Planktothrix agardhii versus Planktothrix rubescens: Separation of Ecological Niches and Consequences of Cyanobacterial Dominance in Freshwater
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Biological and Physical Characteristics of the Studied Lakes
3.2. Physicochemical Characteristics of the Studied Lakes
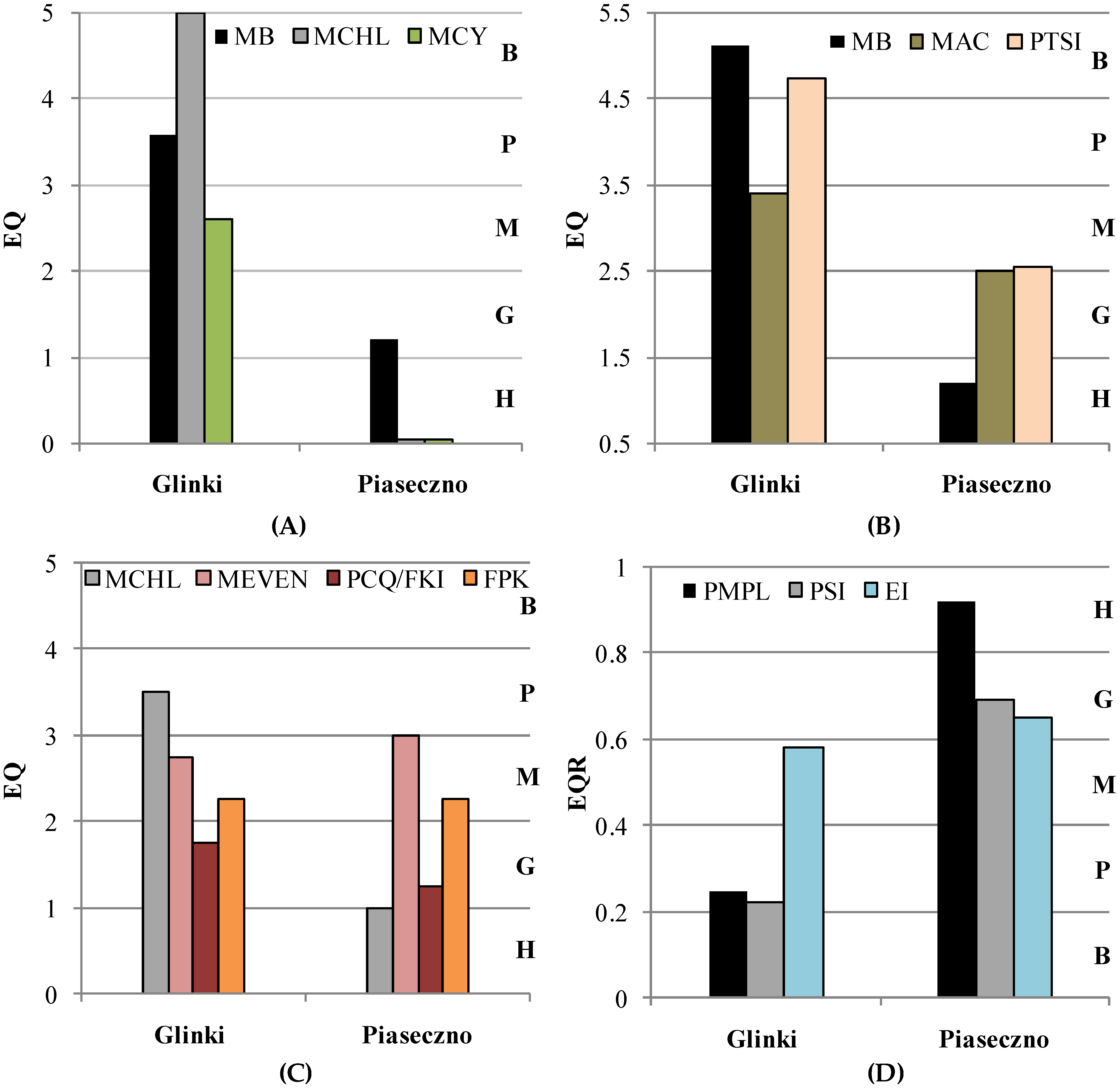
3.3. Assessment of Ecological Status of the Studied Lakes Based on Phytoplankton Indices
4. Discussion
5. Conclusions
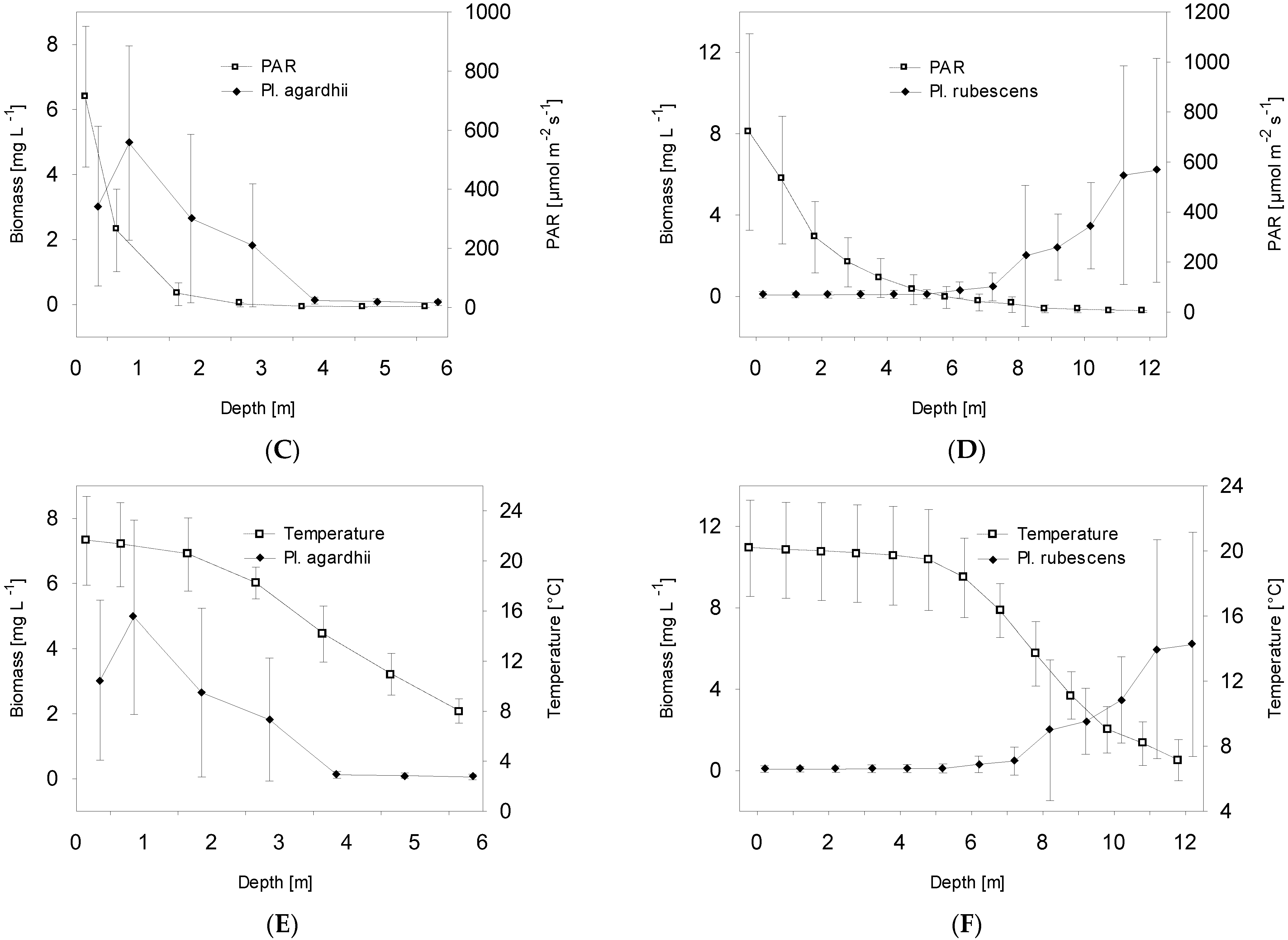
- The development of Planktothrix species is closely related to light conditions. In the studied vegetative periods, the highest biomass of P. agardhii was recorded at a depth of 0.5–1 m under high light intensity, whilst the highest biomass of P. rubescens was recorded at a depth of 11–12 m under very low light intensity, below the boundary of the euphotic layer.
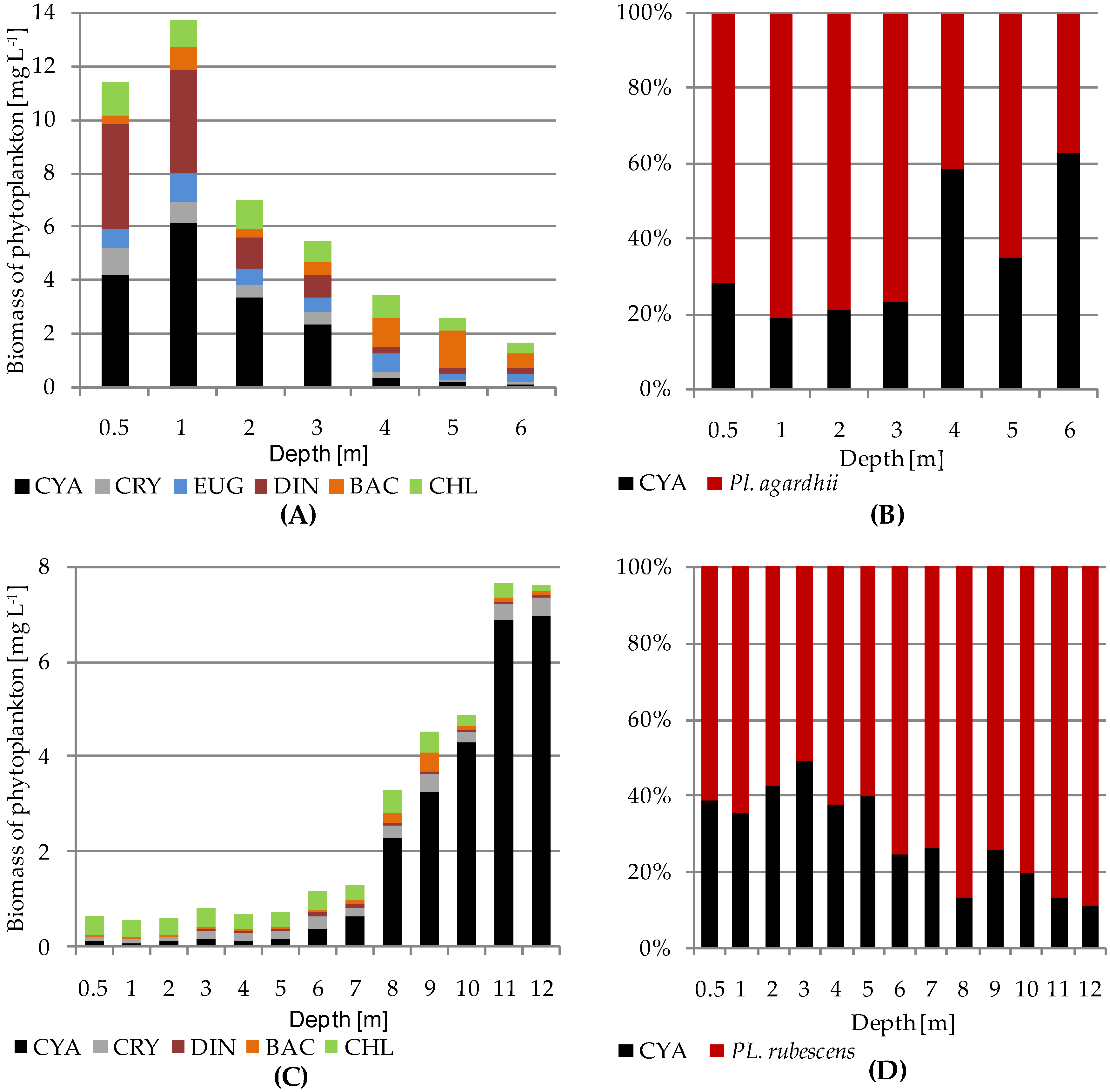
- P. rubescens development led to a rapid and marked decline in biodiversity in the bloom layer (metalimnion) and decreased total phytoplankton biomass in the upper water layers. This implies a significant allelopathic effect of P. rubescens on phytoplankton communities. This effect was not observed for P. agardhii.
- P. rubescens and P. agardhii utilised different dissolved nitrogen fractions for their growth. In particular, P. agardhii in the eutrophic Lake Glinki used ammonium nitrogen, whereas P. rubescens in the mesotrophic Lake Piaseczno used nitrate nitrogen.
- Very low values of dissolved phosphorus fraction were recorded in the bloom layer of P. rubescens, which may produce a potentially limiting effect on the growth of this species. No such effect was observed for P. agardhii.
- PMPL, PSI, and EI are indeed useful for assessing the ecological status of shallow eutrophic lakes, in which phytoplankton blooms occur in the upper water layers. However, problems arise when phytoplankton blooms occur with DCM in the metalimnion of deep lakes. For instance, the highest biomass of P. rubescens occurred outside the euphotic zone. This resulted in a part of the bloom layer being excluded from final calculations based on PMPL and PSI, making it impossible to accurately calculate the ecological status of the lake. Overall, EI, which considers two to three thermal layers of water (depending on the thermal characteristics of a lake), including the phytoplankton bloom layer with DCM, appeared to be the best assessment method.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arvola, L.; George, G.; Livingstone, D.M.; Järvinen, J.; Blenckner, T.; Dokulil, M.T.; Jennings, E.; Aonghusa, C.N.; Nõges, P.; Nõges, T.; et al. The impact of changing climate on thermal characteristics of lakes. In The Impact of Climate Change on European Lakes; George, D.G., Ed.; Aquatic Ecology Series; Springer: Berlin, Germany, 2010; Volume 4, pp. 85–101. [Google Scholar] [CrossRef]
- Dokulil, M.T. Climate impacts on ecohydrological processes in aquatic systems. Ecohydrol. Hydrobiol. 2016, 16, 66–70. [Google Scholar] [CrossRef]
- Woolway, R.I.; Kraemer, B.M.; Lenters, J.D.; Merchant, C.J.; O’Reilly, C.M.; Sharma, S. Global lake responses to climate change. Nat. Rev. Earth Environ. 2020, 1, 388–403. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Munoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Kraemer, B.M.; Pilla, R.M.; Woolway, R.I.; Anneville, O.; Ban, S.; Colom-Montero, W.; Devlin, S.P.; Dokulil, M.T.; Gaiser, E.E.; Hambright, K.D.; et al. Climate change drives widespread shifts in lake thermal habitat. Nat. Clim. Chang. 2021, 11, 521–529. [Google Scholar] [CrossRef]
- Wood, S.A.; Rueckert, A.; Cowman, D.A.; Cary, S.C. Source of edaphic cyanobacterial diversity in the dry valley of Eastern Antarctica. ISME J. 2008, 2, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huismann, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Klähn, S.; Hagemann, M. Compatible solute biosynthesis in cyanobacteria. Environ. Microbiol. 2010, 13, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Dominic, T.K.; Madhusoodanan, P.V. Cyanobacteria from extreme acidic environments. Curr. Sci. 1999, 77, 1021–1023. Available online: http://www.jstor.org/stable/24103571 (accessed on 20 September 2022).
- Pikuta, E.V.; Hoover, R.B.; Tang, J. Microbial extremophiles at the limits of life. Crit. Rev. Microbiol. 2007, 33, 183–209. [Google Scholar] [CrossRef]
- Costa, N.B.; Kolman, M.A.; Giani, A. Cyanobacteria diversity in alkaline saline lakes in the Brazilian Pantanal wetland: A polyphasic approach. J. Plankton Res. 2016, 38, 1389–1403. [Google Scholar] [CrossRef]
- Batista, A.M.M.; Figueredo, C.C.; Giani, A. Variability in a permanent cyanobacterial bloom: Species-specific responses to environmental drivers. FEMS Microbiol. Ecol. 2018, 94, 197. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; Mello, M.M.; van Oosterhout, F.; de Domis, L.; Marinho, M.M. Response of natural cyanobacteria and algae assemblages to a nutrient pulse and elevated temperature. Front. Microbiol. 2018, 9, 1851. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.W.; Reinl, K.L.; Lafrancois, B.M.; Brovold, S.; Miller, T.R. A first assessment of cyanobacterial blooms in oligotrophic Lake Superior. Limnol. Oceanogr. 2020, 65, 2984–2998. [Google Scholar] [CrossRef]
- Reinl, K.L.; Brookes, J.D.; Carey, C.C.; Harris, T.D.; Ibelings, B.W.; Morales-Williams, A.M.; De Senepront Domis, L.N.; Atkins, K.S.; Isles, P.D.F.; Mesman, J.P.; et al. Cyanobacterial blooms in oligotrophic lakes: Shifting the high-nutrient paradigm. Freshw. Biol. 2021, 66, 1846–1859. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, G.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Binding, C.E.; Zastepa, A.; Zeng, C. The impact of phytoplankton community composition on optical properties and satellite observations of the 2017 western Lake Erie algal bloom. J. Great Lakes Res. 2019, 45, 573–586. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Engström-Öst, J.; Viitasalo, M. Turbidity decreases anti-predator behaviour in pike larvae, Esox lucius. Environ. Biol. Fish. 2005, 73, 1–8. [Google Scholar] [CrossRef]
- Scheffer, M.; van Nes, E.H. Shallow lakes theory revisited: Various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 2007, 584, 455–466. [Google Scholar] [CrossRef]
- Wilk-Woźniak, E. An introduction to the ‘micronet’ of cyanobac-terial harmful algal blooms (CyanoHABs): Cyanobacteria, zooplank-ton and microorganisms: A review. Mar. and Freshw. Res. 2020, 71, 636–643. [Google Scholar] [CrossRef]
- Gao, X.; Wang, W.; Ndayishimiye, J.C.; Govaert, L.; Chen, H.; Jeppesen, E.; Xue, Y.; Yu, X.; Yang, J. Invasive and toxic cyanobacteria regulate allochthonous resource use and community niche width of reservoir zooplankton. Freshw. Biol. 2022, 67, 1344–1356. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Macrophytes-cyanobacteria allelopathic interactions and their implications for water resources management—A review. Limnologica 2017, 63, 122–132. [Google Scholar] [CrossRef]
- Al-Sammak, M.A.; Hoagland, K.D.; Cassada, D.; Snow, D.D. Co-occurrence of the Cyanotoxins BMAA, DABA and Anatoxin-a in Nebraska Reservoirs, Fish, and Aquatic Plants. Toxins 2014, 6, 488–508. [Google Scholar] [CrossRef] [PubMed]
- Kubickova, B.; Babica, P.; Hilscherová, K.; Šindlerová, L. Effects of cyanobacterial toxins on the human gastrointestinal tract and the mucosal innate immune system. Environ. Sci. Eur. 2019, 31, 31. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. (Eds.) Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- OJEC. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Commun. 2000, L 327, 1–72. [Google Scholar]
- Pasztaleniec, A. Phytoplankton in the ecological status assessment of European lakes—Advantages and constraints. Environ. Prot. Nat. Resour. 2016, 27, 26–36. [Google Scholar] [CrossRef]
- Komárek, J.; Komárková, J. Taxonomic review of the cyanoprokaryotic genera Planktothrix and Planktothricoides. Czech Phycol. 2004, 4, 1–18. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota-2. Teil/Part 2: Oscillatoriales. In Süßwasserflora von Mitteleuropa; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Spektrum: Berlin/Heidelberg, Germany, 2008; Volume 19/2, pp. 354–361. [Google Scholar]
- Leach, T.H.; Beisner, B.E.; Carey, C.C.; Pernica, P.; Rose, K.C.; Huot, Y.; Brentrup, J.A.; Domaizon, I.; Grossart, H.-P.; Ibelings, B.W.; et al. Patterns and drivers of deep chlorophyll maxima structure in 100 lakes: The relative importance of light and thermal stratification. Limnol. Oceanogr. 2018, 63, 628–646. [Google Scholar] [CrossRef]
- Davis, P.A.; Dent, M.; Parker, J.; Reynolds, C.S.; Walsby, A.E. The annual cycle of growth rate and biomass change in Planktothrix spp. in Blelham Tarn, English Lake District. Freshw. Biol. 2003, 48, 852–867. [Google Scholar] [CrossRef]
- Oberhaus, L.; Briand, J.F.; Leboulanger, C.; Jacquet, S.; Humbert, J.F. Comparative effect of the quality of light and temperature on the growth of Planktothrix agardhii and Planktthrix rubescens. J. Phycol. 2007, 43, 1191–1199. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton (Ecology, Biodiversity and Conservation); Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Salmaso, N.; Naselli-Flores, L.; Padisk, J. Functional classifications and their application in phytoplankton ecology. Freshw. Biol. 2015, 60, 603–619. [Google Scholar] [CrossRef]
- Tooming-Klunderud, A.; Sogge, H.; Rounge, T.B.; Nederbragt, A.J.; Lagesen, K.; Glöcker, G.; Hayes, P.K.; Rohrlack, T.; Jakobsen, K.J. From green to red: Horizontal gene transfer of the phycoerythrin gene cluster between Planktothrix strains. Appl. Environ. Microbiol. 2013, 79, 6803–6812. [Google Scholar] [CrossRef] [PubMed]
- Pancrace, C.; Barny, M.A.; Ueoka, R.; Calteau, A.; Scalvenzi, T.; Pédron, J.; Barbe, V.; Piel, J.; Humbert, J.-F.; Gugger, M. Insights into the Planktothrix genus: Genomic and metabolic comparison of benthic and planktic strains. Sci. Rep. 2017, 7, 41181. [Google Scholar] [CrossRef] [PubMed]
- Oberhaus, L.; Briand, J.F.; Humbert, J.F. Allelopathic growth inhibition by the toxic bloom-forming cyanobacterium Planktothrix rubescens. FEMS Microbiol. Ecol. 2008, 66, 243–249. [Google Scholar] [CrossRef]
- Blom, J.F.; Robinson, J.A.; Jüttner, F. High grazer toxicity of [D-Asp3,(E)-Dhb7] microcystin-RR of Planktothrix rubescens as compared to different microcystins. Toxicon 2001, 39, 1923–1932. [Google Scholar] [CrossRef]
- Ernst, B.; Hoeger, S.J.; O’Brien, E.; Dietrich, D.R. Abundance and toxicity of Planktothrix rubescens in the pre-alpine Lake Ammersee, Germany. Harmful Algae 2009, 8, 329–342. [Google Scholar] [CrossRef]
- Vasas, G.; Farkas, O.; Borics, G.; Felföldi, T.; Sramkó, G.; Batta, G.; Bácsi, I.; Gonda, S. Appearance of Planktothrix rubescens bloom with [D-Asp3, Mdha7]MC–RR in Gravel Pit Pond of a shallow lake-dominated Area. Toxins 2013, 5, 2434–2455. [Google Scholar] [CrossRef]
- Kurmayer, R.; Deng, L.; Entfellner, E. Role of toxic and bioactive secondary metabolites in colonization and bloom formation by filamentous cyanobacteria Planktothrix. Harmful Algae 2016, 54, 69–86. [Google Scholar] [CrossRef]
- Bukowska, A.; Kaliński, T.; Koper, M.; Kostrzewska-Szlakowska, I.; Kwiatkowski, J.; Mazur-Marzec, H.; Jasser, I. Predicting blooms of toxic cyanobacteria in eutrophic lakes with diverse cyanobacterial communities. Sci. Rep. 2017, 7, 8342. [Google Scholar] [CrossRef]
- Kangro, K.; Laugaste, R.; Nõges, P.; Ott, I. Long-term changes and seasonal development of phytoplankton in a strongly stratified hypertrophic lake. Hydrobiologia 2005, 547, 91–103. [Google Scholar] [CrossRef]
- Churro, C.; Azevedo, J.; Vasconcelos, V.; Silva, A. Detection of a Planktothrix agardhii bloom in Portuguese Marine Coastal Waters. Toxins 2017, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Hampel, J.J.; McCarthy, M.J.; Neudeck, M.; Bullerjahn, G.S.; McKay, R.M.L.; Newell, S.E. Ammonium recycling supports toxic Planktothrix blooms in Sandusky Bay, Lake Erie: Evidence from stable isotope and metatranscriptome data. Harmful Algae 2019, 81, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Walsby, A.E.; Schanz, F.; Schmid, M. The Burgundy-blood phenomenon: A model of buoyancy change explains autumnal waterblooms by Planktothrix rubescens in Lake Zürich. New Phytol. 2006, 169, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Yankova, Y.; Neuenschwander, S.; Köster, O.; Posch, T. Abrupt stop of deep water turnover with lake warming: Drastic consequences for algal primary producers. Sci. Rep. 2017, 7, 13770. [Google Scholar] [CrossRef]
- Knapp, D.; Castro, B.F.; Marty, D.; Loher, E.; Köster, O.; Wüest, A.; Posch, T. The red harmful plague in times of climate change: Blooms of the cyanobacterium Planktothrix rubescens triggered by stratification dynamics and irradiance. Front. Microbiol. 2021, 12, 705914. [Google Scholar] [CrossRef]
- Kondracki, J. Geografia Regionalna Polski; Wyd. Nauk. PWN: Warszawa, Poland, 2002. [Google Scholar]
- Wilgat, T.; Michalczyk, Z.; Turczyński, M.; Wojciechowski, K. The Łęczna-Włodawa Lakes. Studia Ośr. Dok. Fizjogr. PAN 1991, 19, 23–140. [Google Scholar]
- Harasimiuk, M.; Michalczyk, Z.; Turczyński, M. Jeziora łęczyńsko-włodawskie. In Biblioteka Monitoringu Środowiska; Studia Ośrodka Dokumentacji Fizjograficznej: Lublin, Poland, 1998. [Google Scholar]
- Michalczyk, Z.; Mięsiak-Wójcik, K.; Sposób, J.; Turczyński, M. The state of and changes in water conditions in the Łęczna-Włodawa Lake District. Przegląd Geogr. 2017, 89, 9–28. [Google Scholar] [CrossRef]
- Nusch, E.A. Comparison of different methods for chlorophyll and phaeopigment determination. Arch. Hydrobiol Beih. Ergebn. Limnol. 1980, 14, 14–36. [Google Scholar]
- Hermanowicz, W.; Dojlido, J.; Dożańska, W.; Koziorowski, B.; Zerbe, J. Fizyczno-Chemiczne Badanie Wody i Ścieków; Arkady: Warszawa, Poland, 1999. [Google Scholar]
- Utermöhl, H. Zur Vervollkommung der quantitative Phytoplankton-Methodik. Mitt. Int. Verein. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Pielou, E.C. Ecological Diversity; John Wiley&Sons: Hoboken, NJ, USA, 1975. [Google Scholar]
- Hutorowicz, A.; Pasztaleniec, A. Phytoplankton metric of ecological status assessment for Polish Lakes and its performance along nutrient gradients. Pol. J. Ecol. 2014, 62, 525–540. [Google Scholar] [CrossRef]
- Kolada, A.; Soszka, H.; Kutyła, S.; Pasztaleniec, A. The typology of Polish lakes after a decade of its use: A critical review and verification. Limnologica 2017, 67, 20–26. [Google Scholar] [CrossRef]
- Mischke, U.; Riedmüller, U.; Hoehn, E.; Schönfelder, I.; Nixdorf, B. Description of the German system for phytoplankton-based assessment of lakes for implementation of the EU Water Framework Directive (WFD). In Gewässerreport (Nr. 10): Bewertung von Seen Mittels Phytoplankton zur Umsetzung der EU-Wasserrahmenrichtlinie; Univ. Cottbus: Berlin, Germany, 2008. [Google Scholar]
- Mathes, J.; Plambeck, G.; Schaumburg, J. Das Typisierungssystem für stehende Gewässer in Deutschland mit Wasserflächenab 0.5 km2 zur Umsetzung der Wasserrahmenrichtlinie. Implementierung der EU-WRRL in Deutschland: Ausgewählte Bewertungsmethoden und Defizite. Aktuelle Reihe 2002, 5, 15–23. [Google Scholar]
- Nygaard, G. Hydrobiological studies on some Danish ponds and lakes II. The quotient hypothesis on some new or little known phytoplankton organisms. Det K. Dan. Vidensk. Selsk. 1949, 7, 1–301. [Google Scholar]
- Ott, I.; Laugaste, R. Fütoplanktoni koondindeks (FKI), üldistus Eesti järvede kohta. Eest. Keskkonnaminist. Infoleht 1996, 3, 7–8. [Google Scholar]
- Kobos, J.; Błaszczyk, A.; Hohlfeld, N.; Toruńska-Sitarz, A.; Krakowiak, A.; Hebel, A.; Sutryk, K.; Grabowska, M.; Toporowska, M.; Kokociński, M.; et al. Cyanobacteria and cyanotoxins in Polish freshwater bodies. Oceanol. Hydrobiol. Stud. 2013, 42, 358–378. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Deep living Planktothrix rubescens modulated by environmental constraints and climate forcing. In Phytoplankton Responses to Human Impacts at Different Scales; Part of the Developments in Hydrobiology book series; Salmaso, N., Naselli-Flores, L., Cerasino, L., Flaim, G., Tolotti, M., Padisák, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 221, pp. 29–46. [Google Scholar] [CrossRef]
- Padisák, J.; Vasas, G.; Borics, G. Phycogeography of freshwater phytoplankton: Traditional knowledge and new molecular tools. Hydrobiologia 2016, 764, 3–27. [Google Scholar] [CrossRef]
- Krupa, D.; Czernaś, K. Mass Appearance of Cyanobacterium Planktothrix rubescens in Lake Piaseczno, Poland. Water Qual. Res. J. Can. 2003, 38, 141–152. [Google Scholar] [CrossRef]
- Lenard, T. Metalimnetic bloom of Planktothrix rubescens in relation to environmental conditions. Oceanol. Hydrobiol. Stud. 2009, 38, 45–53. [Google Scholar]
- Lenard, T.; Ejankowski, W. Natural water brownification as a shift in the phytoplankton community in a deep hard water lake. Hydrobiologia 2017, 787, 153–166. [Google Scholar] [CrossRef]
- Kromkamp, J.C.; Domin, A.; Dubinsky, Z.; Lehmann, C.; Schanz, F. Changes in photosynthetic properties measured by oxygen evolution and variable chlorophyll fluorescence in a simulated entrainment experiment with the cyanobacterium Planktothrix rubescens. Aquat. Sci. 2001, 63, 363–382. [Google Scholar] [CrossRef]
- Walsby, A.E.; Schanz, F. Light-dependent growth rate determines changes in the population of Planktothrix rubescens over the annual cycle in Lake Zürich, Switzerland. New Phytol. 2002, 154, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, S.; Briand, J.F.; Leboulanger, C.; Avois-Jacquet, C.; Oberhaus, L.; Tassin, B.; Vinçon-Leite, B.; Paolini, G.; Druart, J.-C.; Anneville, O.; et al. The proliferation of the toxic cyanobacterium Planktothrix rubescens following restoration of the largest natural French lake (Lac du Bourget). Harmful Algae 2005, 4, 651–672. [Google Scholar] [CrossRef]
- Hingsamer, P.; Peeters, F.; Hofmann, H. The consequences of internal waves for phytoplankton focusing on the distribution and production of Planktothrix rubescens. PLoS ONE 2014, 9, e104359. [Google Scholar] [CrossRef]
- Djediat, C.; Feilke, K.; Brochard, A.; Caramelle, L.; Tiam, S.K.; Sétif, P.; Gauvrit, T.; Yéprémianb, C.; Wilson, A.; Talbot, L.; et al. Light stress in green and red Planktothrix strains: The orange carotenoid protein and its related photoprotective mechanism. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148037. [Google Scholar] [CrossRef]
- Briand, E.; Reubrecht, S.; Mondeguer, F.; Sibat, M.; Hess, P.; Amzil, Z.; Bormans, M. Chemically mediated interactions between Microcystis and Planktothrix: Impact on their growth, morphology and metabolic profiles. Environ. Microbiol. 2019, 21, 1552–1566. [Google Scholar] [CrossRef]
- Savadova-Ratkus, K.; Mazur-Marzec, H.; Karosienė, J.; Kasperovičienė, J.; Paškauskas, R.; Vitonytė, I.; Koreivienė, J. Interplay of nutrients, temperature, and competition of native and alien cyanobacteria species growth and cyanotoxin production in temperate lakes. Toxins 2021, 13, 23. [Google Scholar] [CrossRef]
- Feuillade, M.; Feuillade, J.; Blanc, P. Alkaline phosphatase activity fluctuations and associated factors in a eutrophic lake dominated by Oscillatoria rubescens. Hydrobiology 1990, 207, 233–240. [Google Scholar] [CrossRef]
- Sheridan, C.C.; Steinberg, D.K.; Kling, G.W. The microbial and metazoan community associated with colonies of Trichodesmium spp.: A quantitative survey. J. Plank. Res. 2002, 24, 913–922. [Google Scholar] [CrossRef]
- Pasztaleniec, A.; Ochocka, A. A comparative study of phytoplankton epi- and metalimnetic communities under different light and thermal regimes. Ecohydrol. Hydrobiol. 2021, 21, 760–774. [Google Scholar] [CrossRef]
- Mankiewicz-Boczek, J.; Gągała, I.; Kokociński, M.; Jurczak, T.; Stefaniak, K. Perennial toxigenic Planktothrix agardhii bloom in selected lakes of Western Poland. Environ. Toxicol. 2011, 26, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Toporowska, M.; Pawlik-Skowrońska, B.; Kalinowska, R. Mass development of diazotrophic cyanobacteria (Nostocales) and production of neurotoxic anatoxin-a in a Planktothrix (Oscillatoriales) dominated temperate lake. Water Air Soil Pollut. 2016, 227, 321. [Google Scholar] [CrossRef] [PubMed]
- Pawlik-Skowrońska, B.; Toporowska, M.; Mazur-Marzec, H. Toxic oligopeptides in the cyanobacterium Planktothrix agardhii-dominated blooms and their effects on duckweed (Lemnaceae) development. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 41. [Google Scholar] [CrossRef]


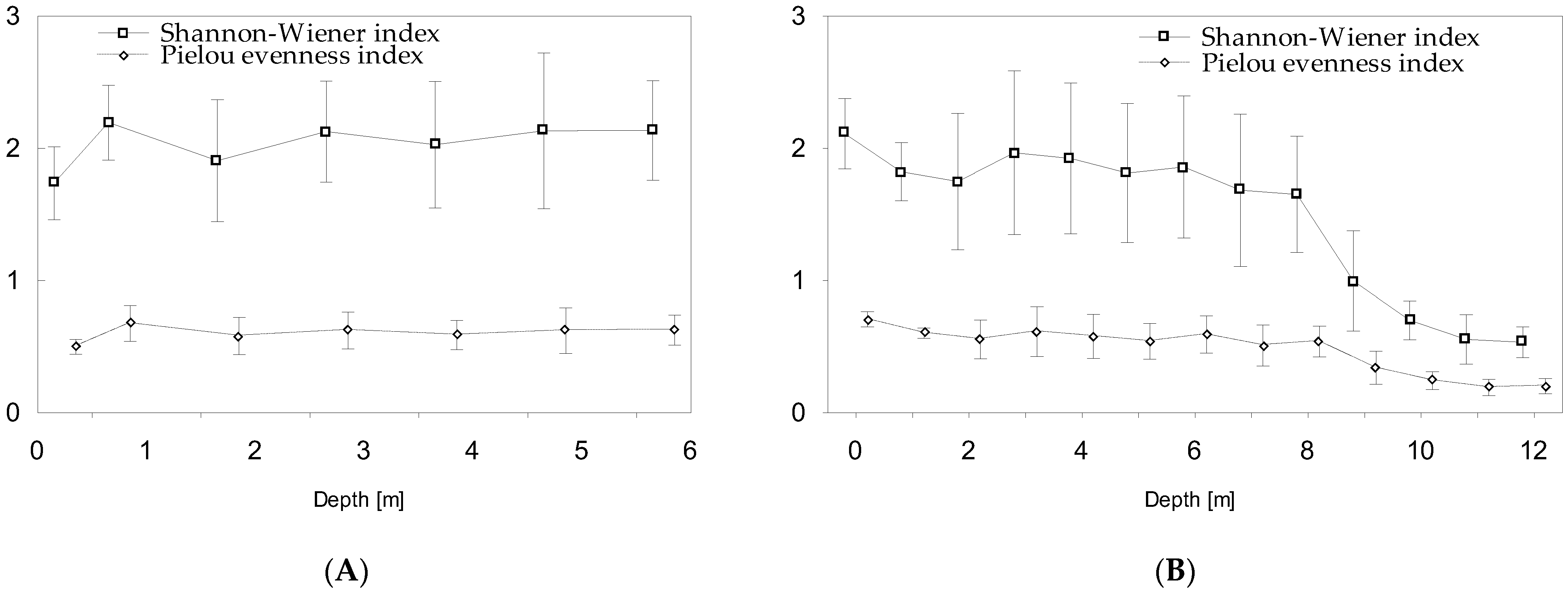

| Parameters | Lake Glinki | Lake Piaseczno |
|---|---|---|
| Area (ha) | 46.9 | 84.7 |
| Maximum depth (m) | 8.8 | 38.8 |
| Mean depth (m) | 2.8 | 12.6 |
| Mean slope inclination | 1° 35′ | 4° 50′ |
| Maximum length (m) | 1031 | 1464 |
| Maximum width (m) | 652 | 819 |
| Mean width (m) | 455 | 579 |
| Length of shoreline (m) | 3018 | 3788 |
| Volume of lake (thousands m3) | 1342 | 10,674 |
| Type of stratification | stratified | stratified |
| Type of water mixing | dimictic | dimictic |
| Catchment area (ha) | 159.7 | 284.9 |
| Parameters | The Biomass of | |
|---|---|---|
| P. agardhii | P. rubescens | |
| Depth | −0.55 | 0.76 |
| Total nitrogen (TN) | 0.42 | 0.27 |
| Ammonium nitrogen(N-NH4) | −0.38 | −0.19 |
| Nitrate nitrogen (N-NO3) | −0.25 | −0.55 |
| Phosphate phosphorus (P-PO4) | −0.41 | −0.38 |
| Total phosphorus (TP) | 0.57 | 0.71 |
| pH | 0.54 | 0.23 |
| Electrolytic conductivity | −0.50 | −0.09 |
| Photosynthetic active radiation (PAR) | 0.41 | −0.68 |
| Temperature of water | 0.58 | −0.87 |
| Concentration of chlorophyll-a | 0.79 | 0.72 |
| Total biomass of phytoplankton | 0.78 | 0.85 |
| Shannon–Wiener diversity index | 0.08 | −0.72 |
| Pielou evenness index | 0.21 | −0.64 |
| Number of species | −0.25 | −0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenard, T.; Poniewozik, M. Planktothrix agardhii versus Planktothrix rubescens: Separation of Ecological Niches and Consequences of Cyanobacterial Dominance in Freshwater. Int. J. Environ. Res. Public Health 2022, 19, 14897. https://doi.org/10.3390/ijerph192214897
Lenard T, Poniewozik M. Planktothrix agardhii versus Planktothrix rubescens: Separation of Ecological Niches and Consequences of Cyanobacterial Dominance in Freshwater. International Journal of Environmental Research and Public Health. 2022; 19(22):14897. https://doi.org/10.3390/ijerph192214897
Chicago/Turabian StyleLenard, Tomasz, and Małgorzata Poniewozik. 2022. "Planktothrix agardhii versus Planktothrix rubescens: Separation of Ecological Niches and Consequences of Cyanobacterial Dominance in Freshwater" International Journal of Environmental Research and Public Health 19, no. 22: 14897. https://doi.org/10.3390/ijerph192214897
APA StyleLenard, T., & Poniewozik, M. (2022). Planktothrix agardhii versus Planktothrix rubescens: Separation of Ecological Niches and Consequences of Cyanobacterial Dominance in Freshwater. International Journal of Environmental Research and Public Health, 19(22), 14897. https://doi.org/10.3390/ijerph192214897






