Higher Risk of Acute Respiratory Distress Syndrome and Risk Factors among Patients with COVID-19: A Systematic Review, Meta-Analysis and Meta-Regression
Abstract
:1. Introduction
1.1. The Objective of This Study
1.2. Research Question
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Quality Assessment
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
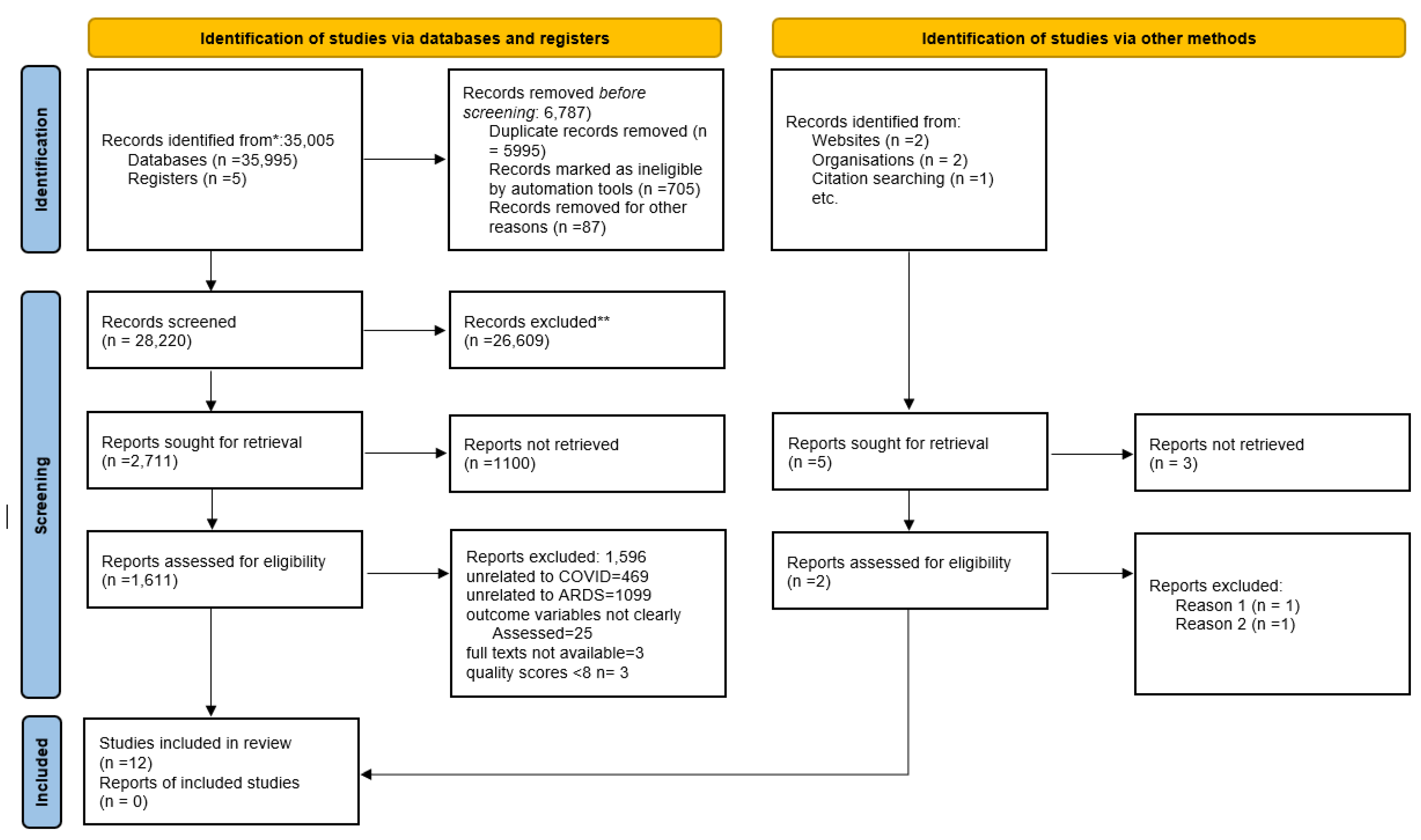
3.1. Study Identification
3.2. Study Characteristics
3.3. Participant Characteristics
3.4. Higher Risk of ARDS among Patients with COVID-19
3.5. Risk Factors of ARDS among COVID-19 through Meta-Regression Analysis
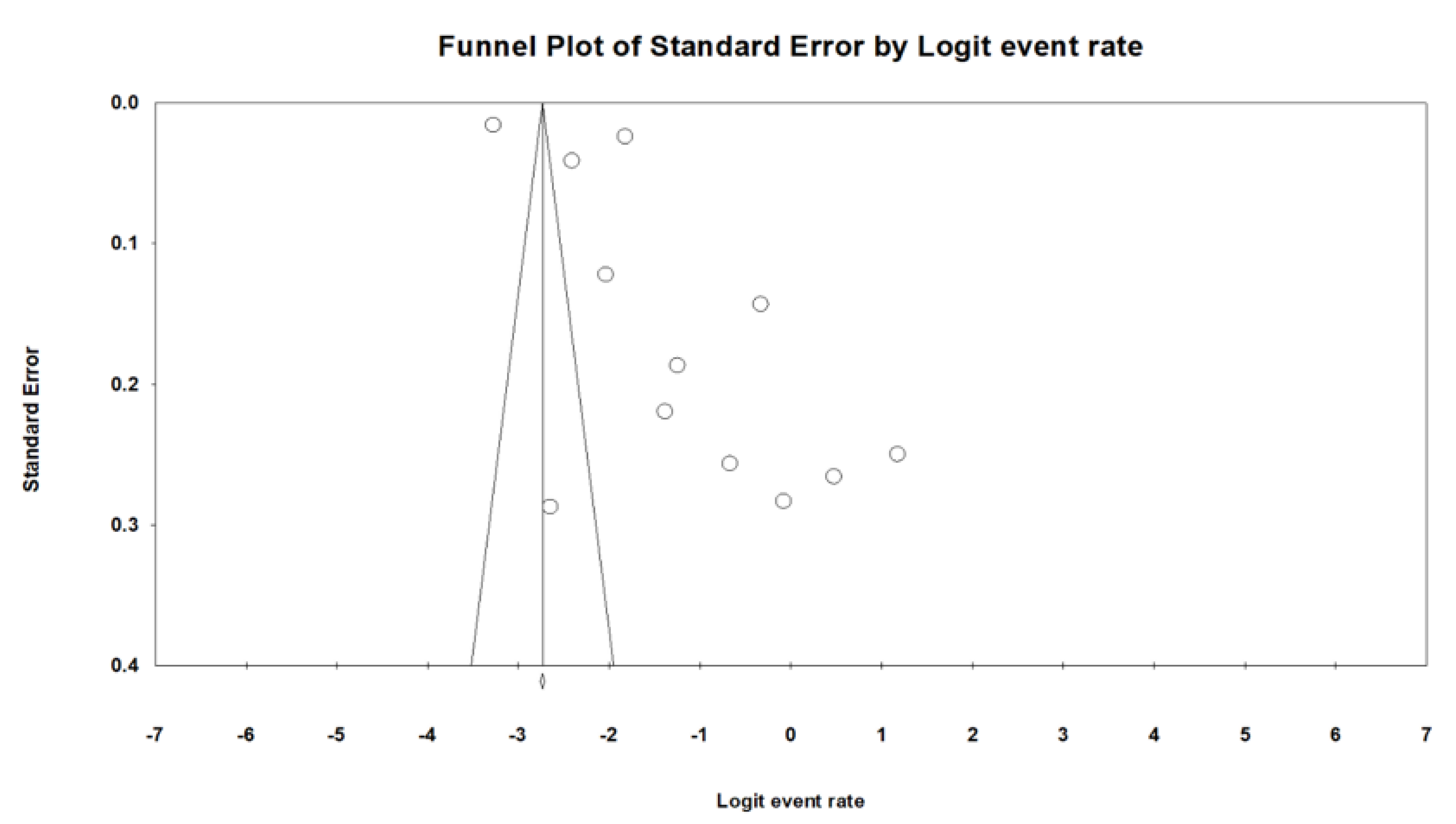
3.6. Publication Bias
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phelan, A.L.; Katz, R.; Gostin, L.O. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA 2020, 323, 709–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536.
- Dreher, M.; Kersten, A.; Bickenbach, J.; Balfanz, P.; Hartmann, B.; Cornelissen, C.; Daher, A.; Stöhr, R.; Kleines, M.; Lemmen, S.W. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Deutsches Ärzteblatt International 2020, 117, 271. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Muntean, E.A.; Mallen, C.D. The impact of COVID-19 on the patient, clinician, healthcare services and society: A patient pathway review. J. Med. Virol. 2022, 94, 3634–3641. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, J.; Webb, E.; Williams, G.A.; Hernández-Quevedo, C.; Maier, C.B.; Panteli, D. European countries’ responses in ensuring sufficient physical infrastructure and workforce capacity during the first COVID-19 wave. Health Policy 2022, 126, 362–372. [Google Scholar] [CrossRef]
- Baj, J.; Karakuła-Juchnowicz, H.; Teresiński, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, E.; Forma, A.; Karakuła, K.; Flieger, W.; Portincasa, P. COVID-19: Specific and non-specific clinical manifestations and symptoms: The current state of knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Zacharis, A.; Keskinidou, C.; Jahaj, E.; Pratikaki, M.; Gallos, P.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. Soluble angiotensin converting enzyme 2 (ACE2) is upregulated and soluble endothelial nitric oxide synthase (eNOS) is downregulated in COVID-19-induced acute respiratory distress syndrome (ARDS). Pharmaceuticals 2021, 14, 695. [Google Scholar] [CrossRef]
- Mizera, L.; Rath, D.; Schoellmann, A.; Petersen-Uribe, A.; Avdiu, A.; Zdanyte, M.; Jaeger, P.; Heinzmann, D.; Müller, K.; Gawaz, M. Deceleration capacity is associated with acute respiratory distress syndrome in COVID-19. Heart Lung 2021, 50, 914–918. [Google Scholar] [CrossRef]
- Gujski, M.; Jankowski, M.; Rabczenko, D.; Goryński, P.; Juszczyk, G. The Prevalence of Acute Respiratory Distress Syndrome (ARDS) and Outcomes in Hospitalized Patients with COVID-19—A Study Based on Data from the Polish National Hospital Register. Viruses 2022, 14, 76. [Google Scholar] [CrossRef]
- Mathews, K.; Soh, H.; Shaefi, S.; Wang, W.; Bose, S.; Coca, S.; Gupta, S.; Hayek, S.; Srivastava, A.; Brenner, S. Study of the Treatment and Outcomes in Critically Ill Patients with Coronavirus Disease (STOP-COVID) Investigators, Prone positioning and survival in mechanically ventilated patients with coronavirus disease 2019-related respiratory failure. Crit. Care Med. 2021, 49, 1026–1037. [Google Scholar] [PubMed]
- Hasan, S.S.; Capstick, T.; Ahmed, R.; Kow, C.S.; Mazhar, F.; Merchant, H.a.; Zaidi, S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2020, 14, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Tzotzos, S.J.; Fischer, B.; Fischer, H.; Zeitlinger, M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit. Care 2020, 24, 516. [Google Scholar] [CrossRef] [PubMed]
- Shih, E.; Squiers, J.J.; DiMaio, J.M.; George, T.; Banwait, J.; Monday, K.; Blough, B.; Meyer, D.; Schwartz, G.S. Outcomes of extracorporeal membrane oxygenation in patients with severe acute respiratory distress syndrome caused by COVID-19 versus influenza. Ann. Thorac. Surg. 2022, 113, 1445–1451. [Google Scholar] [CrossRef]
- Singhal, L.; Garg, Y.; Yang, P.; Tabaie, A.; Wong, A.I.; Mohammed, A.; Chinthala, L.; Kadaria, D.; Sodhi, A.; Holder, A.L. eARDS: A multi-center validation of an interpretable machine learning algorithm of early onset Acute Respiratory Distress Syndrome (ARDS) among critically ill adults with COVID-19. PLoS ONE 2021, 16, e0257056. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Ho, F.K.; Petermann-Rocha, F.; Gray, S.R.; Jani, B.D.; Katikireddi, S.V.; Niedzwiedz, C.L.; Foster, H.; Hastie, C.E.; Mackay, D.F.; Gill, J.M. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS ONE 2020, 15, e0241824. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: A scoping review. Syst. Rev. 2017, 6, 263. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H. Regression in meta-analysis. In Comprehensive Meta Analysis Manual; Biostat, Inc.: Frederick, MD, USA, 2015. [Google Scholar]
- McInnes, M.D.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group*, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; The Joanna Briggs Institute: Adelaide, Australia, 2017; Volume 5. [Google Scholar]
- Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J.; Rücker, G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res. Synth. Methods 2019, 10, 476–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliavaca, C.B.; Stein, C.; Colpani, V.; Barker, T.H.; Munn, Z.; Falavigna, M. How are systematic reviews of prevalence conducted? A methodological study. BMC Med. Res. Methodol 2020, 20, 96. [Google Scholar] [CrossRef] [Green Version]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. Jama 2006, 295, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Borenstein, M. Software for publication bias. In Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 193–220. [Google Scholar]
- Wang, A.; Gao, G.; Wang, S.; Chen, M.; Qian, F.; Tang, W.; Xu, Y.; Song, R.; Zhuang, L.; Ma, X. Clinical characteristics and risk factors of acute respiratory distress syndrome (ARDS) in COVID-19 patients in Beijing, China: A retrospective study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e925971–e925974. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Sun, N.-N.; Gao, H.-N.; Chen, Z.-Y.; Yang, Y.; Ju, B.; Tang, L.-L. Risk factors analysis of COVID-19 patients with ARDS and prediction based on machine learning. Sci. Rep. 2021, 11, 2933. [Google Scholar] [CrossRef]
- Hu, X.-S.; Hu, C.-H.; Zhong, P.; Wen, Y.-J.; Chen, X.-Y. Risk factors associated with acute respiratory distress syndrome in COVID-19 patients outside Wuhan: A double-center retrospective cohort study of 197 cases in Hunan, China. World J. Clin. Cases 2021, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- El-Solh, A.A.; Meduri, U.G.; Lawson, Y.; Carter, M.; Mergenhagen, K.A. Clinical course and outcome of COVID-19 acute respiratory distress syndrome: Data from a national repository. J. Intensive Care Med. 2021, 36, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, T.; Gupta, N.; Kohli, S.; Khurana, A.; Dass, J.; Diwan, S.; Mahendran, A.; Khan, M.; Aggarwal, M.; Subramanian, A. A Prospective Study of Specialized Coagulation Parameters in Admitted COVID-19 Patients and Their Correlation With Acute Respiratory Distress Syndrome and Outcome. Cureus 2021, 13, e17463. [Google Scholar] [CrossRef]
- Seo, J.-W.; Kim, S.E.; Choi, E.Y.; Hong, K.S.; Oh, T.H.; Kim, U.; Kang, S.-J.; Park, K.-H.; Jung, S.-I.; Kim, D.Y. Risk Factors and a Scoring System to Predict ARDS in Patients with COVID-19 Pneumonia in Korea: A Multicenter Cohort Study. Dis. Markers 2021, 2021, 8821697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Cao, J.; Tu, W.-J.; Cheng, W.; Yu, L.; Liu, Y.-K.; Hu, X.; Liu, Q. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 748–755. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Kumari, R.; Bhattacharyya, S. Pandemics and Patients with Chronic Diseases: A Study on the Health Care System in Port Blair, Andaman and Nicobar Islands. J. Anthropol. Surv. India 2022, 2277436X221087535. [Google Scholar] [CrossRef]
- Azoulay, É.; Curtis, J.R.; Kentish-Barnes, N. Ten reasons for focusing on the care we provide for family members of critically ill patients with COVID-19. Intensive Care Med. 2021, 47, 230–233. [Google Scholar] [CrossRef]
- Ramanathan, K.; Antognini, D.; Combes, A.; Paden, M.; Zakhary, B.; Ogino, M.; MacLaren, G.; Brodie, D.; Shekar, K. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir. Med. 2020, 8, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Matthay, M.A.; Aldrich, J.M.; Gotts, J.E. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir. Med. 2020, 8, 433–434. [Google Scholar] [CrossRef]
- Shekar, K.; Slutsky, A.S.; Brodie, D. ECMO for severe ARDS associated with COVID-19: Now we know we can, but should we? Lancet Respir. Med. 2020, 8, 1066–1068. [Google Scholar] [CrossRef]
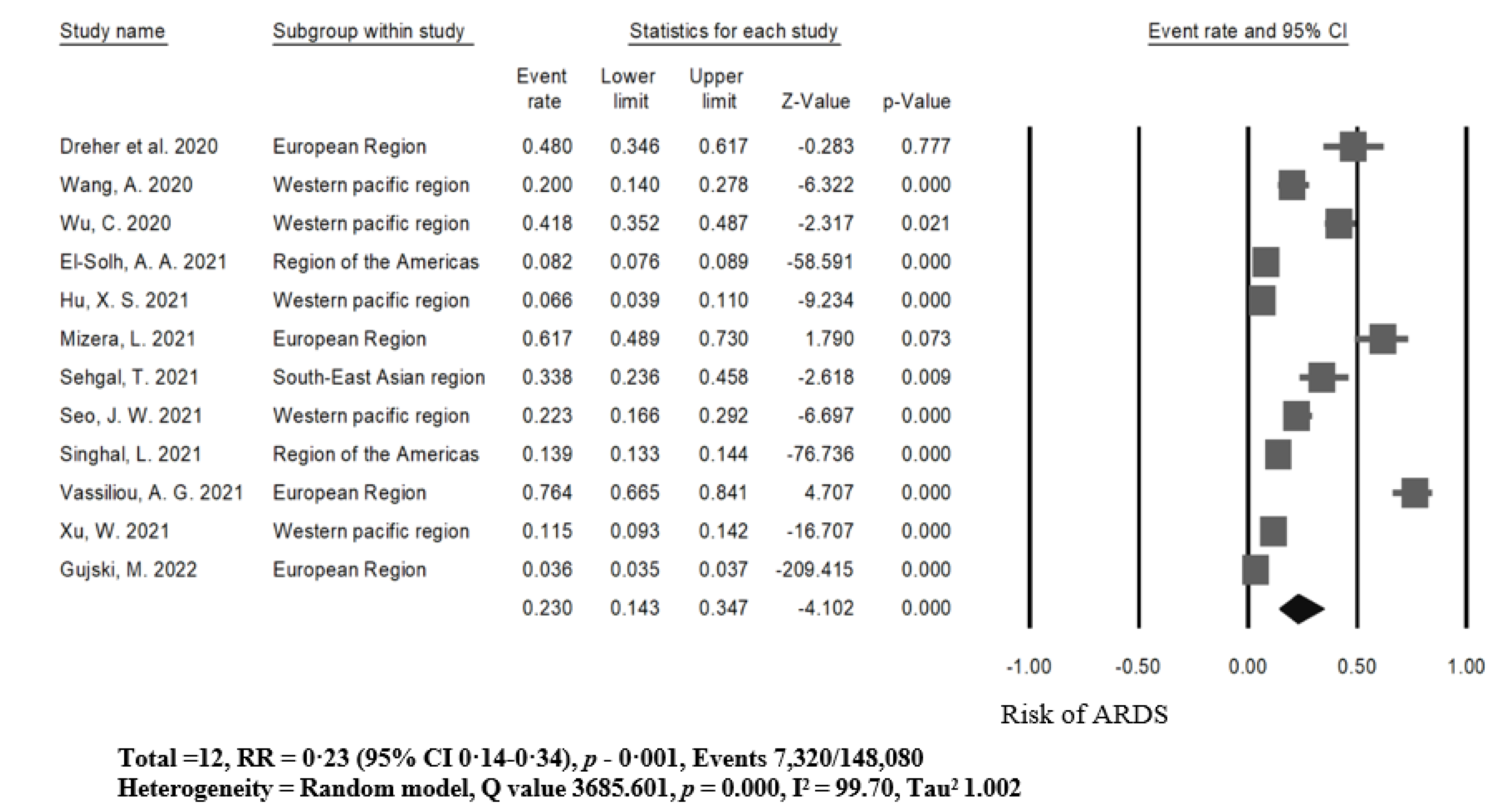
| Author, (Year) Country | WHO Region | Study Design | Ethnicity (n) | Mean Age | Sample Size (n) | Gender (n) | Prevalence Rate of ARDS (n) (%) | Joanna Briggs Institute Score |
|---|---|---|---|---|---|---|---|---|
| Dreher et al. (2020) Germany [3] | European region | Cross- sectional study | Caucasian: 50 | ALL: 65. ARDS: 62. No ARDS: 68. | 50 | Male—33 Female—17 | 24 (48%) | 9 |
| Wang et al. (2020) China [31] | Western Pacific region | Retrospective study | Chinese: 130 | ARDS: 63.5. No ARDS: 40. | 130 | Male—17 Female—128 | 26 (20%) | 9 |
| Wu et al. (2020) China [32] | Western Pacific region | Cohort study | Chinese: 201 | ARDS: 58.5. No ARDS: 48. | 201 | Male—128 Female—73 | 84 (41.80%) | 8 |
| El-Solh et al. (2021) United States [35] | Region of the Americas | Retrospective study | Caucasian:3547 Black: 3264 Latino:989 | ARDS: 69 No ARDS: 70. | 7816 | Male—7387 Female—60 | 643 (8.23%) | 9 |
| Hu et al. (2021) China [34] | Western Pacific region | Retrospective study | Chinese: 197 | ALL: 45. ARDS: 58. No ARDS: 42. | 197 | Male—93 Female—104 | 13 (6.60%) | 9 |
| Mizera et al. (2021) Germany [9] | European region | Cross- sectional study | Caucasian: 60 | ARDS: 69.1. No ARDS: 63.3 | 60 | Male—36 Female—24 | 37 (61.67%) | 8 |
| Sehgal et al. (2021) India [36] | South-East Asian region | Prospective study | Chinese: 68 | NA | 68 | Male—43 Female—25 | 23 (33.82%) | 9 |
| Seo et al. (2021) Korea [37] | Western Pacific region | Cohort study | Korean: 166 | ARDS: 72. No ARDS: 56 | 166 | Male—78 Female—88 | 37 (22.29%) | 8 |
| Singhal et al. (2021) United States [15] | Region of the Americas | Retrospective study | African: 4089 Caucasian:7462 Indian: 258 Asian: 427 Mixed race: 4 Unknown: 2544 | ARDS: 62. No ARDS: 68. | 14,785 | Male—7358 Female—7427 | 2052 (13.88%) | 8 |
| Vassiliou et al. (2021) Greece [8] | European Region | Cross- sectional study | Caucasian: 89 | ARDS: 62. No ARDS: 61. | 89 | Male—70 Female—19 | 68 (76.40%) | 9 |
| Xu et al. (2021) China [33] | Western Pacific region | Retrospective study | Chinese: 659 | ARDS: 56.5. No ARDS: 49. | 659 | Male—332 Female—327 | 76 (11.53%) | 9 |
| Gujski et al. (2022) Poland [10] | European Region | Retrospective study | Caucasian: 116,539 | NA | 116,539 | Male—60,915 Female—55,624 | 4237 (3.60%) | 9 |
| Meta-Analysis | Meta-Regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | No of Study | Sample | Risk Ratio (%) (95% CI) | p-Value | I² | Coefficient | Standardized Error | 95% CI | p-Value |
| Gender | 12 | 147711 | 23.8 (17.7–31.2) | 0.001 | 99.42 | ||||
| Female | 12 | 72860 | 22.8 (14.5–33.9) | 0.001 | 99.05 | Reference | |||
| Male | 12 | 74851 | 25.1 (14.7–39.4) | 0.001 | 99.60 | 0.1048 | 0.4521 | −0.78–0.99 | 0.8166 |
| Age | 5 | 138642 | 10.0 (6.2–15.7) | 0.001 | 99.66 | ||||
| 14–40 years old | 5 | 3,642 | 12.4 (2.6–42.3) | 0.001 | 99.61 | Reference | |||
| ≥41–64 years old | 5 | 51207 | 13.5 (6.7–25.4) | 0.001 | 99.61 | 1.5309 | 0.7094 | 0.14–2.92 | 0.0309 |
| ≥65 years old | 3 | 49793 | 4.4 (2.6–7.3) | 0.001 | 95.73 | 1.1377 | 0.8132 | −0.45–2.73 | 0.1618 |
| Smoking | 4 | 9498 | 14.1 (9.4–20.5) | 0.001 | 95.37 | ||||
| No smoking | 4 | 5587 | 15.5 (6.8–31.5) | 0.001 | 97.49 | Reference | |||
| Smoking | 4 | 3911 | 12.5 (5.5–25.7) | 0.008 | 79.48 | −0.2463 | 0.7439 | −1.70–1.21 | 0.7406 |
| Cluster exposure history | 2 | 735 | 15.9 (8.7–27.4) | 0.001 | 85.31 | ||||
| No exposure history | 2 | 277 | 16.8 (12.8–21.7) | 0.290 | 10.68 | Reference | |||
| Exposure history | 2 | 458 | 20.5 (2.9–68.7) | 0.001 | 91.78 | 0.2372 | 0.8990 | −1.52–1.99 | 0.7919 |
| Fever | 7 | 10139 | 14.8 (9.9–21.7) | 0.001 | 96.16 | ||||
| No fever | 7 | 4404 | 8.5 (4.7–15.1) | 0.001 | 75.26 | Reference | |||
| Fever | 7 | 5735 | 20.9 (12.0–33.7) | 0.001 | 97.09 | 1.0355 | 0.5113 | 0.03–2.03 | 0.0429 |
| Muscular soreness | 3 | 1022 | 11.5 (6.8–18.8) | 0.001 | 83.12 | ||||
| No muscular soreness | 3 | 842 | 13.8 (6.6–26.5) | 0.001 | 91.80 | Reference | |||
| Muscular soreness | 3 | 180 | 8.7 (5.3–14.0) | 0.438 | 0.00 | −0.5387 | 0.6182 | −1.75–0.67 | 0.3835 |
| Cough | 7 | 10029 | 22.2 (15.2–31.3) | 0.001 | 96.51 | ||||
| No cough | 7 | 7010 | 18.0 (9.2–32.1) | 0.001 | 93.50 | Reference | |||
| Cough | 7 | 3019 | 26.8 (15.6–42.1) | 0.001 | 96.50 | 0.5185 | 0.5164 | −0.49–1.53 | 0.3153 |
| Productive cough | 5 | 1317 | 19.3 (12.5–28.5) | 0.001 | 91.20 | ||||
| No productive cough | 5 | 775 | 16.6 (8.7–29.6) | 0.001 | 91.86 | Reference | |||
| Productive cough | 5 | 542 | 22.1 (11.3–38.7) | 0.001 | 91.72 | 0.3575 | 0.5552 | −0.73–1.44 | 0.5196 |
| Sore throat | 4 | 1090 | 14.2 (8.5–22.8) | 0.001 | 85.01 | ||||
| No sore throat | 4 | 957 | 17.3 (8.9–31.0) | 0.001 | 92.93 | Reference | |||
| Sore throat | 4 | 133 | 10.0 (5.9–16.7) | 0.654 | 0.00 | −0.7391 | 0.6484 | −2.01–0.53 | 0.2543 |
| Dyspnea | 4 | 9044 | 19.9 (11.2–32.9) | 0.001 | 97.86 | ||||
| No dyspnea | 4 | 6097 | 8.6 (2.9–22.8) | 0.001 | 95.91 | Reference | |||
| Dyspnea | 4 | 2947 | 38.5 (11.4–75.4) | 0.001 | 98.04 | 1.9171 | 1.0015 | −0.45–3.88 | 0.0556 |
| Fatigue | 3 | 8381 | 9.1 (7.2–11.4) | 0.001 | 76.55 | ||||
| No fatigue | 3 | 6787 | 10.1 (9.4–10.9) | 0.182 | 41.29 | Reference | |||
| Fatigue | 3 | 1594 | 8.6 (5.2–13.9) | 0.010 | 78.34 | −0.1430 | 0.2636 | −0.65–0.37 | 0.5876 |
| Headache | 3 | 1029 | 20.3 (11.0–34.5) | 0.001 | 89.51 | ||||
| No headache | 3 | 876 | 19.6 (10.0–34.9) | 0.001 | 91.03 | Reference | |||
| Headache | 3 | 153 | 23.0 (3.5–71.4) | 0.001 | 92.12 | 0.1005 | 0.8026 | −1.47–1.67 | 0.9003 |
| Diarrhea | 6 | 9854 | 14.7 (10.5–20.0) | 0.001 | 90.32 | ||||
| No diarrhea | 6 | 8936 | 14.9 (9.2–23.2) | 0.001 | 94.83 | Reference | |||
| Diarrhea | 6 | 918 | 11.1 (9.1–13.5) | 0.018 | 63.50 | −0.0371 | 0.4417 | −0.90–0.82 | 0.9330 |
| Nausea and vomiting | 3 | 1022 | 13.6 (8.7–20.5) | 0.001 | 77.02 | ||||
| No nausea and vomiting | 3 | 912 | 12.2 (6.8–20.9) | 0.001 | 87.59 | Reference | |||
| Nausea and vomiting | 3 | 110 | 16.3 (6.6–35.0) | 0.088 | 58.81 | 0.3524 | 0.5670 | −0.75–1.46 | 0.5342 |
| Lung infiltrates or consolidation | 3 | 460 | 14.7 (4.9–36.9) | 0.001 | 90.59 | ||||
| No lung infiltrates or consolidation | 3 | 225 | 6.9 (4.1–11.1) | 0.966 | 0.00 | Reference | |||
| Lung infiltrates or consolidation occurred | 3 | 235 | 24.7 (6.0–62.9) | 0.001 | 91.70 | 1.4722 | 1.1172 | −0.71–3.66 | 0.1876 |
| Multilobe involvement in chest | 2 | 366 | 7.3 (1.6–28.1) | 0.001 | 90.70 | ||||
| Without multilobe involvement in chest | 2 | 116 | 0.8 (0.1–5.8) | 0.986 | 0.00 | Reference | |||
| With multilobe involvement in chest | 2 | 250 | 19.7 (4.3–57.2) | 0.001 | 95.14 | 3.3557 | 1.5330 | 0.35–6.36 | 0.0286 |
| Leucopenia | 2 | 327 | 14.9 (7.1–28.6) | 0.003 | 78.69 | ||||
| Without leucopenia | 2 | 83 | 18.1 (11.2–27.9) | 0.714 | 0.00 | Reference | |||
| With leucopenia | 2 | 244 | 12.4 (3.1–39.0) | 0.001 | 91.60 | −0.5511 | 1.0741 | −2.65–1.55 | 0.6079 |
| Lymphopenia | 2 | 327 | 11.1 (2.6–37.4) | 0.001 | 93.32 | ||||
| Without lymphopenia | 2 | 227 | 3.1 (1.5–6.4) | 0.614 | 0.00 | Reference | |||
| With lymphopenia | 2 | 100 | 30.4 (12.2–5.8) | 0.011 | 84.47 | 2.5987 | 0.7571 | 1.11–4.08 | 0.0006 |
| Underlying medical illnesses | 4 | 1103 | 32.7 (15.8–55.8) | 0.001 | 95.48 | ||||
| Without underlying medical illnesses | 4 | 593 | 34.6 (10.5–70.6) | 0.001 | 95.34 | Reference | |||
| With underlying medical illnesses | 4 | 510 | 31.2 (9.3–66.6) | 0.001 | 95.99 | –0.1561 | 1.0793 | −2.27–1.95 | 0.8850 |
| Diabetes mellitus | 10 | 131086 | 22.7 (16.3–30.6) | 0.001 | 98.69 | ||||
| Without diabetes mellitus | 10 | 119438 | 17.8 (10.0–29.4) | 0.001 | 98.97 | Reference | |||
| With diabetes mellitus | 10 | 11648 | 29.9 (18.9–43.8) | 0.001 | 96.92 | 0.7022 | 0.4680 | –0.21–1.61 | 0.1335 |
| Hypertension | 10 | 131100 | 20.6 (14.9–27.7) | 0.001 | 98.68 | ||||
| Without hypertension | 10 | 111673 | 15.2 (8.5–25.9) | 0.001 | 98.34 | Reference | |||
| With hypertension | 10 | 19427 | 28.5 (16.5–44.5) | 0.001 | 98.59 | 0.7917 | 0.4925 | −0.17–1.75 | 0.1079 |
| Chronic obstructive pulmonary disease | 8 | 130591 | 20.8 (14.1–29.7) | 0.001 | 98.53 | ||||
| Without chronic obstructive pulmonary disease | 8 | 125717 | 19.4 (11.0–32.0) | 0.001 | 99.15 | Reference | |||
| With chronic obstructive pulmonary disease | 8 | 4874 | 21.4 (12.0–35.3) | 0.001 | 89.10 | 0.3264 | 0.5407 | −0.73–1.38 | 0.5461 |
| Asthma | 3 | 777 | 25.4 (12.6–44.4) | 0.001 | 84.17 | ||||
| Without asthma | 3 | 766 | 19.4 (8.8–37.6) | 0.001 | 90.94 | Reference | |||
| With asthma | 3 | 11 | 49.1 (18.7–80.2) | 0.317 | 12.85 | 1.3206 | 0.9398 | −0.52–3.16 | 0.1600 |
| Chronic kidney failure | 8 | 131013 | 20.0 (13.8–28.3) | 0.001 | 98.48 | ||||
| Without chronic kidney failure | 8 | 125421 | 19.9 (11.7–31.9) | 0.001 | 99.16 | Reference | |||
| With chronic kidney failure | 8 | 5592 | 19.1 (10.6–31.8) | 0.739 | 91.53 | 0.1008 | 0.5133 | −0.90–1.10 | 0.8443 |
| Chronic cardiac disease | 7 | 130816 | 13.1 (9.3–18.2) | 0.001 | 98.86 | ||||
| Without chronic cardiac disease | 7 | 99673 | 13.0 (6.5–24.3) | 0.001 | 99.39 | Reference | |||
| With chronic cardiac disease | 7 | 31143 | 11.2 (7.1–17.2) | 0.048 | 92.89 | 0.1858 | 0.5377 | −0.86–1.23 | 0.7296 |
| Coronary artery disease | 3 | 258 | 43.6 (25.4–63.7) | 0.001 | 83.69 | ||||
| Without coronary artery disease | 3 | 235 | 34.5 (16.9–57.8) | 0.001 | 90.22 | Reference | |||
| With coronary artery disease | 3 | 23 | 62.4 (35.3–83.4) | 0.262 | 25.28 | 1.1040 | 0.8250 | −0.51–2.72 | 0.1808 |
| Thyroid disease | 2 | 247 | 25.5 (19.5–32.2) | 0.159 | 42.11 | ||||
| Without thyroid disease | 2 | 236 | 26.0 (16.1–39.2) | 0.073 | 68.96 | Reference | |||
| With thyroid disease | 2 | 11 | 32.0 (8.5–70.5) | 0.179 | 44.66 | 0.2440 | 0.9489 | −1.61–2.10 | 0.7971 |
| Antiviral therapy | 2 | 398 | 24.0 (7.4–55.3) | 0.001 | 94.74 | ||||
| Without antiviral therapy | 2 | 331 | 35.6 (4.9–85.6) | 0.001 | 93.64 | Reference | |||
| With antiviral therapy | 2 | 67 | 15.4 (1.6–66.6) | 0.001 | 97.38 | −1.1104 | 1.7311 | −4.50–2.28 | 0.5212 |
| Antibiotic therapy | 2 | 495 | 15.0 (3.5–46.3) | 0.001 | 94.37 | ||||
| Without antibiotic therapy | 2 | 144 | 7.8 (2.9–19.0) | 0.255 | 22.77 | Reference | |||
| With antibiotic therapy | 2 | 351 | 20.3 (2.8–68.9) | 0.001 | 95.14 | 0.8311 | 1.5353 | −2.17–3.84 | 0.5883 |
| Nasal cannula oxygen therapy | 2 | 398 | 20.1 (4.6–56.7) | 0.001 | 96.64 | ||||
| Without nasal cannula oxygen therapy | 2 | 139 | 42.9 (10.7–82.5) | 0.001 | 94.20 | Reference | |||
| With nasal cannula oxygen therapy | 2 | 259 | 7.8 (1.3–34.9) | 0.001 | 92.14 | −2.1811 | 1.3269 | −4.78–0.41 | 0.1002 |
| Mechanical ventilation oxygen therapy | 4 | 15272 | 47.0 (19.1–76.9) | 0.001 | 99.69 | ||||
| Without mechanical ventilation oxygen therapy | 4 | 13425 | 17.4 (5.3–44.3) | 0.001 | 98.63 | Reference | |||
| With mechanical ventilation oxygen therapy | 4 | 1847 | 83.9 (48.3–96.7) | 0.001 | 87.16 | 3.1785 | 1.0584 | 1.10–5.25 | 0.0027 |
| WHO region | 12 | 148080 | 23.0 (14.3–34.7) | 0.001 | 99.70 | ||||
| Region of theAmericas | 2 | 25296 | 10.7 (6.3–17.6) | 0.001 | 99.34 | Reference | |||
| European region | 4 | 121104 | 39.3 (4.2–90.5) | 0.001 | 99.53 | 1.6336 | 0.7856 | 0.09–3.17 | 0.0376 |
| Western Pacific region | 5 | 1589 | 18.0 (9.1–32.4) | 0.001 | 96.10 | 0.6010 | 0.7562 | −0.88–2.08 | 0.4268 |
| Southeast Asianregion | 1 | 91 | 33.8 (23.6–45.8) | 1.00 | 0.00 | 1.4473 | 1.1285 | −0.76–3.65 | 0.1997 |
| Sample size | 12 | 148080 | 23.0 (14.3–34.7) | 0.001 | 99.70 | ||||
| >500 | 4 | 146807 | 8.4 (3.7–18.1) | 0.088 | 99.89 | Reference | |||
| ≤500 | 8 | 1273 | 35.7 (21.3–53.2) | 0.001 | 95.33 | 1.8006 | 0.5585 | 0.70–2.89 | 0.0013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-T.; Ku, H.-C.; Maithreepala, S.D.; Tsai, Y.-J.; Chen, L.-F.; Ko, N.-Y.; Konara Mudiyanselage, S.P. Higher Risk of Acute Respiratory Distress Syndrome and Risk Factors among Patients with COVID-19: A Systematic Review, Meta-Analysis and Meta-Regression. Int. J. Environ. Res. Public Health 2022, 19, 15125. https://doi.org/10.3390/ijerph192215125
Tsai Y-T, Ku H-C, Maithreepala SD, Tsai Y-J, Chen L-F, Ko N-Y, Konara Mudiyanselage SP. Higher Risk of Acute Respiratory Distress Syndrome and Risk Factors among Patients with COVID-19: A Systematic Review, Meta-Analysis and Meta-Regression. International Journal of Environmental Research and Public Health. 2022; 19(22):15125. https://doi.org/10.3390/ijerph192215125
Chicago/Turabian StyleTsai, Yi-Tseng, Han-Chang Ku, Sujeewa Dilhani Maithreepala, Yi-Jing Tsai, Li-Fan Chen, Nai-Ying Ko, and Sriyani Padmalatha Konara Mudiyanselage. 2022. "Higher Risk of Acute Respiratory Distress Syndrome and Risk Factors among Patients with COVID-19: A Systematic Review, Meta-Analysis and Meta-Regression" International Journal of Environmental Research and Public Health 19, no. 22: 15125. https://doi.org/10.3390/ijerph192215125
APA StyleTsai, Y.-T., Ku, H.-C., Maithreepala, S. D., Tsai, Y.-J., Chen, L.-F., Ko, N.-Y., & Konara Mudiyanselage, S. P. (2022). Higher Risk of Acute Respiratory Distress Syndrome and Risk Factors among Patients with COVID-19: A Systematic Review, Meta-Analysis and Meta-Regression. International Journal of Environmental Research and Public Health, 19(22), 15125. https://doi.org/10.3390/ijerph192215125






