Phytoplankton Community Structure Dynamics in Relation to Water Environmental Factors in Zhalong Wetland
Abstract
:1. Introduction
2. Materials
2.1. Sampling Sites
2.2. Data Processing
2.2.1. Analysis of Species Dominance
2.2.2. One-Way ANOVA
2.2.3. For Canonical Correspondence Analysis
2.2.4. Random Forest Method
3. Results
3.1. Spatiotemporal Distribution Characteristics of Phytoplankton Community within Zhalong Wetland
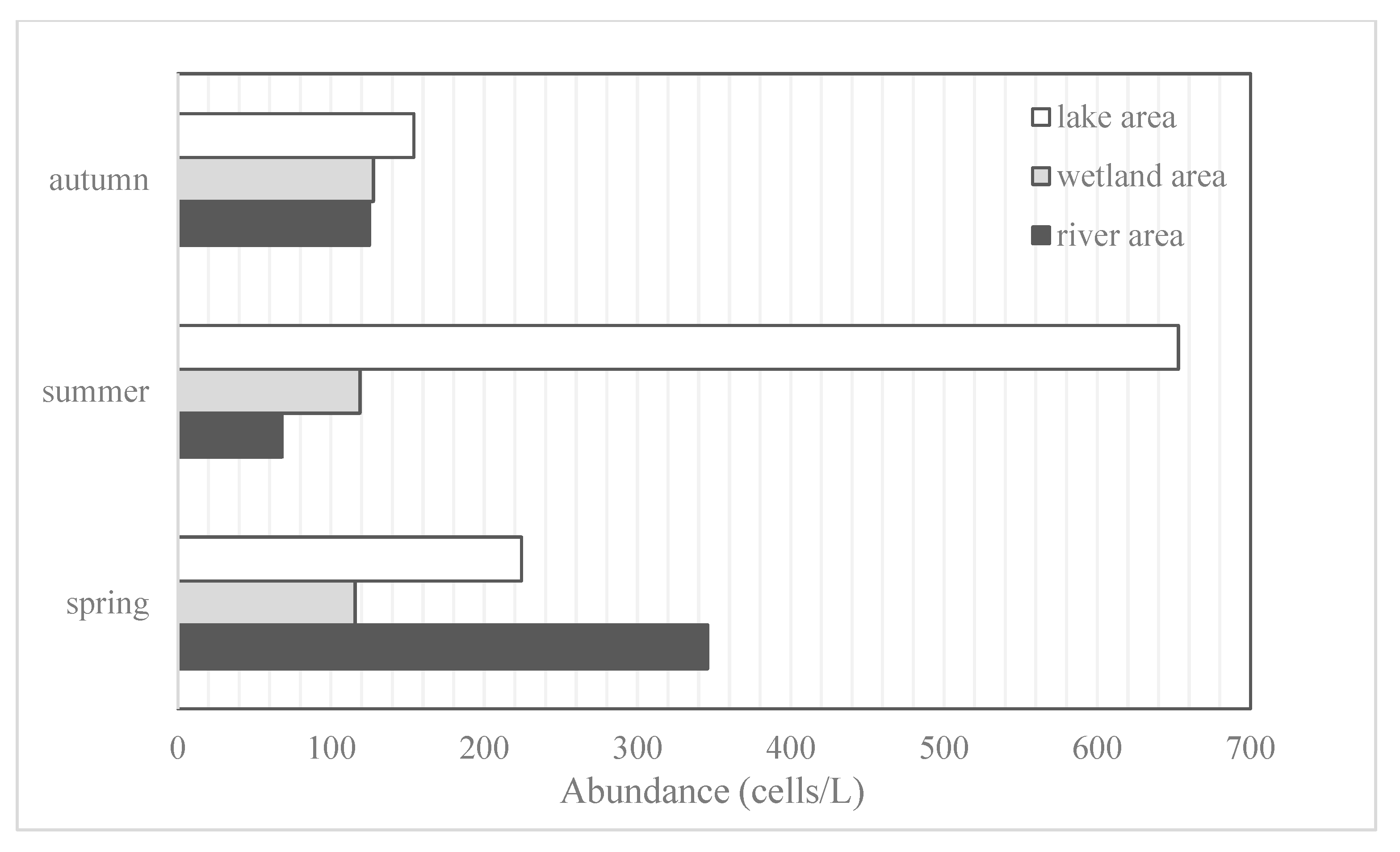
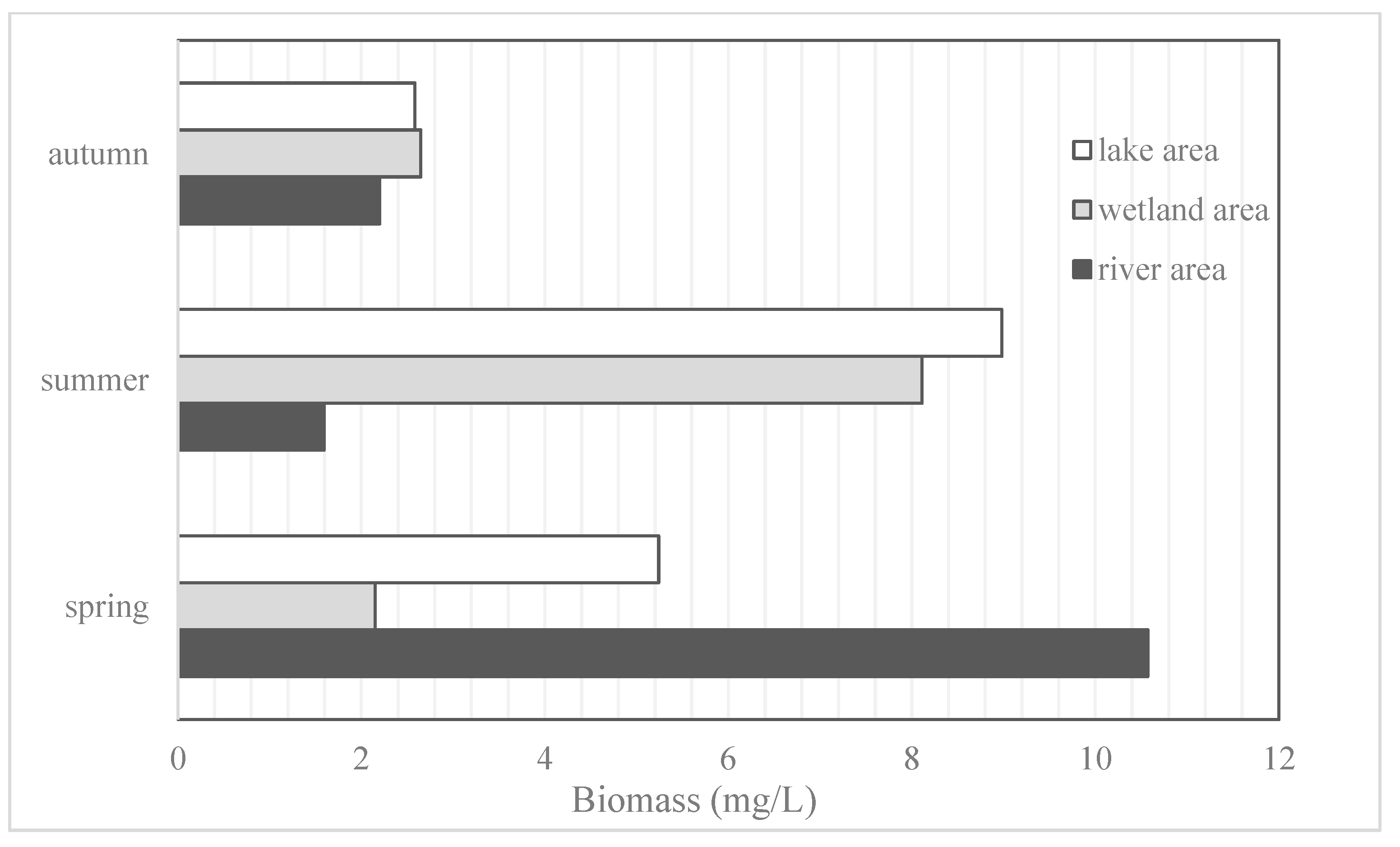
3.2. Spatiotemporal Variation of Phytoplankton Abundance and Biomass within Zhalong Wetland
3.2.1. Phytoplankton Biomass
3.2.2. Temporal and Spatial Distribution Characteristics of Dominant Species of Phytoplankton within Zhalong Wetland
3.3. Temporal and Spatial Distribution Characteristics of Water Environmental Factors within Zhalong Wetland
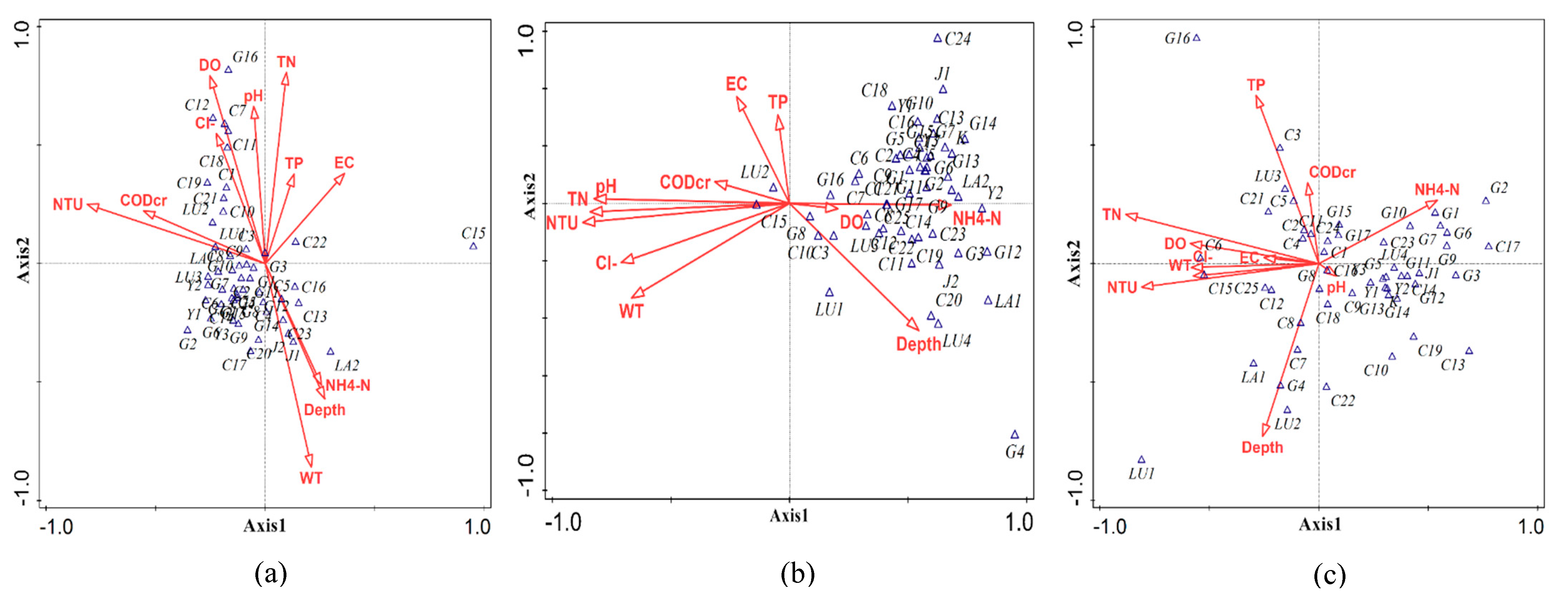
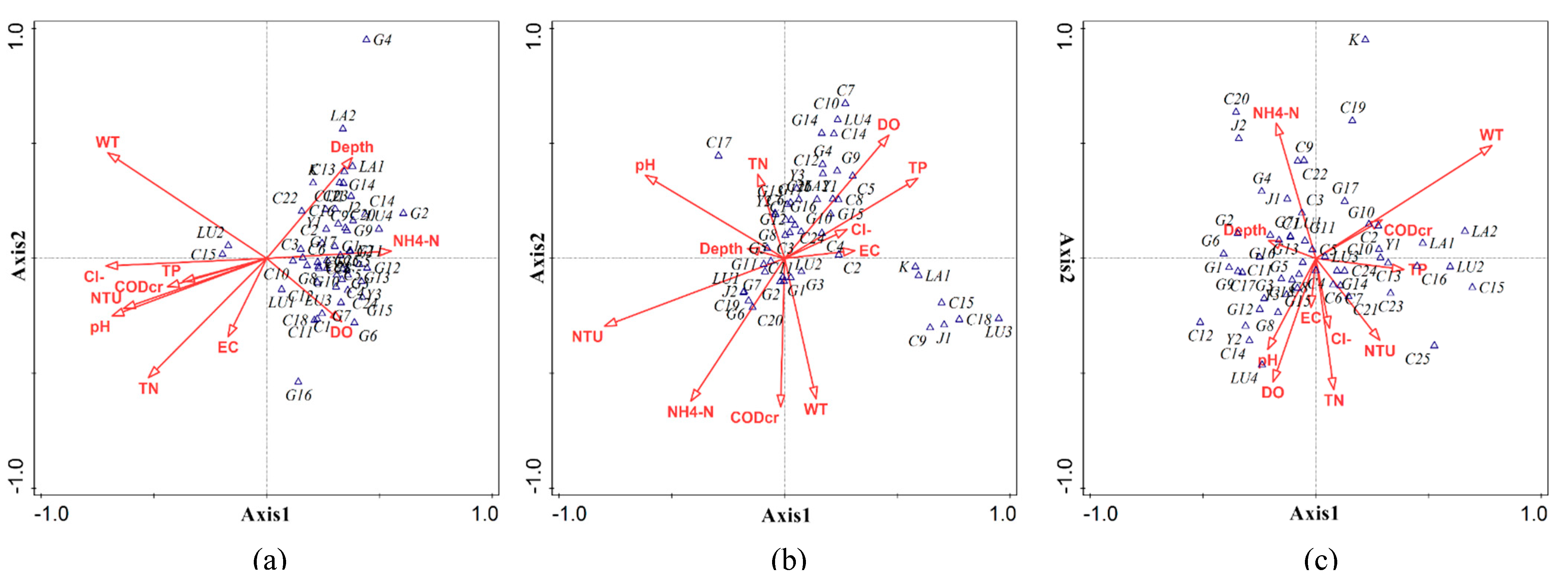
3.4. CCA of Phytoplankton Community Structure and Environmental Factors within the Zhalong Wetland
3.4.1. CCA of Phytoplankton in Spring, Summer, and Autumn
3.4.2. CCA of Phytoplankton in Lake, River, and Wetland Areas
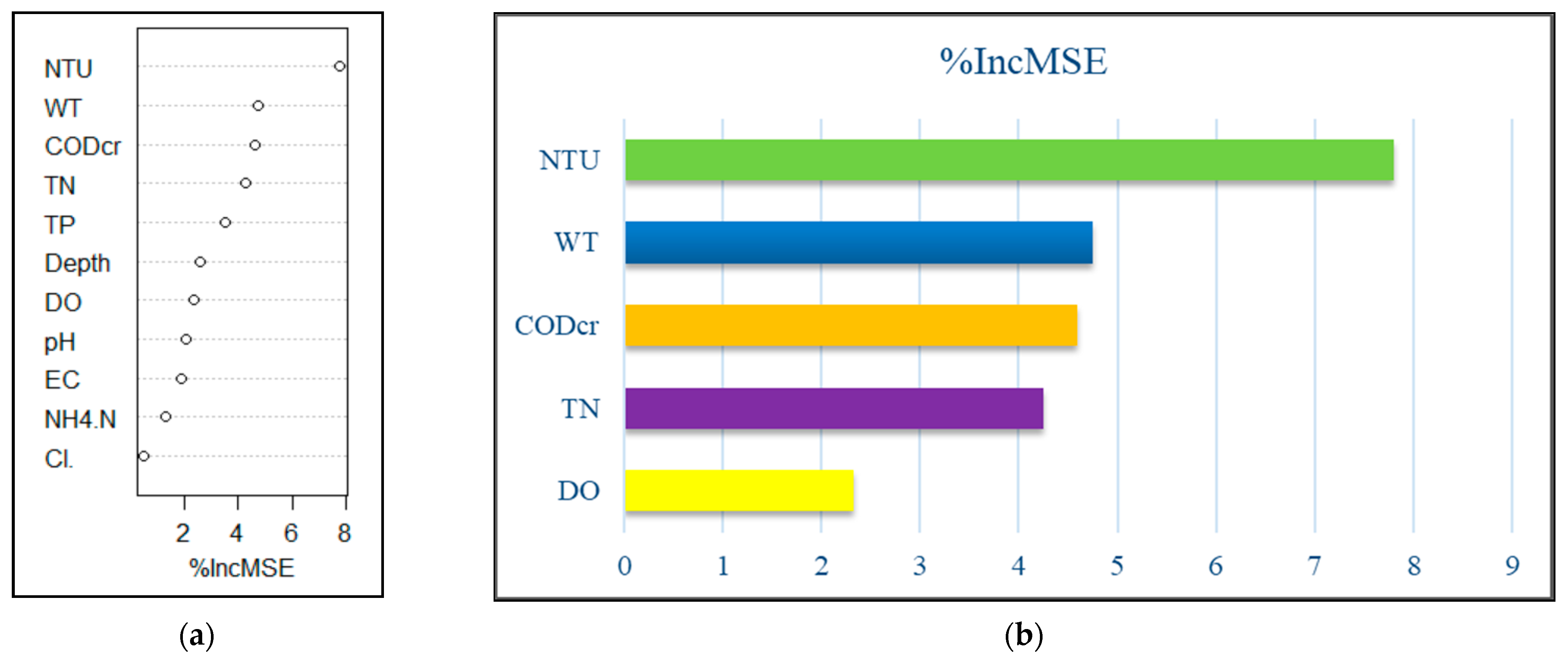
3.5. Analysis of the Influence of Water Environmental Factors on Phytoplankton Abundance Using the Random Forest Method
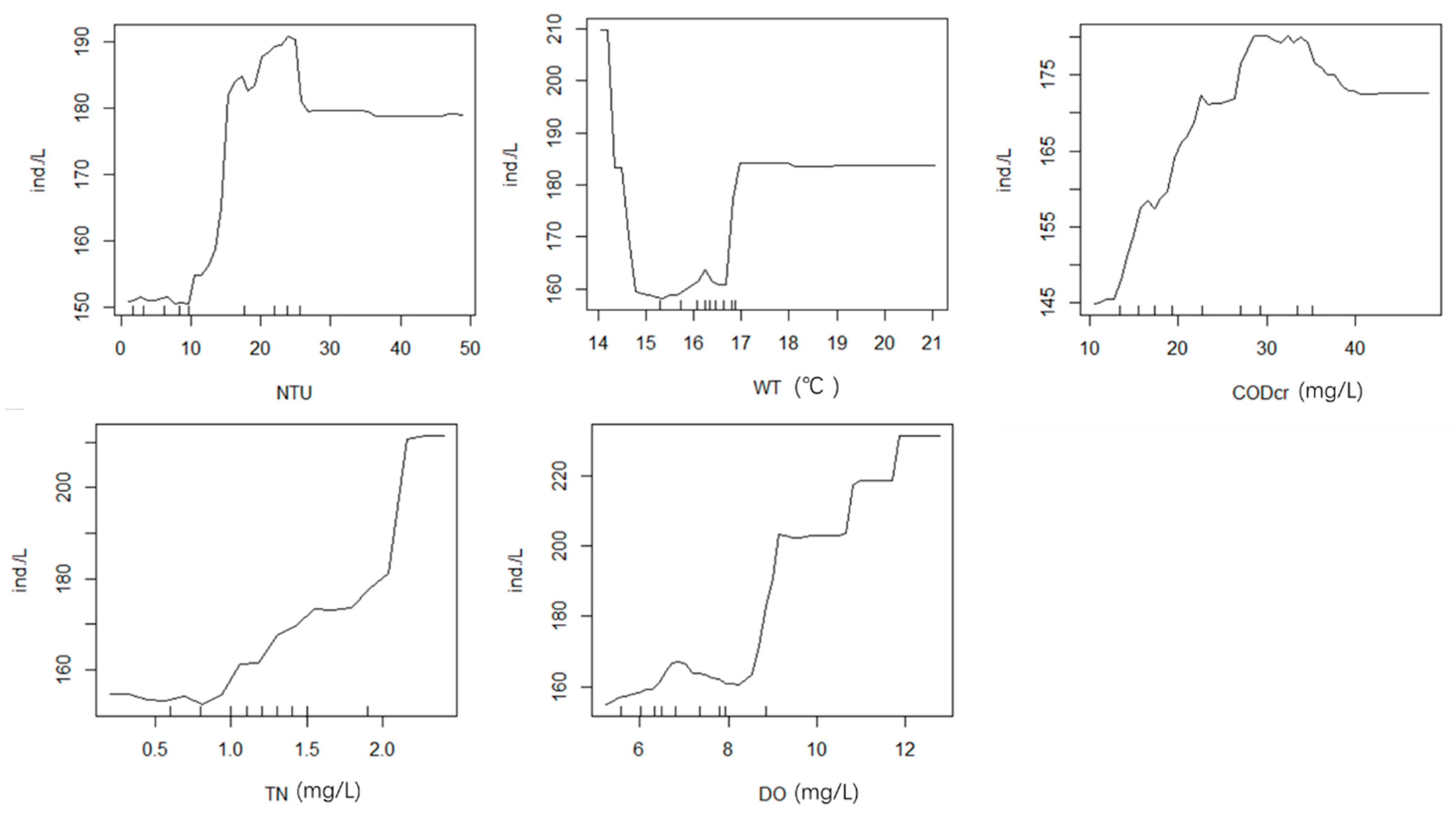
3.5.1. Random Forest Analysis of Phytoplankton and Environmental Factors in Spring
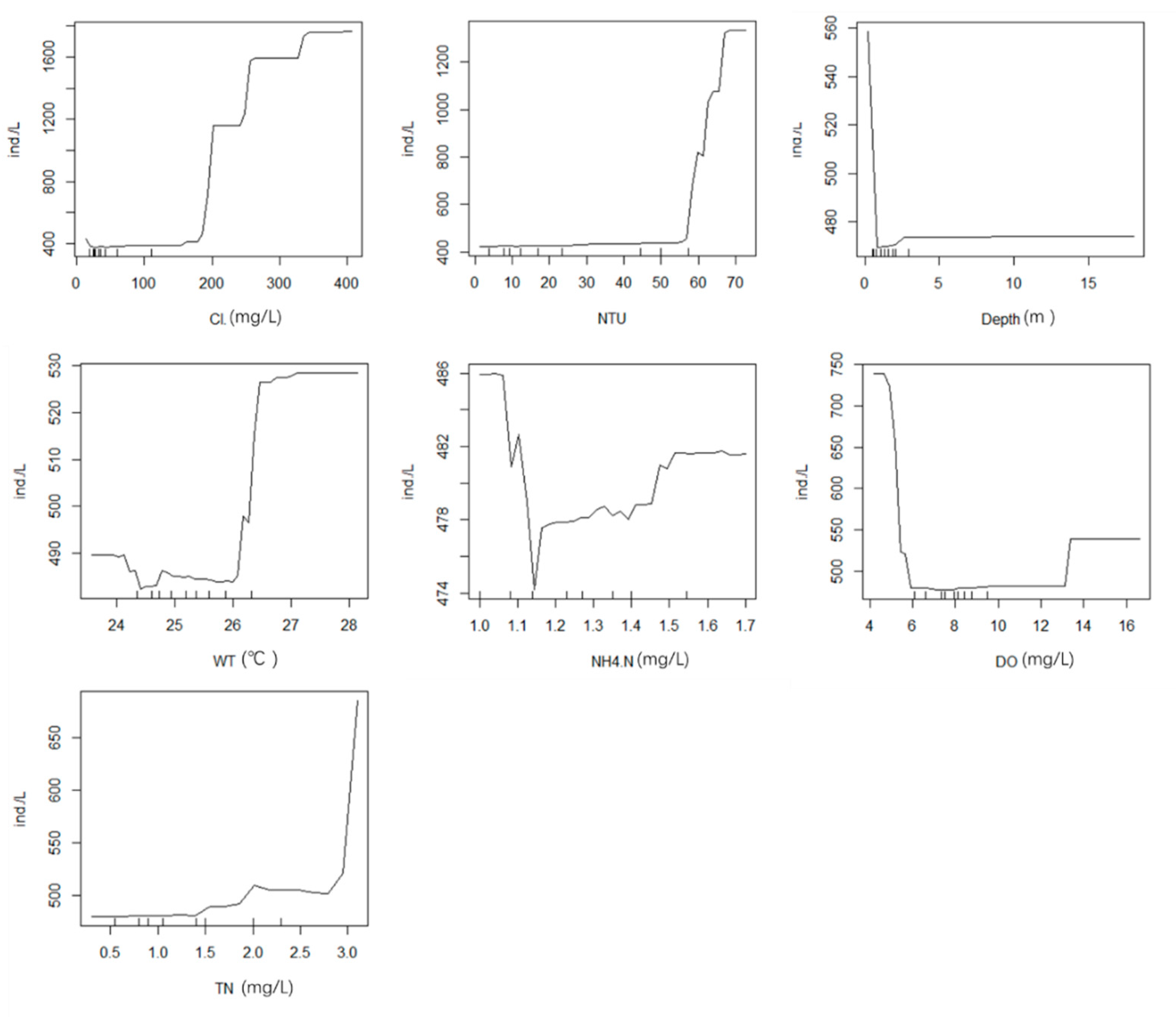
3.5.2. Random Forest Analysis of Phytoplankton and Environmental Factors in Summer
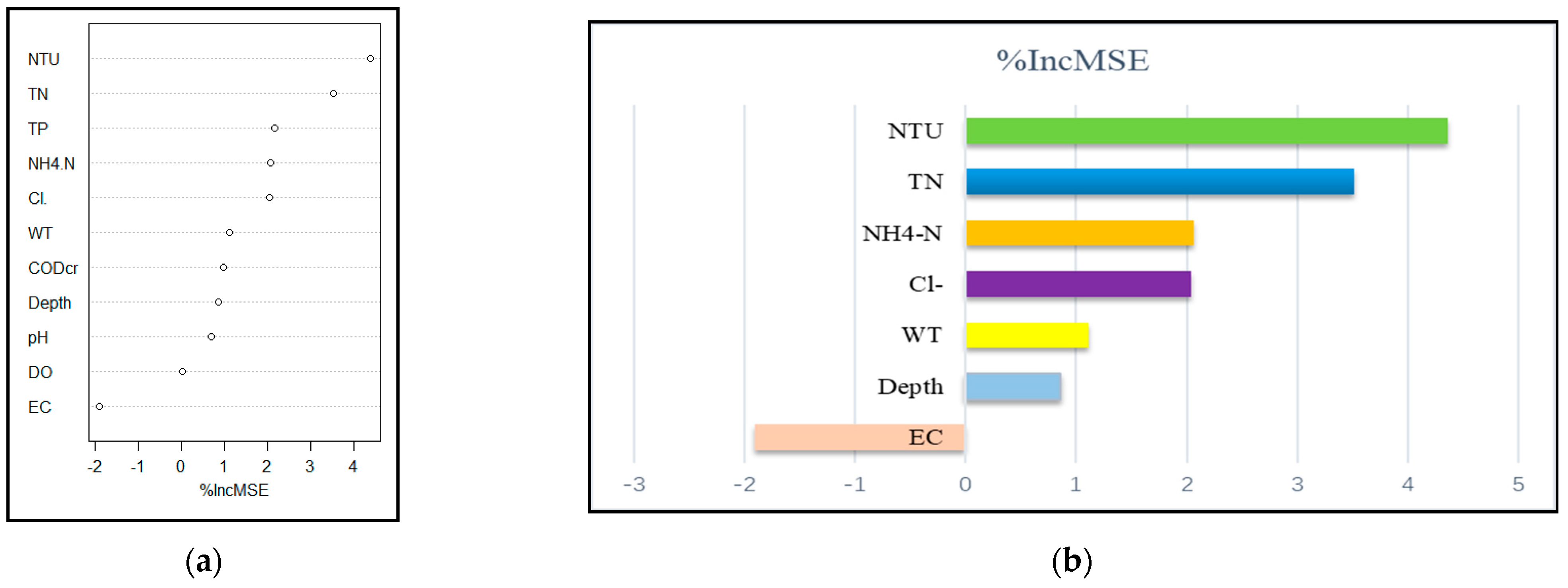
3.5.3. Random Forest Analysis of Phytoplankton and Environmental Factors in Autumn
3.5.4. Random Forest Analysis of Phytoplankton and Environmental Factors in Lake, River, and Wetland Areas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Phylum | Species | Code | Phylum | Species | Code |
|---|---|---|---|---|---|
| Cyanophyta | Anabaena azotica | LA1 | Euglenophyta | Euglena pisciformis | LU4 |
| Cyanophyta | Leptolynbya sp. | LA2 | Pyrrophyta | Glenodinium sp. | K |
| Bacillariophyta | Fragilaria brevistriata | G1 | Chlorophyta | Chlamydomonas ovalis | C1 |
| Bacillariophyta | Fragilaria capucina | G2 | Chlorophyta | Chlamydomonas globosa | C2 |
| Bacillariophyta | Fragilaria virescens | G3 | Chlorophyta | Chloromonas sp. | C3 |
| Bacillariophyta | Melosira granulata var.angustissima | G4 | Chlorophyta | Ankistrodesmus angustus | C4 |
| Bacillariophyta | Synedra acus | G5 | Chlorophyta | Ankistrodesmus acicularis | C5 |
| Bacillariophyta | Synedra tabulata | G6 | Chlorophyta | Tetrastrum staurogeniae | C6 |
| Bacillariophyta | Synedra ulna | G7 | Chlorophyta | Scenedesmus dimorphus | C7 |
| Bacillariophyta | Cyclotella meneghiniana | G8 | Chlorophyta | Scenedesmus quadricauda | C8 |
| Bacillariophyta | Navicula radiosa | G9 | Chlorophyta | Scenedesmus bijuga | C9 |
| Bacillariophyta | Navicula cincta | G10 | Chlorophyta | Pediastrum boryanum | C10 |
| Bacillariophyta | Navicula exigua | G11 | Chlorophyta | Kirchneriella lunaris | C11 |
| Bacillariophyta | Navicula pupula | G12 | Chlorophyta | Crucigenia tetrapedia | C12 |
| Bacillariophyta | Navicula cryptocephata | G13 | Chlorophyta | Staurastrum sp. | C13 |
| Bacillariophyta | Cymbella affinis | G14 | Chlorophyta | Cosmarium depressum | C14 |
| Bacillariophyta | Gyrosigma acuminatum | G15 | Chlorophyta | Chlorella vulgaris | C15 |
| Bacillariophyta | Ceratoneis closterium | G16 | Chlorophyta | Westella botryoides | C16 |
| Bacillariophyta | Cocconeis placentula | G17 | Chlorophyta | Mougeotia sp. | C17 |
| Chrysophyta | Dinobryon divergens | J1 | Chlorophyta | Oocystis elliptica | C18 |
| Chrysophyta | Kephyrion boreale | J2 | Chlorophyta | Oocystis lacustris | C19 |
| Cryptophyta | Cryptomonas ovata | Y1 | Chlorophyta | Haematococcus pluvialis | C20 |
| Cryptophyta | Chroomonas caudata | Y2 | Chlorophyta | Tetraedron minimum | C21 |
| Cryptophyta | Rhodomonas lacustris | Y3 | Chlorophyta | Tetraedron trilobulatum | C22 |
| Euglenophyta | Euglena oxyuris | LU1 | Chlorophyta | Tetraedron trigonum | C23 |
| Euglenophyta | Euglena caudata | LU2 | Chlorophyta | Golenkinia radiata | C24 |
| Euglenophyta | Euglena acus | LU3 | Chlorophyta | Tetrachlorella alternans | C25 |
References
- Bhat, N.A.; Wanganeo, A.; Raina, R. Seasonal dynamics of phytoplankton community in a tropical wetland. Environ. Monit. Assess. 2015, 187, 4136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Zang, S.Y. Characteristics of phytoplankton distribution for assessment of water quality in the Zhalong Wetland, China. Int. J. Environ. Sci. Technol. 2015, 12, 3657–3664. [Google Scholar] [CrossRef] [Green Version]
- Ochs, C.A.; Capello, H.E.; Pongruktham, O. Bacterial production in the Lower Mississippi River: Importance of suspended sediment and phytoplankton biomass. Hydrobiologia 2010, 637, 19–31. [Google Scholar] [CrossRef]
- Andrew, S.M.; Strzepek, R.F.M.; Whitney, S.; Chow, W.; Ellwood, M.J. Divergent physiological and molecular responses of light- and iron-limited Southern Ocean phytoplankton. Limnol. Oceanogr. Lett. 2021, 7, 150–158. [Google Scholar] [CrossRef]
- Mesquita, M.C.B.; Prestes, A.C.C.; Gomes, A.M.A.; Marinho, M.M. Direct Effects of Temperature on Growth of Different Tropical Phytoplankton Species. Microb. Ecol. 2020, 79, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, Z.; Yang, G.; Chen, Y. Effects of resuspension and eutrophication level on summer phytoplankton dynamics in two hypertrophic areas of Lake Taihu, China. Aquat. Ecol. 2010, 44, 41–54. [Google Scholar] [CrossRef]
- Rodríguez, P.; Pizarro, H. Phytoplankton and periphyton production and its relation to temperature in a humic lagoon. Limnologica 2015, 55, 9–12. [Google Scholar] [CrossRef]
- Verhamme, E.M.; Redder, T.M.; Schlea, D.A.; Grush, J.; Bratton, J.F.; DePinto, J.V. Development of the Western Lake Erie Ecosystem Model (WLEEM): Application to connect phosphorus loads to cyanobacteria biomass. J. Great Lakes Res. 2016, 42, 1193–1205. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhang, Y.; Wu, X.; Yang, Y.; Xu, X.; Zheng, B.; Deng, W.; Shao, Z.; Lu, L.; Wang, L.; et al. A study on the relationship between metabolism of Cyanobacteria and chemical oxygen demand in Dianchi Lake, China. Water Environ. Res. 2019, 91, 1650–1660. [Google Scholar] [CrossRef]
- Lu, X.; Sui, F.; Liu, Y.; Fan, Y. Comparison of three functional classification approaches to characterize phytoplankton response to environmental heterogeneity: A case study in NE China wetlands. J. Freshw. Ecol. 2022, 37, 103–116. [Google Scholar] [CrossRef]
- Ma, Y.; Li, G.; Li, J.; Zhou, H.; Jiang, B. Seasonal succession of phytoplankton community and its relationship with environmental factors of North Temperate Zone water of the Zhalong Wetland, in China. Ecotoxicology 2014, 23, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, B.; Li, Z.; Cheng, G.; Zhou, L. Dynamic simulation of a duckweed-dominated wetland in north China based on a system dynamics model. Ecol. Indic. 2018, 92, 268–277. [Google Scholar] [CrossRef]
- Maloufi, S.; Catherine, A.; Mouillot, D.; Louvard, C.; Coute, A.; Bernard, C.; Troussellier, M. Environmental heterogeneity among lakes promotes hyper -diversity across phytoplankton communities. Freshw. Biol. 2016, 61, 633–645. [Google Scholar] [CrossRef]
- Dalu, T.; Chauke, R. Assessing macroinvertebrate communities in relation to environmental variables: The case of Sambandou wetlands, Vhembe Biosphere Reserve. Appl. Water Sci. 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Gogoi, P.; Kumari, S.; Sarkar, U.K.; Lianthuamluaia, L.; Puthiyottil, M.; Bhattacharjya, B.K.; Das, B.K. Dynamics of phytoplankton community in seasonally open and closed wetlands in the Teesta-Torsa basin, India, and management implications for sustainable utilization. Environ. Monit. Assess. 2021, 193, 810. [Google Scholar] [CrossRef] [PubMed]
- Istvánovics, V.; Honti, M. Stochastic simulation of phytoplankton biomass using eighteen years of daily data—Predictability of phytoplankton growth in a large, shallow lake. Sci. Total Environ. 2021, 764, 143636. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, H.; Xiao, W.; Wang, L.; Huang, B. A machine-learning approach to modeling picophytoplankton abundances in the South China Sea. Prog. Oceanogr. 2020, 189, 102456. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Wang, H.; Ma, C. Phytoplankton community structure in relation to environmental factors and ecological assessment of water quality in the upper reaches of the Genhe River in the Greater Hinggan Mountains. Environ. Sci. Pollut. Res. 2019, 26, 17512–17519. [Google Scholar] [CrossRef]
- Hu, H.J.; Wei, Y.X. The Freshwater Algae of China: Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006; pp. 1–1023. (In Chinese) [Google Scholar]
- Zhao, W. Hydrobiology; China Agriculture Press: Beijing, China, 2005; pp. 501–507. (In Chinese) [Google Scholar]
- Wei, F.S.; Qi, W.Q.; Sun, Z.G.; Huang, Y.R.; Shen, Y.W. Water and Wastewater Monitoring and Analysis Method; China Environmental Science Press: Beijing, China, 2002; pp. 211–284. (In Chinese) [Google Scholar]
- Hu, R.; Han, B.; Naselli-Flores, L. Comparing biological classifications of freshwater phytoplankton: A case study from South China. Hydrobiologia 2013, 701, 219–233. [Google Scholar] [CrossRef]
- Morabito, G.; Oggioni, A.; Caravati, E.; Panzani, P. Seasonal morphological plasticity of phytoplankton in Lago Maggiore (N. Italy). Hydrobiologia 2007, 578, 47–57. [Google Scholar] [CrossRef]
- Fan, Y.; Li, J.; Men, X.; Liu, Y. Preliminary description of diatom community and its relationship with water physicochemical variables in Qixinghe Wetland. Chin. J. Oceanol. Limnol. 2012, 30, 379–387. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.M.; Ilyas, M.; Alqarawi, A.A.; Ahmad, Z.; Abd-Allah, E.F. Plant species and communities assessment in interaction with edaphic and topographic factors; an ecological study of the mount Eelum District Swat, Pakistan. Saudi J. Biol. Sci. 2017, 24, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L.; Cutler, A. Random Forest: Breiman and Cutler’s Random Forests for Classification and Regression; 2008. Available online: https://www.stat.berkeley.edu/~breiman/RandomForests/ (accessed on 12 October 2022).
- Liaw, A.; Wiener, M. Classification and regression by random Forest. R News 2001, 2, 18–22. [Google Scholar]
- Edwards, J.A.; Santos-Medellin, C.M.; Liechty, Z.S.; Bao, N.; Lurie, E.; Eason, S.; Phillips, G.; Sundaresan, V. Compositional shifts in root-associated bacterial and archaeal microbiota track the plant life cycle in field-grown rice. PLoS Biol. 2018, 16, e2003862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Liu, Y.; Zang, S. Relationships between phytoplankton community in different functional regions and environmental factors in Zhalong Wetland, Heilongjiang Province. J. Lake Sci. 2016, 28, 554–565. (In Chinese) [Google Scholar]
- Guo, C.; Liu, Y.; Fan, Y.; Li, H. Characteristics and diversity of phytoplankton community structures in the Sanjiang Plain Wetland (Fuyuan region) in the summe. J. Lake Sci. 2014, 26, 759–766. (In Chinese) [Google Scholar]
- Yang, Y.; Gao, Y.; Chen, Y.; Li, S.; Zhan, A. Interactome-based abiotic and biotic impacts on biodiversity of plankton communities in disturbed wetlands. Divers. Distrib. 2019, 25, 1416–1428. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Liu, L.; Shu, J.; Zhu, Y.; Zhou, J. Phytoplankton and Eutrophication Degree Assessment of Baiyangdian Lake Wetland, China. Sci. World J. 2013, 2013, 436965. [Google Scholar] [CrossRef]
- Li, X.C.; Sun, X.; Ma, C.X.; Yu, H.X. Phytoplankton functional groups and their relationship with environmental factors in a eutrophic and high-alkalinity lake in the cold regions of northern china. Appl. Ecol. Environ. Res. 2021, 19, 1549–1569. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, C.; Mwagona, P.C.; Li, Z.; Liu, Z.; Dou, H.; Zhou, X.; Bhadha, J.H. Bottom-up and top-down effects on phytoplankton functional groups in Hulun Lake, China. Ann. Limnol. Int. J. Limnol. 2021, 57, 3. [Google Scholar] [CrossRef]
- Ba, Q.; Li, X.; Liu, Y.; Dai, C.; Fan, Y. Algal community structure and water quality evaluation in zhalong wetland. Oceanol. Limnol. Sin. 2016, 47, 924–934. (In Chinese) [Google Scholar]
- Dai, C.; Yi, Y.; Liu, Y.; Ba, Q.; Fan, Y. Seasonal changes of diatom community structure in the Zhalong wetland and its relationship with environmental conditions. Acta Ecol. Sin. 2017, 37, 2818–2827. (In Chinese) [Google Scholar]



| Species | Dominant Index | ||||||
|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Lake Area | River Area | Wetland Area | ||
| Bacillariophyta | Synedra acus | 0.116 | 0.112 | 0.048 | 0.165 | 0.117 | |
| Bacillariophyta | Synedra tabulata | 0.099 | |||||
| Bacillariophyta | Cyclotella meneghiniana | 0.132 | 0.042 | 0.163 | 0.076 | 0.285 | 0.120 |
| Bacillariophyta | Fragilaria brevistriata | 0.025 | 0.038 | ||||
| Bacillariophyta | Fragilaria capucina | 0.020 | |||||
| Bacillariophyta | Ceratoneis closterium | 0.021 | |||||
| Chlorophyta | Ankistrodesmus angustus | 0.027 | 0.052 | 0.024 | 0.026 | 0.033 | |
| Chlorophyta | Chlorella vulgaris | 0.310 | 0.067 | 0.418 | 0.045 | ||
| Chlorophyta | Chlamydomonas globosa | 0.031 | |||||
| Cryptophyta | Rhodomonas lacustris | 0.021 | 0.046 | ||||
| Cryptophyta | Cryptomonas ovata | 0.028 | 0.074 | ||||
| Chrysophyta | Dinobryon divergens | 0.034 | |||||
| Environmental Factors | Spring | Summer | Autumn | p-Value |
|---|---|---|---|---|
| TN (mg/L) | 1.22 ± 0.05 | 1.22 ± 0.07 | 1.83 ± 0.11 | 0.000 ** |
| TP (mg/L) | 0.10 ± 0.01 | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.000 ** |
| CODcr (mg/L) | 24.07 ± 1.02 | 27.10 ± 0.54 | 24.99 ± 0.75 | 0.023 * |
| WT (°C) | 16.27 ± 0.10 | 25.20 ± 0.09 | 9.60 ± 0.08 | 0.000 ** |
| Cl− (mg/L) | 35.19 ± 2.54 | 68.34 ± 10.97 | 101.14 ± 11.68 | 0.000 ** |
| pH | 9.43 ± 0.03 | 9.36 ± 0.05 | 9.66 ± 0.05 | 0.000 ** |
| SD (cm) | 58.12 ± 5.18 | 59.60 ± 5.44 | 63.37 ± 4.92 | 0.762 |
| NH4+-N (mg/L) | 1.51 ± 0.03 | 1.24 ± 0.02 | 1.13 ± 0.01 | 0.000 ** |
| EC (ms/cm) | 0.41 ± 0.03 | 0.46 ± 0.03 | 0.51 ± 0.03 | 0.029 * |
| NTU | 15.20 ± 1.61 | 26.66 ± 2.58 | 17.35 ± 2.30 | 0.001 ** |
| DO (mg/L) | 7.15 ± 0.17 | 7.84 ± 0.18 | 9.91 ± 0.22 | 0.000 ** |
| Depth (m) | 1.13 ± 0.07 | 1.94 ± 0.33 | 1.31 ± 0.08 | 0.011 * |
| Spring | Summer | Autumn | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Environmental | Lake Area | River Area | Wetland Area | Lake Area | River Area | Wetland Area | Lake Area | River Area | Wetland Area |
| Factors | |||||||||
| TN | 1.31 ± 0.07 | 1.23 ± 0.10 | 0.99 ± 0.08 | 1.25 ± 0.09 | 1.10 ± 0.27 | 1.17 ± 0.13 | 1.85 ± 0.15 | 1.84 ± 0.19 | 1.77 ± 0.16 |
| TP | 0.11 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.17 ± 0.01 | 0.26 ± 0.03 | 0.27 ± 0.01 | 0.17 ± 0.01 | 0.23 ± 0.04 | 0.25 ± 0.02 |
| CODcr | 20.61 ± 0.96 | 32.30 ± 2.04 | 30.04 ± 2.26 | 26.56 ± 0.61 | 28.57 ± 2.58 | 28.37 ± 1.16 | 24.29 ± 0.94 | 29.40 ± 1.67 | 25.27 ± 1.48 |
| WT | 16.08 ± 0.12 | 16.57 ± 0.13 | 16.67 ± 0.24 | 25.46 ± 0.11 | 25.14 ± 0.20 | 24.58 ± 0.08 | 9.66 ± 0.10 | 9.45 ± 0.22 | 9.50 ± 0.15 |
| Cl− | 38.52 ± 3.48 | 30.38 ± 8.19 | 28.40 ± 3.05 | 80.19 ± 16.32 | 30.75 ± 6.26 | 46.78 ± 7.00 | 116.06 ± 16.96 | 43.81 ± 6.28 | 82.60 ± 10.64 |
| pH | 9.47 ± 0.03 | 9.55 ± 0.07 | 9.29 ± 0.07 | 9.52 ± 0.05 | 9.14 ± 0.13 | 8.99 ± 0.07 | 9.76 ± 0.07 | 9.49 ± 0.11 | 9.44 ± 0.07 |
| NH4+-N | 1.52 ± 0.03 | 1.45 ± 0.06 | 1.49 ± 0.10 | 1.20 ± 0.02 | 1.31 ± 0.09 | 1.33 ± 0.05 | 1.11 ± 0.01 | 1.20 ± 0.03 | 1.18 ± 0.02 |
| EC | 0.44 ± 0.03 | 0.37 ± 0.09 | 0.34 ± 0.05 | 0.41 ± 0.03 | 0.61 ± 0.10 | 0.53 ± 0.06 | 0.51 ± 0.03 | 0.37 ± 0.07 | 0.57 ± 0.06 |
| NTU | 16.94 ± 2.29 | 20.51 ± 3.20 | 9.04 ± 1.41 | 31.32 ± 3.37 | 19.86 ± 8.52 | 14.22 ± 3.55 | 21.16 ± 3.34 | 9.64 ± 2.61 | 10.32 ± 1.51 |
| DO | 7.48 ± 0.23 | 6.32 ± 0.31 | 6.59 ± 0.17 | 8.13 ± 0.24 | 7.59 ± 0.44 | 7.18 ± 0.21 | 10.04 ± 0.26 | 8.62 ± 0.41 | 10.01 ± 0.52 |
| Depth | 1.21 ± 0.10 | 1.16 ± 0.17 | 0.90 ± 0.09 | 2.36 ± 0.49 | 1.10 ± 0.27 | 1.18 ± 0.13 | 1.21 ± 0.10 | 1.54 ± 0.19 | 1.50 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, Y.; Chai, F.; Yu, H.; Sun, X.; Liu, D. Phytoplankton Community Structure Dynamics in Relation to Water Environmental Factors in Zhalong Wetland. Int. J. Environ. Res. Public Health 2022, 19, 14996. https://doi.org/10.3390/ijerph192214996
Li X, Zhao Y, Chai F, Yu H, Sun X, Liu D. Phytoplankton Community Structure Dynamics in Relation to Water Environmental Factors in Zhalong Wetland. International Journal of Environmental Research and Public Health. 2022; 19(22):14996. https://doi.org/10.3390/ijerph192214996
Chicago/Turabian StyleLi, Xiaoyu, Yuxi Zhao, Fangying Chai, Hongxian Yu, Xu Sun, and Di Liu. 2022. "Phytoplankton Community Structure Dynamics in Relation to Water Environmental Factors in Zhalong Wetland" International Journal of Environmental Research and Public Health 19, no. 22: 14996. https://doi.org/10.3390/ijerph192214996





