Identification of Potential Artefacts in In Vitro Measurement of Vanadium-Induced Reactive Oxygen Species (ROS) Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of NaVO3 and VOSO4 Stock Solutions
2.3. Antioxidant Activity of Mammalian Cell Culture Media
2.3.1. Ferric (III) Reducing Antioxidant Power (RP)
2.3.2. Ability to Quench ABTS Radicals (ABTS)
2.3.3. Ability to Quench Hydroxyl Radicals (OH)
2.3.4. Ability to Quench DPPH Radicals (DPPH)
2.4. Measurement of H2O2 Production in Cell Culture Media
2.5. Oxidation of DCFH-DA and DHR123 Fluorogens in Cell Culture Media
2.6. Data Analysis
3. Results and Discussion
3.1. Antioxidant Capacity of Mammalian Cell Culture Media
3.2. Prooxidant Abilities of Mammalian Cell Culture Media
3.3. NaVO3 and VOSO4 Modulate the Level of H2O2 in Mammalian Cell Culture Media
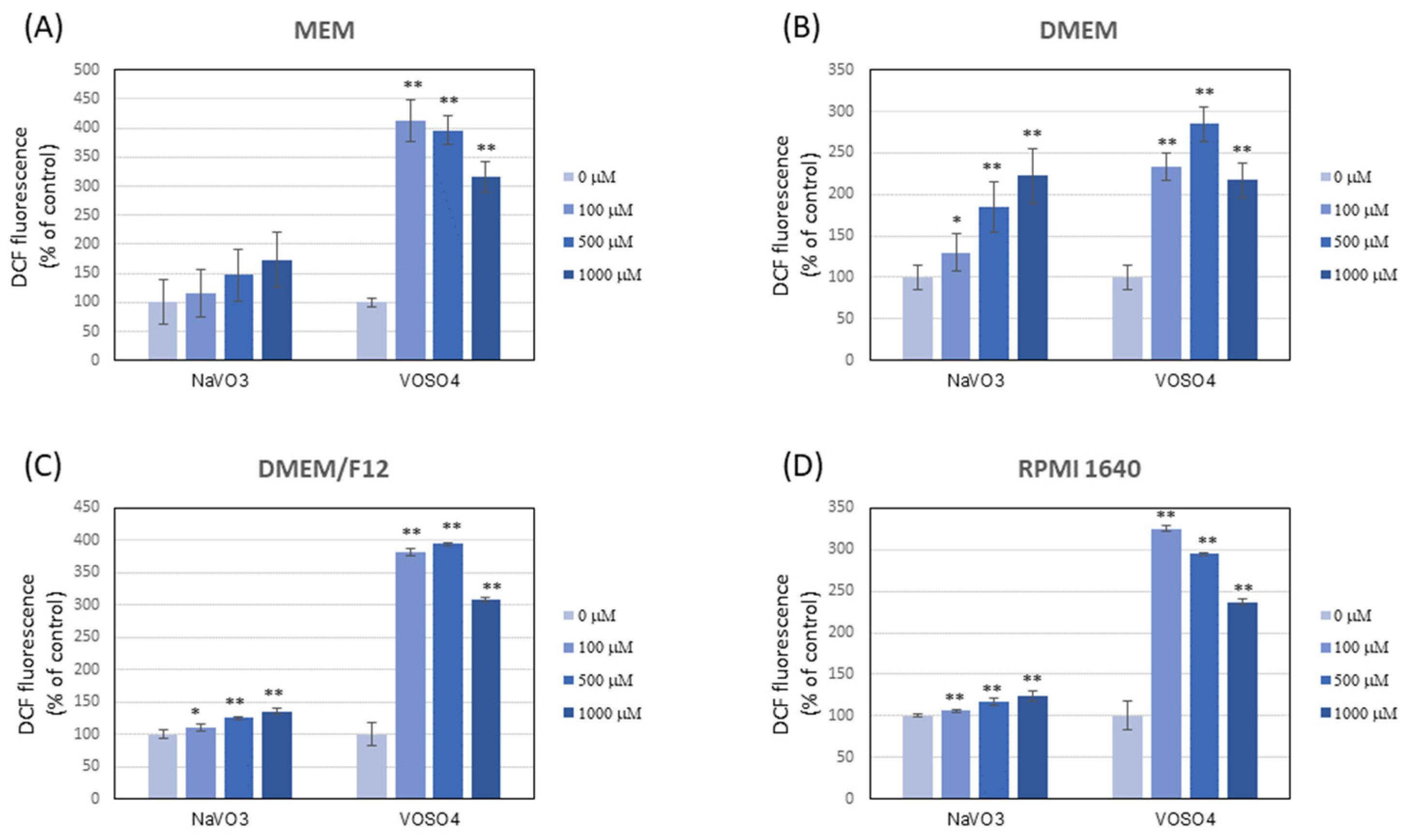
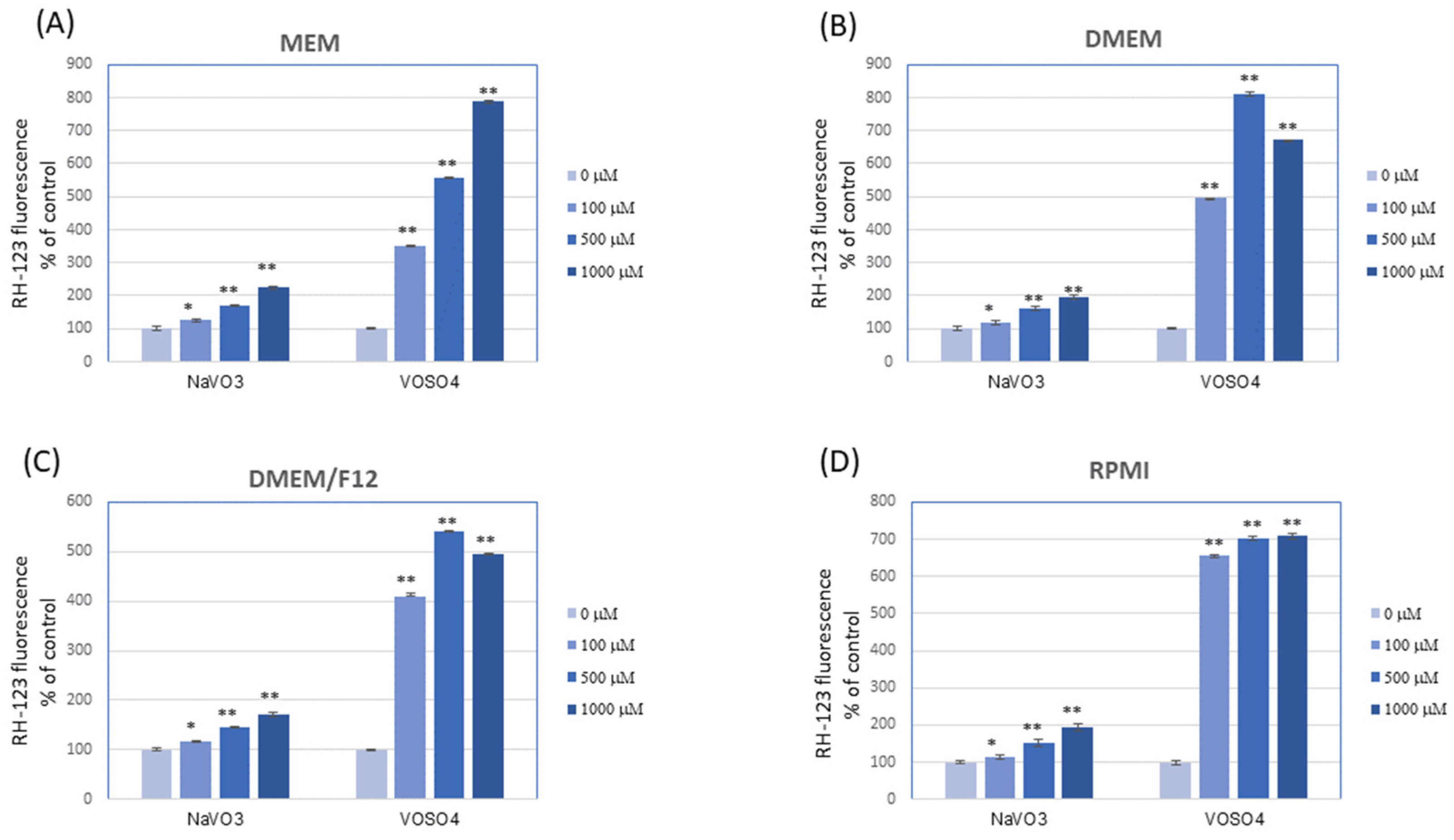
3.4. NaVO3 and VOSO4 Significantly Increase the DCF and RH-123 Fluorescence in Mammalian Cell Culture Media (in the Absence of Cells)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and Possible Benefits in the Light of a Comprehensive Overview of Its Pharmacotoxicological Mechanisms and Multi-Applications with a Summary of Further Research Trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global Biogeochemical Cycle of Vanadium. Proc. Natl. Acad. Sci. USA 2017, 114, E11092–E11100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehder, D. The Potentiality of Vanadium in Medicinal Applications. Future Med. Chem. 2012, 4, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. Protective Effects of Dietary Antioxidants against Vanadium-Induced Toxicity: A Review. Oxidative Med. Cell. Longev. 2020, 2020, 1490316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, J.A.J.; Burke, I.T.; Edwards, R.A.; Malcolm, H.M.; Mayes, W.M.; Olszewska, J.P.; Pan, G.; Graham, M.C.; Heal, K.V.; Rose, N.L.; et al. Vanadium: A Re-Emerging Environmental Hazard. Environ. Sci. Technol. 2018, 52, 11973–11974. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Lemus, M.; López-Valdez, N.; Bizarro-Nevares, P.; González-Villalva, A.; Ustarroz-Cano, M.; Zepeda-Rodríguez, A.; Pasos-Nájera, F.; García-Peláez, I.; Rivera-Fernández, N.; Fortoul, T.I. Toxic Effects of Inhaled Vanadium Attached to Particulate Matter: A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 8457. [Google Scholar] [CrossRef]
- Fatola, O.I.; Olaolorun, F.A.; Olopade, F.E.; Olopade, J.O. Trends in Vanadium Neurotoxicity. Brain Res. Bull. 2019, 145, 75–80. [Google Scholar] [CrossRef]
- Woodin, M.A.; Liu, Y.; Neuberg, D.; Hauser, R.; Smith, T.J.; Christiani, D.C. Acute Respiratory Symptoms in Workers Exposed to Vanadium-Rich Fuel-Oil Ash. Am. J. Ind. Med. 2000, 37, 353–363. [Google Scholar] [CrossRef]
- Patel, M.M.; Hoepner, L.; Garfinkel, R.; Chillrud, S.; Reyes, A.; Quinn, J.W.; Perera, F.; Miller, R.L. Ambient Metals, Elemental Carbon, and Wheeze and Cough in New York City Children through 24 Months of Age. Am. J. Respir. Crit. Care Med. 2009, 180, 1107–1113. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Samet, J.M.; Dominici, F. Hospital Admissions and Chemical Composition of Fine Particle Air Pollution. Am. J. Respir. Crit. Care Med. 2009, 179, 1115–1120. [Google Scholar] [CrossRef]
- Hu, J.; Peng, Y.; Zheng, T.; Zhang, B.; Liu, W.; Wu, C.; Jiang, M.; Braun, J.M.; Liu, S.; Buka, S.L.; et al. Effects of Trimester-Specific Exposure to Vanadium on Ultrasound Measures of Fetal Growth and Birth Size: A Longitudinal Prospective Prenatal Cohort Study. Lancet Planet Health 2018, 2, e427–e437. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhou, D.; Zhang, Q.; Feng, C.; Zheng, W.; He, K.; Lan, Y. Vanadium Exposure-Induced Neurobehavioral Alterations among Chinese Workers. Neurotoxicology 2013, 36, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer (Ed.) Cobalt in Hard Metals and Cobalt Sulfate, Gallium Arsenide, Indium Phosphide and Vanadium Pentoxide: This Publication Represents the Views and Expert Opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Which Met in Lyon, 7–14 October 2003; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2006. [Google Scholar]
- Todorich, B.; Olopade, J.O.; Surguladze, N.; Zhang, X.; Neely, E.; Connor, J.R. The Mechanism of Vanadium-Mediated Developmental Hypomyelination Is Related to Destruction of Oligodendrocyte Progenitors Through a Relationship with Ferritin and Iron. Neurotox. Res. 2011, 19, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Cramer, H.; Albrecht, C.; Schins, R.; Rahman, Q.; Zimmermann, U.; Dopp, E. Vanadium Pentoxide-Coated Ultrafine Titanium Dioxide Particles Induce Cellular Damage and Micronucleus Formation in V79 Cells. J. Toxicol. Environ. Health Part A 2008, 71, 976–980. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, C.; Li, J.; Leonard, S.S.; Lanciotti, R.; Butterworth, L.; Shi, X. Vanadate-Induced Cell Growth Regulation and the Role of Reactive Oxygen Species. Arch. Biochem. Biophys. 2001, 392, 311–320. [Google Scholar] [CrossRef]
- Guerrero-Palomo, G.; Rendón-Huerta, E.P.; Montaño, L.F.; Fortoul, T.I. Vanadium Compounds and Cellular Death Mechanisms in the A549 Cell Line: The Relevance of the Compound Valence. J. Appl. Toxicol. 2019, 39, 540–552. [Google Scholar] [CrossRef]
- Xi, W.-S.; Tang, H.; Liu, Y.-Y.; Liu, C.-Y.; Gao, Y.; Cao, A.; Liu, Y.; Chen, Z.; Wang, H. Cytotoxicity of Vanadium Oxide Nanoparticles and Titanium Dioxide-Coated Vanadium Oxide Nanoparticles to Human Lung Cells. J. Appl. Toxicol. 2020, 40, 567–577. [Google Scholar] [CrossRef]
- Capella, L.S.; Gefé, M.R.; Silva, E.F.; Affonso-Mitidieri, O.; Lopes, A.G.; Rumjanek, V.M.; Capella, M.A.M. Mechanisms of Vanadate-Induced Cellular Toxicity: Role of Cellular Glutathione and NADPH. Arch. Biochem. Biophys. 2002, 406, 65–72. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Z.; Ding, M.; Li, J.; Ye, J.; Leonard, S.S.; Shen, H.M.; Butterworth, L.; Lu, Y.; Costa, M.; et al. Vanadate Induces P53 Transactivation through Hydrogen Peroxide and Causes Apoptosis. J. Biol. Chem. 2000, 275, 32516–32522. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Medan, D.; Mercer, R.; Overmiller, D.; Leornard, S.; Castranova, V.; Shi, X.; Ding, M.; Huang, C.; Rojanasakul, Y. Vanadium-Induced Apoptosis and Pulmonary Inflammation in Mice: Role of Reactive Oxygen Species. J. Cell. Physiol. 2003, 195, 99–107. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, G.; Husain, S.A. Oxidants, Antioxidants and Carcinogenesis. Indian J. Exp. Biol. 2002, 40, 1213–1232. [Google Scholar] [PubMed]
- Ścibior, A.; Szychowski, K.A.; Zwolak, I.; Dachowska, K.; Gmiński, J. In Vitro Effect of Vanadyl Sulfate on Cultured Primary Astrocytes: Cell Viability and Oxidative Stress Markers. J. Appl. Toxicol. 2020, 40, 737–747. [Google Scholar] [CrossRef]
- Shukla, R.; Barve, V.; Padhye, S.; Bhonde, R. Reduction of Oxidative Stress Induced Vanadium Toxicity by Complexing with a Flavonoid, Quercetin: A Pragmatic Therapeutic Approach for Diabetes. Biometals 2006, 19, 685–693. [Google Scholar] [CrossRef]
- Zwolak, I.; Wnuk, E. Effects of Sodium Pyruvate on Vanadyl Sulphate-Induced Reactive Species Generation and Mitochondrial Destabilisation in CHO-K1 Cells. Antioxidants 2022, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Leon, I.E.; Porro, V.; Di Virgilio, A.L.; Naso, L.G.; Williams, P.A.M.; Bollati-Fogolín, M.; Etcheverry, S.B. Antiproliferative and Apoptosis-Inducing Activity of an Oxidovanadium(IV) Complex with the Flavonoid Silibinin against Osteosarcoma Cells. J. Biol. Inorg. Chem. 2014, 19, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Crans, D.C. Decavanadate (V10 O28 6-) and Oxovanadates: Oxometalates with Many Biological Activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Su, X.-Y.; Wang, Z.-Y.; Liu, J.-R. In Vitro and in Vivo Antioxidant Activity of Pinus Koraiensis Seed Extract Containing Phenolic Compounds. Food Chem. 2009, 117, 681–686. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Tarpey, M.M.; Wink, D.A.; Grisham, M.B. Methods for Detection of Reactive Metabolites of Oxygen and Nitrogen: In Vitro and in Vivo Considerations. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 286, R431–R444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülçin, İ. Antioxidant Activity of Food Constituents: An Overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Wnuk, M.; Slota, E.; Bartosz, G. Total Anti-Oxidant Capacity of Cell Culture Media. Clin. Exp. Pharmacol. Physiol. 2007, 34, 781–786. [Google Scholar] [CrossRef]
- Halliwell, B. Cell Culture, Oxidative Stress, and Antioxidants: Avoiding Pitfalls. Biomed. J. 2014, 37, 99–105. [Google Scholar] [CrossRef]
- Güngör, N.; Ozyürek, M.; Güçlü, K.; Cekiç, S.D.; Apak, R. Comparative Evaluation of Antioxidant Capacities of Thiol-Based Antioxidants Measured by Different in Vitro Methods. Talanta 2011, 83, 1650–1658. [Google Scholar] [CrossRef]
- Somogyi, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant Measurements. Physiol. Meas. 2007, 28, R41–R55. [Google Scholar] [CrossRef]
- Zigler, J.S.; Lepe-Zuniga, J.L.; Vistica, B.; Gery, I. Analysis of the Cytotoxic Effects of Light-Exposed HEPES-Containing Culture Medium. In Vitro Cell. Dev. Biol. 1985, 21, 282–287. [Google Scholar] [CrossRef]
- Lopalco, A.; Dalwadi, G.; Niu, S.; Schowen, R.L.; Douglas, J.; Stella, V.J. Mechanism of Decarboxylation of Pyruvic Acid in the Presence of Hydrogen Peroxide. J. Pharm. Sci. 2016, 105, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Grzelak, A.; Rychlik, B.; Bartosz, G. Light-Dependent Generation of Reactive Oxygen Species in Cell Culture Media. Free. Radic. Biol. Med. 2001, 30, 1418–1425. [Google Scholar] [CrossRef]
- Martín-Romero, F.J.; Miguel-Lasobras, E.M.; Domínguez-Arroyo, J.A.; González-Carrera, E.; Alvarez, I.S. Contribution of Culture Media to Oxidative Stress and Its Effect on Human Oocytes. Reprod. BioMed. Online 2008, 17, 652–661. [Google Scholar] [CrossRef]
- Hempel, S.L.; Buettner, G.R.; O’Malley, Y.Q.; Wessels, D.A.; Flaherty, D.M. Dihydrofluorescein Diacetate Is Superior for Detecting Intracellular Oxidants: Comparison with 2′,7′-Dichlorodihydrofluorescein Diacetate, 5(and 6)-Carboxy-2′,7′-Dichlorodihydrofluorescein Diacetate, and Dihydrorhodamine 123. Free Radic. Biol. Med. 1999, 27, 146–159. [Google Scholar] [CrossRef]
- Myhre, O.; Andersen, J.M.; Aarnes, H.; Fonnum, F. Evaluation of the Probes 2′,7′-Dichlorofluorescin Diacetate, Luminol, and Lucigenin as Indicators of Reactive Species Formation. Biochem. Pharmacol. 2003, 65, 1575–1582. [Google Scholar] [CrossRef]
- Brubacher, J.L.; Bols, N.C. Chemically De-Acetylated 2′,7′-Dichlorodihydrofluorescein Diacetate as a Probe of Respiratory Burst Activity in Mononuclear Phagocytes. J. Immunol. Methods 2001, 251, 81–91. [Google Scholar] [CrossRef]
- Shankar, H.N.R.; Ramasarma, T. Multiple Reactions in Vanadyl-V(IV) Oxidation by H2O2. Mol Cell Biochem 1993, 129, 9–29. [Google Scholar] [CrossRef]
- Liochev, S.I.; Fridovich, I. The Roles of O2−, HO., and Secondarily Derived Radicals in Oxidation Reactions Catalyzed by Vanadium Salts. Arch. Biochem. Biophys. 1991, 291, 379–382. [Google Scholar] [CrossRef]
- Hamada, T. Vanadium Induced Hemolysis of Vitamin E Deficient Erythrocytes in Hepes Buffer. Experientia 1994, 50, 49–53. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic Activity of Metals in Heterogeneous Fenton-like Oxidation of Wastewater Contaminants: A Review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Shi, X.; Dalal, N.S. One-Electron Reduction of Chromate by NADPH-Dependent Glutathione Reductase. J. Inorg. Biochem. 1990, 40, 1–12. [Google Scholar] [CrossRef]
- Keller, R.J.; Coulombe, R.A.; Sharma, R.P.; Grover, T.A.; Piette, L.H. Oxidation of NADH by Vanadium Compounds in the Presence of Thiols. Arch. Biochem. Biophys. 1989, 271, 40–48. [Google Scholar] [CrossRef]
- Sanna, D.; Micera, G.; Garribba, E. On the Transport of Vanadium in Blood Serum. Inorg. Chem. 2009, 48, 5747–5757. [Google Scholar] [CrossRef] [PubMed]
- Okeson, C.D.; Riley, M.R.; Riley-Saxton, E. In Vitro Alveolar Cytotoxicity of Soluble Components of Airborne Particulate Matter: Effects of Serum on Toxicity of Transition Metals. Toxicol. In Vitro 2004, 18, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Liebling, E.J.; Burger, R.F.; Zuckerbraun, H.L.; Schuck, A.G. Choice of DMEM, Formulated with or without Pyruvate, Plays an Important Role in Assessing the in Vitro Cytotoxicity of Oxidants and Prooxidant Nutraceuticals. In Vitro Cell. Dev. Biol. Anim. 2009, 45, 226–233. [Google Scholar] [CrossRef]
- Dugas, T.R.; Morel, D.W.; Harrison, E.H. Novel Cell Culture Medium for Use in Oxidation Experiments Provides Insights into Mechanisms of Endothelial Cell-Mediated Oxidation of LDL. In Vitro Cell. Dev. Biol. Anim. 2000, 36, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Reiniers, M.J.; van Golen, R.F.; Bonnet, S.; Broekgaarden, M.; van Gulik, T.M.; Egmond, M.R.; Heger, M. Preparation and Practical Applications of 2′,7′-Dichlorodihydrofluorescein in Redox Assays. Anal. Chem. 2017, 89, 3853–3857. [Google Scholar] [CrossRef]
- Brömme, H.-J.; Zühlke, L.; Silber, R.-E.; Simm, A. DCFH2 Interactions with Hydroxyl Radicals and Other Oxidants—Influence of Organic Solvents. Exp. Gerontol. 2008, 43, 638–644. [Google Scholar] [CrossRef] [Green Version]
- Ravishankar, H.N.; Kalyani, P.; Ramasarma, T. NADH Oxidation Is Stimulated by an Intermediate Formed during Vanadyl-H2O2 Interaction. Biochim. Biophys. Acta 1994, 1201, 289–297. [Google Scholar] [CrossRef]
- Cortizo, A.M.; Bruzzone, L.; Molinuevo, S.; Etcheverry, S.B. A Possible Role of Oxidative Stress in the Vanadium-Induced Cytotoxicity in the MC3T3E1 Osteoblast and UMR106 Osteosarcoma Cell Lines. Toxicology 2000, 147, 89–99. [Google Scholar] [CrossRef]

| Medium | Antioxidant Activity | |||
|---|---|---|---|---|
| DPPH (mg TE/mL) | ABTS (mg TE/mL) | OH (mg TE/mL) | RP (mg TE/mL) | |
| DMEM | 0.111 ± 0.006 a | 0.844 ± 0.053 a | 0.640 ± 0.038 a | 0.072 ± 0.003 a |
| MEM | 0.025 ± 0.002 b | 0.492 ± 0.023 b | 0.150 ± 0.017 c | 0.013 ± 0.002 b |
| RPMI 1640 | 0.029 ± 0.006 b | 0.319 ± 0.027 c | 0.321 ± 0.061 b | 0.013 ± 0.002 b |
| DMEM/F-12 | 0.043 ± 0.009 c | 0.561 ± 0.031 d | 0.301 ± 0.054 b | 0.018 ± 0.004 c |
| [H2O2] µM at 5 min in | |||||
|---|---|---|---|---|---|
| Compounds Added (µM) | DMEM | MEM | RPMI | DMEM/F12 | |
| none | 9.20 ± 1.47 | 1.58 ± 0.77 | 1.25 ± 0.21 | 0.26 ± 0.01 | |
| VOSO4 | 100 | 0.57 ± 0.41 *** | 0.94 ± 0.15 | 0.87 ± 0.10 * | 1.02 ± 0.14 *** |
| 500 | 0.77 ± 0.05 *** | 2.62 ± 0.50 | 1.33 ± 0.04 | 2.28 ± 0.42 *** | |
| 1000 | 1.15 ± 0.09 *** | 4.11 ± 1.62 | 1.43 ± 0.02 | 3.25 ± 0.85 ** | |
| NaVO3 | 100 | 5.50 ± 1.14 *** | 1.06 ± 0.47 | 0.73 ± 0.17 ** | 0.07 ± 0.01 * |
| 500 | 5.11 ± 1.10 *** | 0.90 ± 0.40 | 0.60 ± 0.15 *** | 0 | |
| 1000 | 3.98 ± 0.61 *** | 0.68 ± 0.27 | 0.47 ± 0.11 *** | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwolak, I.; Wnuk, E.; Świeca, M. Identification of Potential Artefacts in In Vitro Measurement of Vanadium-Induced Reactive Oxygen Species (ROS) Production. Int. J. Environ. Res. Public Health 2022, 19, 15214. https://doi.org/10.3390/ijerph192215214
Zwolak I, Wnuk E, Świeca M. Identification of Potential Artefacts in In Vitro Measurement of Vanadium-Induced Reactive Oxygen Species (ROS) Production. International Journal of Environmental Research and Public Health. 2022; 19(22):15214. https://doi.org/10.3390/ijerph192215214
Chicago/Turabian StyleZwolak, Iwona, Ewa Wnuk, and Michał Świeca. 2022. "Identification of Potential Artefacts in In Vitro Measurement of Vanadium-Induced Reactive Oxygen Species (ROS) Production" International Journal of Environmental Research and Public Health 19, no. 22: 15214. https://doi.org/10.3390/ijerph192215214






