Conduct Problems and Hair Cortisol Concentrations Decrease in School-Aged Children after VIPP-SD: A Randomized Controlled Trial in Two Twin Cohorts
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Intervention
2.3.1. Randomization
2.3.2. VIPP-SD for Twins
2.3.3. Control Condition
2.4. Measures
2.4.1. Strengths and Difficulties Questionnaire (SDQ; [29])
2.4.2. Hair Cortisol Concentrations (HCC)
2.4.3. Genotyping and Imputation
2.4.4. Polygenic Score (PGS) of Differential Susceptibility
2.4.5. Control Variables
2.5. Data Analysis
3. Results
3.1. Preliminary Analyses
3.2. Main Results
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section/Topic | Item No | Checklist Item | As Reported on Page No |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomized trial in the title | 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | 1 | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of the rationale | 1–3 |
| 2b | Specific objectives or hypotheses | 3 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 3–5 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | 7, Supplement | |
| Participants | 4a | Eligibility criteria for participants | 3–5 |
| 4b | Settings and locations where the data were collected | 3–5 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were administered | 5 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 4–6 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | 7, Supplement | |
| Sample size | 7a | How sample size was determined | 3 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | Not applicable | |
| Randomization: | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 5 |
| 8b | Type of randomization; details of any restriction (such as blocking and block size) | 5 | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 5 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 5 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 5 |
| 11b | If relevant, description of the similarity of interventions | Not applicable | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 7 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 7 | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome | 4 |
| 13b | For each group, losses and exclusions after randomization, together with reasons | 4 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 4 |
| 14b | Why the trial ended or was stopped | Not applicable | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 4 |
| Numbers analyzed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 4,7 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 8–10 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | Not applicable | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | 10–11, Supplement |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | Not applicable |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, the multiplicity of analyses | 12 |
| Generalizability | 21 | Generalizability (external validity, applicability) of the trial findings | 11,12 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 10–12 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | 5 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | Not applicable |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 13 |
References
- Juffer, F. Pairing Attachment Theory and Social Learning Theory in Video-Feedback Intervention to Promote Positive Parenting. Curr. Opin. Psychol. 2017, 6, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Van IJzendoorn, M.H.; Schuengel, C.; Wang, Q.; Bakermans-Kranenburg, M.J. Improving Parenting, Child Attachment, and Externalizing Behaviors: Meta-Analysis of the First 25 Randomized Controlled Trials on the Effects of Video-Feedback Intervention to Promote Positive Parenting and Sensitive Discipline. Dev. Psychopathol. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Groh, A.M.; Fearon, R.M.P.; Van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J.; Roisman, G.I. Attachment in the Early Life Course: Meta-Analytic Evidence for Its Role in Socioemotional Development. Child Dev. Perspect. 2017, 11, 70–76. [Google Scholar] [CrossRef]
- Gunnar, M.R. Social Buffering of Stress in Development: A Career Perspective. Perspect. Psychol. Sci. 2017, 12, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.L.; Clarke-Stewart, K.A. Trajectories of Externalizing Behavior from Age 2 to Age 9: Relations with Gender, Temperament, Ethnicity, Parenting, and Rater. Dev. Psychol. 2008, 44, 771–786. [Google Scholar] [CrossRef]
- Cooke, J.E.; Deneault, A.-A.; Devereux, C.; Eirich, R.; Fearon, R.M.P.; Madigan, S. Parental Sensitivity and Child Behavioral Prolems: A Meta-Analytic Review. Child Dev. 2022, 93, 1231–1248. [Google Scholar] [CrossRef]
- Hostinar, C.E.; Sullivan, R.M.; Gunnar, M.R. Psychobiological Mechanisms Underlying the Social Buffering of the Hypothalamic–Pituitary–Adrenocortical Axis: A Review of Animal Models and Human Studies across Development. Psychol. Bull. 2014, 140, 256–282. [Google Scholar] [CrossRef]
- Simmons, J.G.; Azpitarte, F.; Roost, F.D.; Dommers, E.; Allen, N.B.; Havighurst, S.; Haslam, N. Correlates of Hair Cortisol Concentrations in Disadvantaged Young Children. Stress Health 2019, 35, 104–111. [Google Scholar] [CrossRef]
- O’Farrelly, C.; Barker, B.; Watt, H.; Babalis, D.; Bakermans-Kranenburg, M.; Byford, S.; Ganguli, P.; Grimås, E.; Iles, J.; Mattock, H.; et al. A Video-Feedback Parenting Intervention to Prevent Enduring Behaviour Problems in at-Risk Children Aged 12–36 Months: The Healthy Start, Happy Start RCT. Health Technol. Assess. 2021, 25, 1–84. [Google Scholar] [CrossRef]
- Bernard, K.; Hostinar, C.E.; Dozier, M. Intervention Effects on Diurnal Cortisol Rhythms of Child Protective Services–Referred Infants in Early Childhood: Preschool Follow-up Results of a Randomized Clinical Trial. JAMA Pediatr. 2015, 169, 112. [Google Scholar] [CrossRef]
- Bakermans-Kranenburg, M.J.; Van IJzendoorn, M.H.; Mesman, J.; Alink, L.R.A.; Juffer, F. Effects of an Attachment-Based Intervention on Daily Cortisol Moderated by Dopamine Receptor D4: A Randomized Control Trial on 1- to 3-Year-Olds Screened for Externalizing Behavior. Dev. Psychopathol. 2008, 20, 805–820. [Google Scholar] [CrossRef]
- Martins, R.C.; Blumenberg, C.; Tovo-Rodrigues, L.; Gonzalez, A.; Murray, J. Effects of Parenting Interventions on Child and Caregiver Cortisol Levels: Systematic Review and Meta-Analysis. BMC Psychiatry 2020, 20, 370. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C. Analysis of Cortisol in Hair—State of the Art and Future Directions. Brain. Behav. Immun. 2012, 26, 1019–1029. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Hellhammer, D.H. Salivary Cortisol in Psychoneuroendocrine Research: Recent Developments and Applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Strahler, J.; Skoluda, N.; Kappert, M.B.; Nater, U.M. Simultaneous Measurement of Salivary Cortisol and Alpha-Amylase: Application and Recommendations. Neurosci. Biobehav. Rev. 2017, 83, 657–677. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Kino, T. Glucocorticoid Action Networks and Complex Psychiatric and/or Somatic Disorders. Stress 2007, 10, 213–219. [Google Scholar] [CrossRef]
- Poehlmann-Tynan, J.; Engbretson, A.; Vigna, A.B.; Weymouth, L.A.; Burnson, C.; Zahn-Waxler, C.; Kapoor, A.; Gerstein, E.D.; Fanning, K.A.; Raison, C.L. Cognitively-Based Compassion Training for Parents Reduces Cortisol in Infants and Young Children. Infant Ment. Health J. 2020, 41, 126–144. [Google Scholar] [CrossRef]
- Ellis, B.J.; Boyce, W.T.; Belsky, J.; Bakermans-Kranenburg, M.J.; Ijzendoorn, M.H.V. Differential Susceptibility to the Environment: An Evolutionary–Neurodevelopmental Theory. Dev. Psychopathol. 2011, 23, 7–28. [Google Scholar] [CrossRef]
- Belsky, J.; Bakermans-Kranenburg, M.J.; Van IJzendoorn, M.H. For Better and For Worse: Differential Susceptibility to Environmental Influences. Curr. Dir. Psychol. Sci. 2007, 16, 300–304. [Google Scholar] [CrossRef]
- Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H. The Hidden Efficacy of Interventions: Gene×Environment Experiments from a Differential Susceptibility Perspective. Annu. Rev. Psychol. 2015, 66, 381–409. [Google Scholar] [CrossRef]
- Belsky, J.; van IJzendoorn, M.H. Genetic Differential Susceptibility to the Effects of Parenting. Curr. Opin. Psychol. 2017, 15, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Keers, R.; Coleman, J.R.I.; Lester, K.J.; Roberts, S.; Breen, G.; Thastum, M.; Bögels, S.; Schneider, S.; Heiervang, E.; Meiser-Stedman, R.; et al. A Genome-Wide Test of the Differential Susceptibility Hypothesis Reveals a Genetic Predictor of Differential Response to Psychological Treatments for Child Anxiety Disorders. Psychother. Psychosom. 2016, 85, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Lemery-Chalfant, K.; Clifford, S.; Dishion, T.J.; Shaw, D.S.; Wilson, M.N. Genetic Moderation of the Effects of the Family Check-Up Intervention on Children’s Internalizing Symptoms: A Longitudinal Study with a Racially/Ethnically Diverse Sample. Dev. Psychopathol. 2018, 30, 1729–1747. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.; Speed, D.; Sluis, S.; Østergaard, S.D. Genome-wide Association Study of the Sensitivity to Environmental Stress and Adversity Neuroticism Cluster. Acta Psychiatr. Scand. 2020, 141, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Euser, S.; Bakermans-Kranenburg, M.J.; van den Bulk, B.G.; Linting, M.; Damsteegt, R.C.; Vrijhof, C.I.; van Wijk, I.C.; Crone, E.A.; van IJzendoorn, M.H. Efficacy of the Video-Feedback Intervention to Promote Positive Parenting and Sensitive Discipline in Twin Families (VIPP-Twins): Study Protocol for a Randomized Controlled Trial. BMC Psychol. 2016, 4, 33. [Google Scholar] [CrossRef]
- van der Meulen, M.; Steinbeis, N.; Achterberg, M.; van IJzendoorn, M.H.; Crone, E.A. Heritability of Neural Reactions to Social Exclusion and Prosocial Compensation in Middle Childhood. Dev. Cogn. Neurosci. 2018, 34, 42–52. [Google Scholar] [CrossRef]
- Euser, S.; Vrijhof, C.I.; Van den Bulk, B.G.; Vermeulen, R.; Bakermans-Kranenburg, M.J.; Van IJzendoorn, M.H. Video-Feedback Promotes Sensitive Limit-Setting in Parents of Twin Preschoolers: A Randomized Controlled Trial. BMC Psychol. 2021, 9, 46. [Google Scholar] [CrossRef]
- Runze, J.; Van IJzendoorn, M.H.; Vrijhof, C.I.; Bakermans-Kranenburg, M.J. Replicating a Randomized Trial With Video-Feedback to Promote Positive Parenting in Parents of School-Aged Twins. J. Fam. Psychol. 2022, 36, 490–501. [Google Scholar] [CrossRef]
- Goodman, R. The Strengths and Difficulties Questionnaire: A Research Note. J. Child Pschiatry 1997, 38, 581–586. [Google Scholar] [CrossRef]
- Rippe, R.C.A.; Noppe, G.; Windhorst, D.A.; Tiemeier, H.; van Rossum, E.F.C.; Jaddoe, V.W.V.; Verhulst, F.C.; Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H.; van den Akker, E.L.T. Splitting Hair for Cortisol? Associations of Socio-Economic Status, Ethnicity, Hair Color, Gender and Other Child Characteristics with Hair Cortisol and Cortisone. Psychoneuroendocrinology 2016, 66, 56–64. [Google Scholar] [CrossRef]
- Gao, W.; Stalder, T.; Foley, P.; Rauh, M.; Deng, H.; Kirschbaum, C. Quantitative Analysis of Steroid Hormones in Human Hair Using a Column-Switching LC–APCI–MS/MS Assay. J. Chromatogr. B 2013, 928, 1–8. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Choi, S.W.; O’Reilly, P.F. PRSice-2: Polygenic Risk Score Software for Biobank-Scale Data. GigaScience 2019, 8, giz082. [Google Scholar] [CrossRef]
- Kahan, B.C.; Jairath, V.; Doré, C.J.; Morris, T.P. The Risks and Rewards of Covariate Adjustment in Randomized Trials: An Assessment of 12 Outcomes from 8 Studies. Trials 2014, 15, 139. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide 2017, 8th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2017. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Bosmans, G.; Van Vlierberghe, L.; Bakermans-Kranenburg, M.J.; Kobak, R.; Hermans, D.; van IJzendoorn, M.H. A Learning Theory Approach to Attachment Theory: Exploring Clinical Applications. Clin. Child Fam. Psychol. Rev. 2022, 25, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Steudte-Schmiedgen, S.; Alexander, N.; Klucken, T.; Vater, A.; Wichmann, S.; Kirschbaum, C.; Miller, R. Stress-Related and Basic Determinants of Hair Cortisol in Humans: A Meta-Analysis. Psychoneuroendocrinology 2017, 77, 261–274. [Google Scholar] [CrossRef]
- Boyce, W.T.; Ellis, B.J. Biological Sensitivity to Context: I. An Evolutionary–Developmental Theory of the Origins and Functions of Stress Reactivity. Dev. Psychopathol. 2005, 17, 271–301. [Google Scholar] [CrossRef]
- Zhang, X.; Widaman, K.; Belsky, J. Beyond Orchids and Dandelions: Susceptibility to Environmental Influences Is Not Bimodal. Dev. Psychopathol. 2021, 1–13. [Google Scholar] [CrossRef]
- Belsky, J.; Zhang, X.; Sayler, K. Differential Susceptibility 2.0: Are the Same Children Affected by Different Experiences and Exposures? Dev. Psychopathol. 2022, 34, 1025–1033. [Google Scholar] [CrossRef]
- Windhorst, D.A.; Mileva-Seitz, V.R.; Linting, M.; Hofman, A.; Jaddoe, V.W.V.; Verhulst, F.C.; Tiemeier, H.; Van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. Differential Susceptibility in a Developmental Perspective: DRD4 and Maternal Sensitivity Predicting Externalizing Behavior: G × E Effects of DRD4 and Sensitivity Over Time. Dev. Psychobiol. 2015, 57, 35–49. [Google Scholar] [CrossRef]
- Slagt, M.; Dubas, J.S.; Deković, M.; van Aken, M.A.G. Differences in Sensitivity to Parenting Depending on Child Temperament: A Meta-Analysis. Psychol. Bull. 2016, 142, 1068–1110. [Google Scholar] [CrossRef] [PubMed]
- Duncan, L.E.; Keller, M.C. A Critical Review of the First 10 Years of Candidate Gene-by-Environment Interaction Research in Psychiatry. Am. J. Psychiatry 2011, 168, 1041–1049. [Google Scholar] [CrossRef]
- Moffitt, T.E.; Caspi, A.; Rutter, M. Measured Gene-Environment Interactions in Psychopathology. Perspect. Psychol. Sci. 2006, 1, 23. [Google Scholar] [CrossRef] [PubMed]
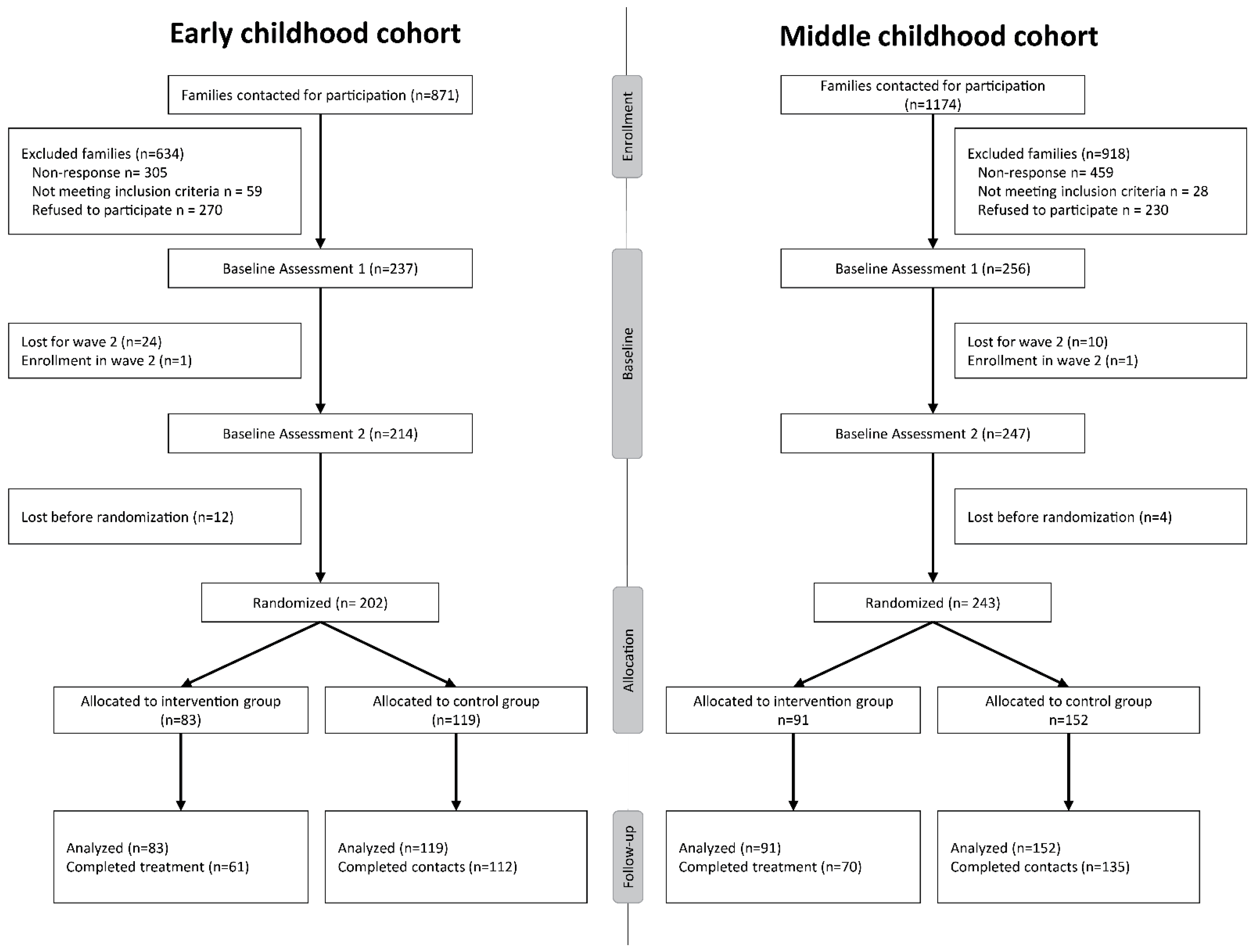


| Early Childhood Cohort | Middle Childhood Cohort | |||||
|---|---|---|---|---|---|---|
| Total (n = 202) | Intervention Group (n = 83) | Control Group (n = 119) | Total (n = 243) | Intervention Group (n = 91) | Control Group (n = 152) | |
| Twin characteristics | ||||||
| Age M (SD) | 3.76 (0.57) | 3.74 (0.62) | 3.77 (0.53) | 7.92 (0.66) | 7.94 (0.66) | 7.92 (0.67) |
| Sex (% boys) | 45 | 45.8 | 44.5 | 48.6 | 49.5 | 48.0 |
| Country of birth (% the Netherlands) | 99.5 | 100 | 99.2 | 99.2 | 100 | 98.7 |
| Zygosity (% MZ) | 60.4 | 66.3 | 56.3 | 55.1 | 50.5 | 57.9 |
| Family characteristics | ||||||
| Primary parent (%) | ||||||
| Biological mother | 91.6 | 88.0 | 94.1 | 90.5 | 87.9 | 92.1 |
| Adoptive mother | 0 | 0 | 0 | 0.8 | 1.1 | 0.7 |
| Biological father | 8.4 | 12.0 | 5.9 | 8.6 | 11.0 | 7.2 |
| Age primary parent M (SD) | 36.87 (4.69) | 36.89 (4.82) | 36.82 (4.62) | 40.48 (4.66) | 40.77 (4.78) | 40.32 (4.60) |
| Country of birth (% the Netherlands) | 96.0 | 98.8 | 94.1 | 97.5 | 96.7 | 98.0 |
| Educational level primary parent | ||||||
| Lower and Intermediate vocational | 30.2 | 37.3 | 25.2 | 34.3 | 35.2 | 33.8 |
| Higher vocational, university bachelor | 42.1 | 36.1 | 46.2 | 41.7 | 39.6 | 43.0 |
| Post-higher vocational, university master | 27.7 | 26.5 | 28.6 | 24.0 | 25.3 | 23.2 |
| Primary parents’ marital status (%) | ||||||
| Two-parent household | 96.5 | 96.4 | 96.6 | 93.8 | 93.4 | 94.1 |
| Single parent household | 3.5 | 3.6 | 3.4 | 6.2 | 6.6 | 5.9 |
| T1 | T2 | T3 | T4 | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Hair cortisol (HCC) in pg/mg | ||||
| Intervention group | na | 3.25 (4.09) | 3.04 (4.60) | na |
| Control group | na | 3.08 (3.51) | 3.04 (4.47) | na |
| Conduct problems | ||||
| Intervention group | 1.30 (0.27) | 1.29 (0.27) | 1.26 (0.26) | 1.21 (0.23) |
| Control group | 1.30 (0.28) | 1.29 (0.29) | 1.26 (0.26) | 1.23 (0.26) |
| Predictor | Est | SE | p | 95% CIs |
|---|---|---|---|---|
| Intercept | 0.05 | 0.07 | 0.74 | −0.06–0.16 |
| Time | 0.09 | 0.10 | 0.33 | −0.06–0.25 |
| Time2 | −0.24 | 0.10 | 0.01 | −0.38–−0.08 |
| Condition | 0.02 | 0.06 | 0.27 | −0.08–0.11 |
| Condition × Time2 | −0.08 | 0.04 | 0.03 | −0.13–−0.02 |
| Condition × PGS-ES × Time2 | −0.00 | 0.00 | 0.41 | −0.11–0.04 |
| Condition × PGS-SESA × Time2 | −0.05 | 0.04 | 0.20 | −0.09–0.01 |
| Predictor | Est | SE | p | 95% CIs |
|---|---|---|---|---|
| Intercept | 0.09 | 0.09 | 0.32 | −0.06–0.23 |
| Time | −0.12 | 0.05 | 0.02 | −0.20–−0.04 |
| Condition | 0.50 | 0.19 | 0.01 | 0.20–0.81 |
| Condition × Time | −0.44 | 0.19 | 0.02 | −0.75–−0.13 |
| Condition × PGS-ES × Time | 0.06 | 0.06 | 0.34 | −0.04–0.15 |
| Condition × PGS-SESA × Time | 0.00 | 0.06 | 0.95 | −0.09–0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Runze, J.; Pappa, I.; Van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. Conduct Problems and Hair Cortisol Concentrations Decrease in School-Aged Children after VIPP-SD: A Randomized Controlled Trial in Two Twin Cohorts. Int. J. Environ. Res. Public Health 2022, 19, 15026. https://doi.org/10.3390/ijerph192215026
Runze J, Pappa I, Van IJzendoorn MH, Bakermans-Kranenburg MJ. Conduct Problems and Hair Cortisol Concentrations Decrease in School-Aged Children after VIPP-SD: A Randomized Controlled Trial in Two Twin Cohorts. International Journal of Environmental Research and Public Health. 2022; 19(22):15026. https://doi.org/10.3390/ijerph192215026
Chicago/Turabian StyleRunze, Jana, Irene Pappa, Marinus H. Van IJzendoorn, and Marian J. Bakermans-Kranenburg. 2022. "Conduct Problems and Hair Cortisol Concentrations Decrease in School-Aged Children after VIPP-SD: A Randomized Controlled Trial in Two Twin Cohorts" International Journal of Environmental Research and Public Health 19, no. 22: 15026. https://doi.org/10.3390/ijerph192215026
APA StyleRunze, J., Pappa, I., Van IJzendoorn, M. H., & Bakermans-Kranenburg, M. J. (2022). Conduct Problems and Hair Cortisol Concentrations Decrease in School-Aged Children after VIPP-SD: A Randomized Controlled Trial in Two Twin Cohorts. International Journal of Environmental Research and Public Health, 19(22), 15026. https://doi.org/10.3390/ijerph192215026






