The kinetic analyses of these photocatalytic reactions were further explored, and the results suggested that they all conformed to the first-order reaction kinetic equations (Figure 6b). The corresponding kinetic rate constant of {010}BiVO4 was estimated to be 0.0064 min−1 (Figure 6c), and with the continuous g-C3N4 loading, the kinetic rate constant of {010}BiVO4/0.2g-C3N4 increased to be 0.0130 min−1. While for {012}BiVO4, the kinetic rate constant only achieved 0.0037 min−1 and increased to 0.0051 min−1 for {012}BiVO4/0.1g-C3N4. Furthermore, the cycling experiments showed that after four cycles (Figure 6d), the degradation efficiency of {010}BiVO4/g-C3N4 exhibited merely a 3% reduction, which represents a relatively stable performance in the field of photocatalysis [48,49,50]. The minor decline in degradation efficiency may be ascribed to the attenuation of surface adsorption with recycling. As shown in Figure S3, the BET spectrum indicates that the specific surface area of {010}BiVO4/g-C3N4 is about 9.6816 m2/g, which represents excellent adsorption performance and can conduce to the photocatalytic activity [51]. However, during the photodegradation of TC, some macromolecular substances should be inevitably absorbed on the surface of {010}BiVO4/g-C3N4 ((Figure 7b) and affect the photocatalytic activity, which could not be emancipate by water clean during the recycle experiment. Therefore, a light degradation efficiency reduction emerged with the recycling of photocatalysis [52].
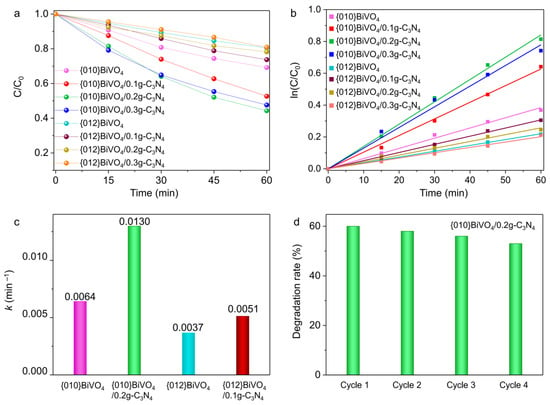
Figure 6.
(a) The photocatalytic degradation curves of TC on {010}BiVO4 and {012}BiVO4 with different g-C3N4 loading amounts. (b) The kinetic analyses of TC degradation curves with (c) the degradation rate constant. (d) Stability tests of TC degradation over {010}BiVO4/g-C3N4.
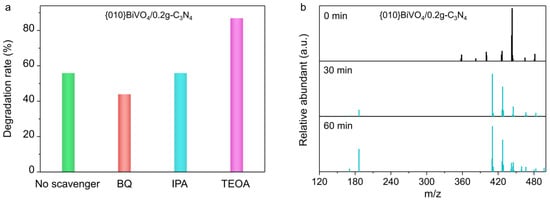
Figure 7.
(a) Trapping experiments of active species during the photocatalytic degradation of TC over {010}BiVO4/0.2g-C3N4. (b) LC-MS spectra of intermediates over {010}BiVO4/0.2g-C3N4 during TC degradation.
3.5. Photocatalytic Degradation Mechanism
To thoroughly and exhaustively explain the mechanism, the roles of radicals were tested. Taking benzoquinone (BQ), isopropyl alcohol (IPA), and triethanolamine (TEOA) as O2•−, •OH, and photoholes capturers, the photocatalytic degradation of TC were performed. As shown in Figure 7a, the TC degradation by {010}BiVO4/0.2g-C3N4 was reduced from 56% to 48% when O2•− trapping agent BQ was introduced. Moreover, the degradation rate was almost unchanged by the addition of •OH trapping agent IPA. This indicates that O2•− rather than •OH is the active radical species for TC degradation over BiVO4/g-C3N4 photocatalyst. The introduction of the hole-trapping agent of TEOA, the degradation rate increased to 83%. This is because TEOA could trap a portion of the holes, inhibiting the recombination of photogenerated carriers, thus leaving more electrons to participate in O2•− generation for TC degradation.
The TC degradation process was speculated based on the degradation intermediates detection by LC-MS. As shown in Figure 7b and Figure S4, the degradation intermediates of TC over {010}BiVO4, {010}BiVO4/0.2g-C3N4, {012}BiVO4, and {012}BiVO4/0.1g-C3N4 are almost identical. Through the molecular debris detection with different m/z values, we speculate that O2•− first promotes the dehydroxylation and demethylation of TC to produce the products with m/z values of 410 and 427. Subsequently, they continued to undergo catalytic ring-opening reactions, decomposing into small molecules with an m/z value of 186, which were eventually broken down completely into CO2 and H2O.
Combined with the band structure, heterojunction type, free radical type, and TC degradation intermediates, the photocatalytic mechanisms on {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 can be speculated (Figure 8). Through the band combination between {010}BiVO4 or {012}BiVO4 with g-C3N4, the type II heterojunctions can be formed. Under visible light irradiation, the photoelectrons generated from {010}BiVO4 and {012}BiVO4 can both flow into the conduction band of g-C3N4 to engage the formation of O2•− radical. While the photoholes from g-C3N4 ingrate into the valence bands of {010}BiVO4 and {012}BiVO4. Since {010}BiVO4 and {012}BiVO4 are encased in g-C3N4 sheets, the trapped photoholes are hard to participate in some reactions. Therefore, O2•− became the main active radical for TC degradation in both {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 systems. Finally, the TC molecules go through dihydroxylation, demethylation, and ring-opening, being decomposed into CO2 and H2O molecules.
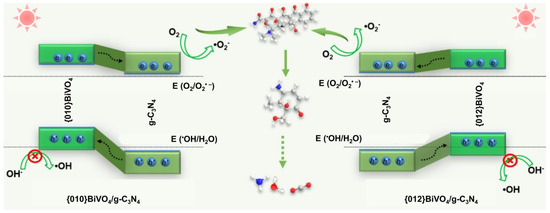
Figure 8.
Photocatalytic mechanism speculations over {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4.
4. Conclusions
In summary, to figure out the confusing heterojunction type between BiVO4 and g-C3N4, we rationally designed {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 as core-shell structures, allowing only photoelectrons or photoelectrodes to participate in oxidation or reduction reactions. Through energy band establishment, radical species investigation, photocatalytic TC degradation performances, and LC-MS tests, both {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 exhibit the type II heterojunction structures rather than the previously reported Z-scheme heterojunctions. The O2•− was proven as the main active radical for TC decomposition in both {010}BiVO4/g-C3N4 and {012}BiVO4/g-C3N4 systems. Moreover, owing to the more negative conduction band of {010}BiVO4 compared with {012}BiVO4, the {010}BiVO4/g-C3N4 demonstrated higher TC degradation efficiency than {012}BiVO4/g-C3N4. Finally, the photocatalytic mechanism of TC degradation was proposed based on the band structure, heterojunction type, free radical changes, and TC degradation intermediates. We expect that this work can become a reference for the construction of heterogeneous complexes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192214770/s1.
Author Contributions
X.Z., X.X.: conceptualization, methodology, investigation, data curation, writing–original draft. J.L.: methodology, investigation, data curation. D.H. (Dongfang Han): investigation, data curation. Y.M.: conceptualization, software, validation. Y.F.: conceptualization, methodology, writing—review and editing, supervision, project administration. D.H. (Dongxue Han): project administration. L.N.: project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was sponsored by the National Natural Science Foundation of China (22172040, 21974031), the Department of Science and Techniques of Guangdong Province (2021A1515010180, 2019B010933001), the Department of Guangdong Provincial Public Security (GZQC20-PZ11-FD084), Science and Technology Projects in Guangzhou (202201000002), Guangzhou Municipal Science and Technology Bureau (202102010449), and Innovation team scientific research project of Guangzhou Education Bureau (202235344).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gobel, A.; McArdell, C.S.; Joss, A.; Siegrist, H.; Giger, W. Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies. Sci. Total Environ. 2007, 372, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Q.; Xiong, G.; Ding, F.; He, Y.; Ren, B.; You, L.; Fan, X.; Hardacre, C.; Sun, Y. Bakelite-type anionic microporous organic polymers with high capacity for selective adsorption of cationic dyes from water. Chem. Eng. J. 2019, 366, 404–414. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Miao, X.S.; Bishay, F.; Chen, M.; Metcalfe, C.D. Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ. Sci. Technol. 2004, 38, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.; Paulo, M.; Silva, L.J.G.; Seifrtová, M.; Lino, C.M.; Solich, P. Tetracycline antibiotics in hospital and municipal wastewaters: A pilot study in Portugal. Anal. Bioanal. Chem. 2010, 396, 2929–2936. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor photocatalysis-past, present, and future outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl. Catal. B Environ. 2015, 162, 494–500. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Wan, C.; Zhou, L.; Xu, S.; Jin, B.; Ge, X.; Qian, X.; Xu, L.; Chen, F.; Zhan, X.; Yang, Y.; et al. Defect engineered mesoporous graphitic carbon nitride modified with AgPd nanoparticles for enhanced photocatalytic hydrogen evolution from formic acid. Chem. Eng. J. 2022, 429, 132388. [Google Scholar]
- Wan, C.; Zhou, L.; Sun, L.; Xu, L.; Cheng, D.; Chen, F.; Zhan, X.; Yang, Y. Boosting visible-light-driven hydrogen evolution from for mic acid over AgPd/2D g-C3N4 nanosheets Mott-Schottky photocatalyst. Chem. Eng. J. 2020, 396, 125229. [Google Scholar] [CrossRef]
- Wang, M.; Ioccozia, J.; Sun, L.; Lin, C.; Lin, Z. Inorganic-modified semiconductor TiO2 nanotube arrays for photocatalysis. Energy Environ. Sci. 2014, 7, 2182–2202. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Shim, J.; Akkinepally, B.; Cho, M.; Yoo, K.; Kim, D. Excellent visible-light driven photocatalyst of (Al, Ni) Co-doped ZnO structures for organic dye degradation. Catal. Today 2020, 340, 277–285. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Zhang, J.; Niu, J.; Zhao, J.; Wei, Y.; Yao, B. Photocatalytic degradation of ciprofloxacin using Zn-doped Cu2O particles: Analysis of degradation pathways and intermediates. Chem. Eng. J. 2019, 374, 316–327. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Cai, B.; Gan, S.; Han, D.; Niu, L.; Wu, T. Hierarchically Z-scheme photocatalyst of Ag@AgCl decorated on BiVO4 (040) with enhancing photoelectrochemical and photocatalytic performance. Appl. Catal. B Environ. 2015, 170–171, 206–214. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Wei, R.; Tang, N.; Jiang, L.; Yang, J.; Guo, J.; Yuan, X.; Liang, J.; Zhu, Y.; Wu, Z.; Li, H. Bimetallic nanoparticles meet polymeric carbon nitride: Fabrications, catalytic applications and perspectives. Coord. Chem. Rev. 2022, 462, 214500. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, G.; Liu, T.; Su, Y.; Ren, H.; Zhang, X.; Xia, A.; Lv, L.; Liu, Y. Photocatalytic properties of the g-C3N4/{010} facets BiVO4 interface Z-Scheme photocatalysts induced by BiVO4 surface heterojunction. Appl. Catal. B Environ. 2018, 234, 37–49. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Li, X.; Zeng, G.; Wang, D.; Niu, C.; Zhao, J.; An, H.; Xie, T.; Deng, Y. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4 (040) Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl. Catal. B Environ. 2017, 200, 330–342. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Wang, J.; François-Xavier, C.P.; Wintgens, T. Highly efficient Z-scheme structured visible-light photocatalyst constructed by selective doping of Ag@AgBr and Co3O4 separately on {010} and {110} facets of BiVO4: Pre-separation channel and hole-sink effects. Appl. Catal. B Environ. 2019, 250, 31–41. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (0 4 0) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Wang, M.; Guo, P.; Zhang, Y.; Liu, T.; Li, S.; Xie, Y.; Wang, Y.; Zhu, T. Eu doped g-C3N4 nanosheet coated on flower-like BiVO4 powders with enhanced visible light photocatalytic for tetracycline degradation. Appl. Surf. Sci. 2018, 453, 11–22. [Google Scholar] [CrossRef]
- Li, C.; Che, H.; Liu, C.; Che, G.; Charpentier, P.A.; Xu, W.Z.; Wang, X.; Liu, L. Facile fabrication of g-C3N4 QDs/BiVO4 Z-scheme heterojunction towards enhancing photodegradation activity under visible light. J. Taiwan Inst. Chem. Eng. 2019, 95, 669–681. [Google Scholar] [CrossRef]
- Li, Z.; Jin, C.; Wang, M.; Kang, J.; Wu, Z.; Yang, D.; Zhu, T. Novel rugby-like g-C3N4/BiVO4 core/shell Z-scheme composites prepared via low-temperature hydrothermal method for enhanced photocatalytic performance. Sep. Purif. Technol. 2020, 232, 115937. [Google Scholar] [CrossRef]
- Le Minh Tri, N.; Cam, N.T.D.; Pham, H.D.; Van Thuan, D.; Pham, T.D.; Nguyen, V.T.; Trung, N.T.; Tung, M.H.T.; Phuong, T.T.T.; Nguyen, T.T.P.; et al. Development of g-C3N4/BiVO4 binary component heterojunction as an advanced visible light-responded photocatalyst for polluted antibiotics degradation. Top. Catal. 2020, 63, 1206–1214. [Google Scholar] [CrossRef]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef]
- LaPara, T.M.; Madson, M.; Borchardt, S.; Lang, K.S.; Johnson, T.J. Multiple discharges of treated municipal wastewater have a small effect on the quantities of numerous antibiotic resistance determinants in the Upper Mississippi river. Environ. Sci. Technol. 2015, 49, 11509–11515. [Google Scholar] [CrossRef]
- Carlesi Jara, C.; Fino, D.; Specchia, V.; Saracco, G.; Spinelli, P. Electrochemical removal of antibiotics from wastewaters. Appl. Catal. B Environ. 2007, 70, 479–487. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Lu, Z.; Wang, G.; Li, P.; Gao, Y.; Huang, X.; Huang, W.; Uji, H.; Lu, G. Synthesis of 42-faceted bismuth vanadate microcrystals for enhanced photocatalytic activity. J. Colloid Interface Sci. 2019, 542, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Praus, P.; Lang, J.; Martaus, A.; Svoboda, L.; Matějka, V.; Kormunda, M.; Šihor, M.; Reli, M.; Kočí, K. Composites of BiVO4 and g-C3N4: Synthesis, properties and photocatalytic decomposition of azo dye AO7 and nitrous oxide. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1219–1234. [Google Scholar] [CrossRef]
- Yu, Y.M.; Liu, K.; Zhang, Y.Y.; Xing, X.; Li, H. Synthesis of g-C3N4/BiVO4 heterojunction composites for photocatalytic degradation of nonylphenol ethoxylate. Sep. Purif. Technol. 2020, 250, 8. [Google Scholar]
- Zhu, J.Y.; Zhu, Y.Y.; Chen, Z.; Wu, S.J.; Fang, X.J.; Yao, Y. Efficient photoelectrochemical performance of gamma irradiated g-C3N4 and its g-C3N4@BiVO4 heterojunction for Solar water splitting. J. Phys. Chem. C 2019, 123, 9013–9026. [Google Scholar]
- Xiao, F.; Xu, J.; Cao, L.; Jiang, S.; Zhang, Q.; Wang, L. In situ hydrothermal fabrication of visible light-driven g-C3N4/SrTiO3 composite for photocatalytic degradation of TC. Environ. Sci. Pollut. Res. Int. 2020, 27, 5788–5796. [Google Scholar] [CrossRef]
- Xu, X.; Zou, Q.; Yuan, Y.; Ji, F.; Fan, Z.; Zhou, B. Preparation of BiVO4-graphene nanocomposites and their photocatalytic activity. J. Nanomater. 2014, 2014, 77. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, J.; Ai, S.; Li, L. As a new peroxidase mimetics: The synthesis of selenium doped graphitic carbon nitride nanosheets and applications on colorimetric detection of H2O2 and xanthine. Sens. Actuators B Chem. 2015, 216, 418–427. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, Y.; Zhang, W.; Zhang, X.; Han, D.; Niu, L.; Ivaska, A. Nanoengineering Construction of Cu2O Nanowire Arrays Encapsulated with g-C3N4 as 3D Spatial Reticulation All-Solid-State Direct Z-Scheme Photocatalysts for Photocatalytic Reduction of Carbon Dioxide. ACS Catal. 2020, 10, 6367–6376. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, X.; Chen, L. BiVO4/BiO0.67F1.66 heterojunction enhanced charge carrier separation to boost photocatalytic activity. J. Nanopart. Res. 2019, 21, 61. [Google Scholar] [CrossRef]
- Wang, G.L.; Zhang, W.Q.; Li, J.Y.; Dong, X.L.; Zhang, X.F. Carbon quantum dots decorated BiVO4 quantum tube with enhanced photocatalytic performance for efficient degradation of organic pollutants under visible and near-infrared light. J. Mater. Sci. 2019, 54, 6488–6499. [Google Scholar] [CrossRef]
- Kuang, P.Y.; Su, Y.Z.; Chen, G.F.; Luo, Z.S.; Xing, Y.; Li, N.; Liu, Z.Q. g-C3N4 decorated ZnO nanorod arrays for enhanced photoelectrocatalytic performance. Appl. Surf. Sci. 2015, 358, 296–303. [Google Scholar] [CrossRef]
- Xu, J.J.; Feng, B.B.; Wang, Y.; Qi, Y.D.; Niu, J.F.; Chen, M.D. BiOCl decorated NaNbO3 nanocubes: A novel p-n heterojunction photocatalyst with Improved activity for ofloxacin degradation. Front. Chem. 2015, 6, 393. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Svenum, I.H.; Ronning, M.; Yang, J. Core-Shell Nanostructures of graphene-wrapped CdS nanoparticles and TiO2 (CdS@G@TiO2): The role of graphene in enhanced photocatalytic H2 generation. Catalysts 2020, 10, 358. [Google Scholar] [CrossRef]
- Akple, M.S.; Ishigaki, T.; Madhusudan, P. Bio-inspired honeycomb-like graphitic carbon nitride for enhanced visible light photocatalytic CO2 reduction activity. Environ. Sci. Pollut. Res. 2020, 27, 22604–22618. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Zhang, L. New insight into daylight photocatalysis of AgBr@Ag: Synergistic effect between semiconductor photocatalysis and plasmonic photocatalysis. Chem. Eur. J. 2012, 18, 6360–6369. [Google Scholar] [CrossRef]
- Li, S.; Xue, B.; Wang, C.; Jiang, W.; Hu, S.; Liu, Y.; Wang, H.; Liu, J. Facile fabrication of flower-like BiOI/BiOCOOH p–n hetero junctions for highly efficient visible-light-driven photocatalytic removal of harmful antibiotics. Nanomaterials 2019, 9, 1571. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, F.; Zhang, H.; Zhang, J.; Han, L. Photocatalytic degradation of 4-chlorophenol over Ag/MFe2O4 (M = Co, Zn, Cu, and Ni) prepared by a modified chemical co-precipitation method: A comparative study. RSC Adv. 2015, 5, 55499–55512. [Google Scholar] [CrossRef]
- Fu, H.; Yang, L.; Hu, D.; Yu, C.; Ling, Y.; Xie, Y.; Li, S.; Zhao, J. Titanium dioxide nano-heterostructure with nanoparticles decorat ing nanowires for high-performance photocatalysis. Int. J. Hydrogen Energy 2018, 43, 10359–10367. [Google Scholar] [CrossRef]
- Nagaraju, P.; Puttaiah, S.H.; Wantala, K.; Shahmoradi, B. Preparation of modified ZnO nanoparticles for photocatalytic degradation of chlorobenzene. Appl. Water Sci. 2020, 10, 137. [Google Scholar] [CrossRef]
- Ganeshbabu, M.; Kannan, N.; Venkatesh, P.S.; Paulraj, G.; Jeganathan, K.; MubarakAli, D. Synthesis and characterization of BiVO4 nanoparticles for environmental applications. RSC Adv. 2020, 10, 18315–18322. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).