Abstract
Avascular osteonecrosis (AVN) is caused by the disrupted blood supply to the bone. Most AVN cases occur in the femoral head, but other sites might be affected as well, including the jaw or distal bones of the extremities. Previous studies suggested that diabetes could increase the risk of AVN of the jaw, but the relationship between diabetes and AVN in other bone sites is unclear. This systematic review and meta-analysis aimed to summarize the evidence from studies that had reported on the occurrence of AVN in sites other than the jaw, depending on the diagnosis of diabetes. Overall, we included 6 observational studies carried out in different populations: primary or secondary AVN of the femoral head, Takayasu arteritis, general population, kidney transplant recipients, systemic lupus erythematosus, and primary brain tumors. A random-effects meta-analysis showed that the risk of AVN in sites other than the jaw was non-significantly increased in patients with diabetes (odds ratio: 1.90, 95% confidence interval: 0.93–3.91). The pooled estimate increased and was significant after the exclusion of one study (2.46, 1.14–5.32). There was a significant heterogeneity (I2 = 65%, tau2 = 0.48, p = 0.01; prediction interval, 0.21–16.84). There was no significant publication bias (p = 0.432). In conclusion, diabetes could increase the risk of AVN in sites other than the jaw, but the available evidence is limited. There is a need for large, well-designed, population-based studies.
1. Introduction
Avascular osteonecrosis (AVN) refers to the death of bone cells due to disruption of the blood supply, which typically occurs in bone areas with limited collateral circulation [1]. The death of bone cells is followed by the disappearance of the articular surface and subsequent degenerative arthritis, which may lead to progressive disability [2,3,4]. Moreover, AVN may increase the risk of tumorigenesis in the affected sites in the long-term [5]. AVN occurs in various bones, such as the femoral head, jaw, knee, talus, or humerus [6,7,8]. The femoral head is the most common site of AVN, with an estimated incidence in the United States between 10,000 and 20,000 cases per year [9]. Femoral-head AVN is diagnosed mostly in young adults (mean age of onset ~40 years) [10] and is associated with a substantial disease burden [11]. Magnetic resonance imaging (MRI) is a highly effective method for the early detection and staging of the disease [12]. AVN of the jaw is a debilitating disease that occurs predominantly in people using bisphosphonates, which are medications that inhibit bone resorption [13,14]. The treatment of early-stage AVN primarily involves physiotherapy, whereas late-stage disease often requires surgery [15,16,17].
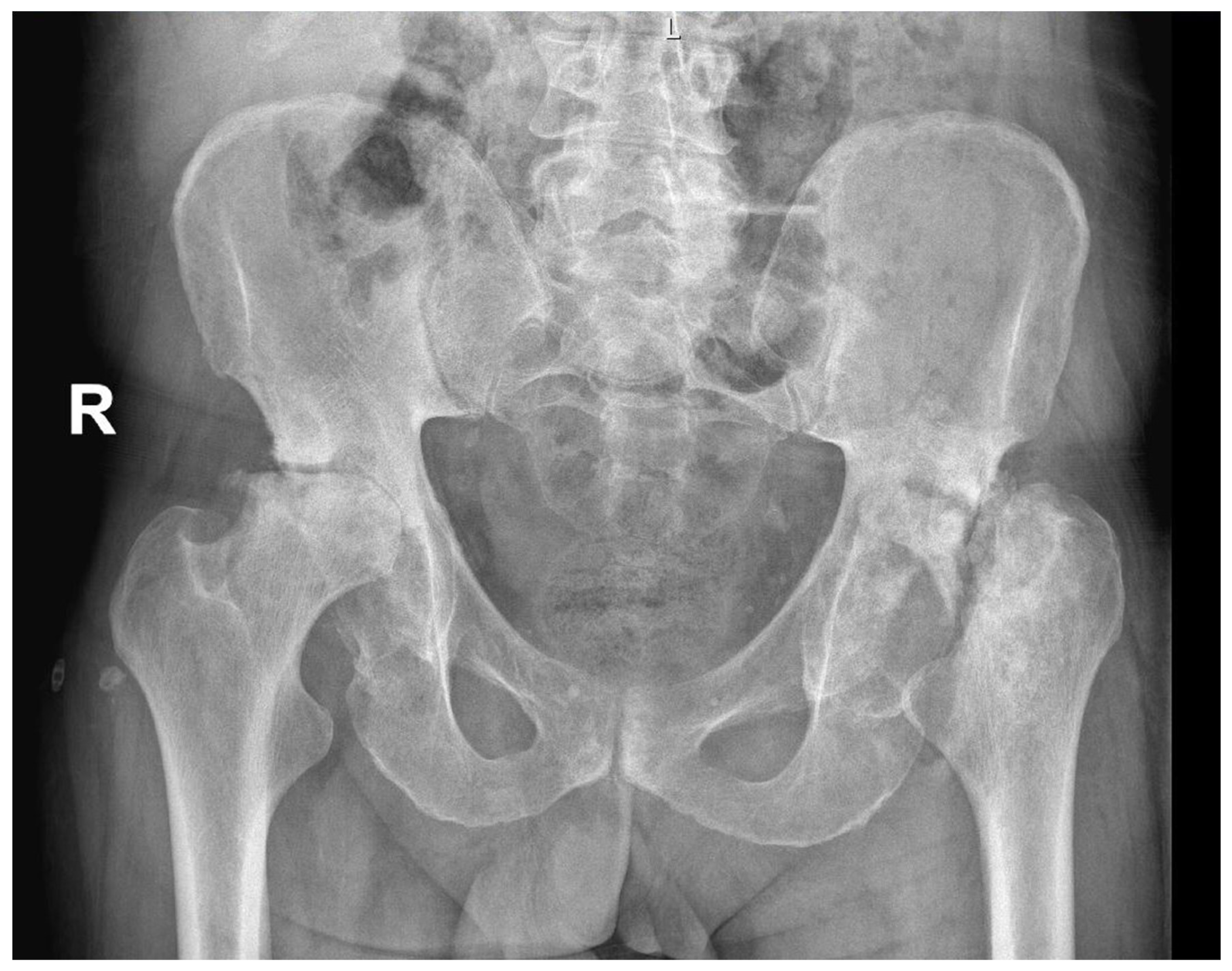
AVN may be caused by trauma, such as fracture, particularly displaced fractures [18], or it can be non-traumatic. The pathogenesis of non-traumatic osteonecrosis is unclear, but there are several established risk factors such as corticosteroid treatment, alcohol abuse, rheumatic diseases, bone-marrow transplantation, or antiretroviral treatment [15,19,20,21,22,23]. Diabetes mellitus (DM), which causes disease of both large vessels (macroangiopathy) and small vessels (microangiopathy) [24], can also increase the risk of avascular osteonecrosis [25,26,27]. Various pathogenic mechanisms in diabetes might additionally impair bone formation, accelerate the apoptosis of bone cells, and reduce the formation of new micro-vessels [1]. Figure 1 presents a case of bilateral AVN in a 62-year-old patient with a history of DM with no other comorbidities.
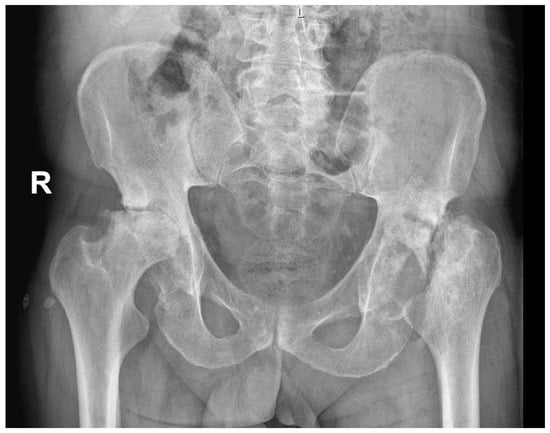
Figure 1.
Radiological image of bilateral avascular osteonecrosis in a patient with diabetes mellitus with no other comorbidities. R—right.
Previous studies on medication-related osteonecrosis of the jaw reported that 15–70% of patients with this condition had diabetes [28,29]. In contrast, the evidence on the association between diabetes and the risk of AVN in sites other that the jaw is less clear. Therefore, we conducted a systematic review and a meta-analysis of studies that had compared cohorts of patients with or without AVN in sites other that the jaw, reporting the proportions of patients with and without diabetes.
2. Materials and Methods
2.1. Protocol and Registration
The protocol for this systematic review and meta-analysis was prepared in compliance with the preferred reporting items for systematic review and meta-analysis protocols (PRISMA) guidance [30]. The protocol is available as Supplementary File S2.
2.2. Search Strategy
We searched the PubMed and EMBASE databases for studies published until 29 July 2022 in English, Polish, or Spanish. We used the following keywords: “AVN”, “sterile necrosis”, “osteonecrosis”, “diabetes”, “hyperglycemia”, and “risk factor”. The exact search phrases are shown in the study protocol. The titles and abstracts were screened to select studies for full-text review. When studies were carried out in overlapping samples, we chose the one with the greatest sample size.
2.3. Study Selection
Two investigators assessed the eligibility of the studies. The more experienced investigator decided on the eligibility when there was a discrepancy. Included were studies that reported sufficient data to obtain or calculate the total number of cases and the number of AVN events in groups with diabetes and without diabetes. Eligible were studies with a score of 5 or more on the Newcastle–Ottawa Scale to exclude studies with a high risk of bias (scores 4 or lower). Case reports, case series among 15 patients or fewer, reviews, and letters to the editor were excluded.
2.4. Data Extraction and Quality Assessment
Two investigators independently extracted the following data: the name of the first author, the publication year, the study design, and characteristics of AVN cases (corticosteroid use, the mean age, and the proportion of men).
2.5. Data Synthesis and Analysis
We calculated odds ratios (OR) using inverse variance for the risk of AVN in patients with diabetes vs. without diabetes (OR > 1 indicated an increased risk of AVN in patients with diabetes). A random-effects meta-analysis was carried out with the restricted maximum-likelihood estimator for tau2 and the Q-profile method for the confidence interval of tau2 and tau. Heterogeneity was expressed with the I2 and τ2 statistics, and it was evaluated with Cochran’s Q test. A prediction interval was estimated to take heterogeneity into account. Sensitivity analyses included leave-one-out analyses. Publication bias was assessed using the Eggers regression test and by inspection of a funnel plot and using fail-safe N analysis. A p-value of less than 0.05 was considered statistically significant. The R software (version 4.1.3) was used for all analyses.
3. Results
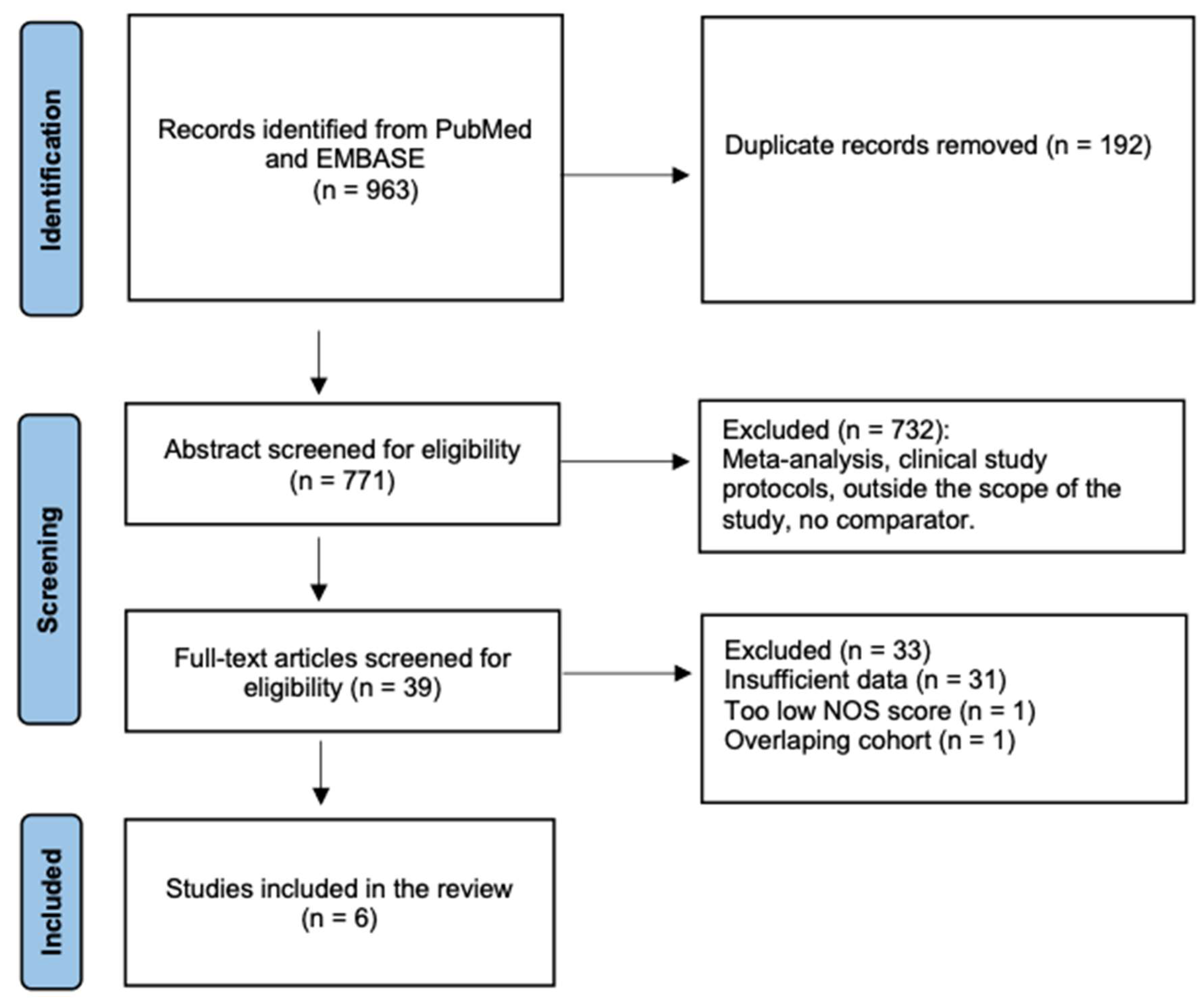
A total of 963 citations were identified, and 39 articles were retrieved in full text. After a full-text review, 31 studies were excluded due to insufficient data, 1 due to poor quality (low NOS score) and 1 due to an overlapping cohort (Figure 2). Overall, 6 studies were included [31,32,33,34,35,36].
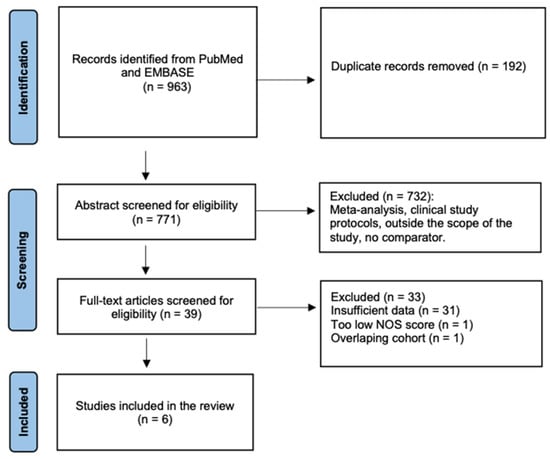
Figure 2.
Flow chart of the study selection.
The studies were carried out in various populations: primary or secondary AVN of the femoral head, Takayasu arteritis, general population, kidney transplant recipients, systemic lupus erythematosus, and primary brain tumors. Nearly all of the AVN cases were reported in the femoral head, with few cases in the femoral condyle, humerus, and ankle (see Table 1). The mean age among AVN cases ranged from 34 to 57 years, and the proportion of males ranged from 13% to 100%. Corticosteroid use was reported in a substantial proportion of AVN cases. The detailed characteristics of included studies are given in Table 1.

Table 1.
Characteristics of individual studies.
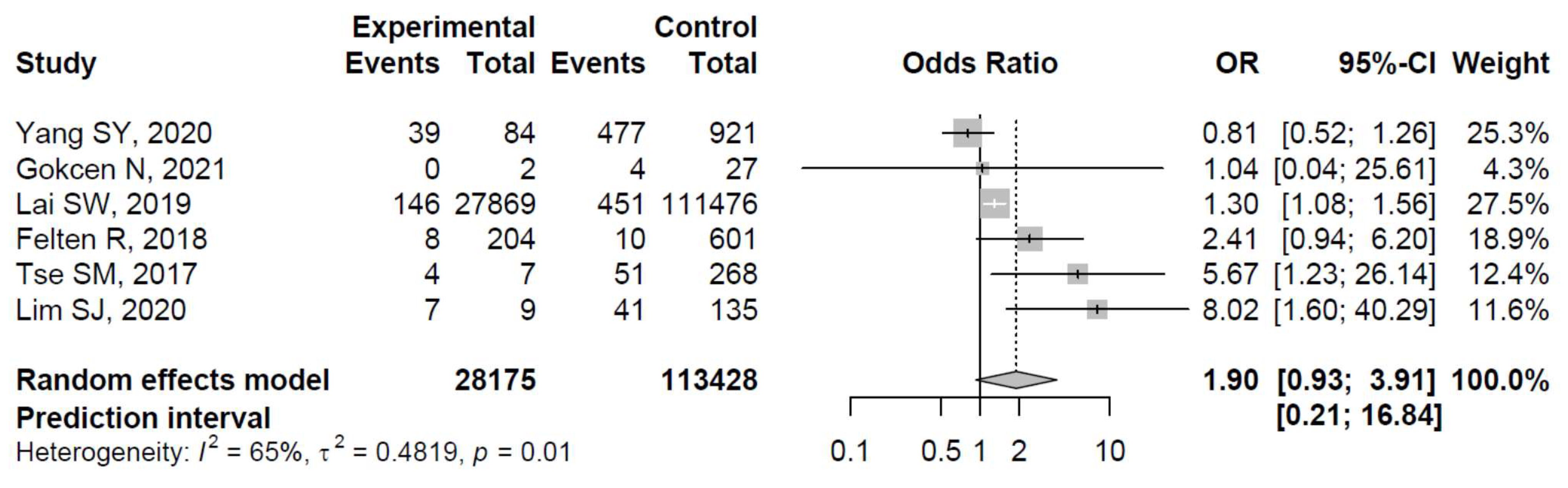
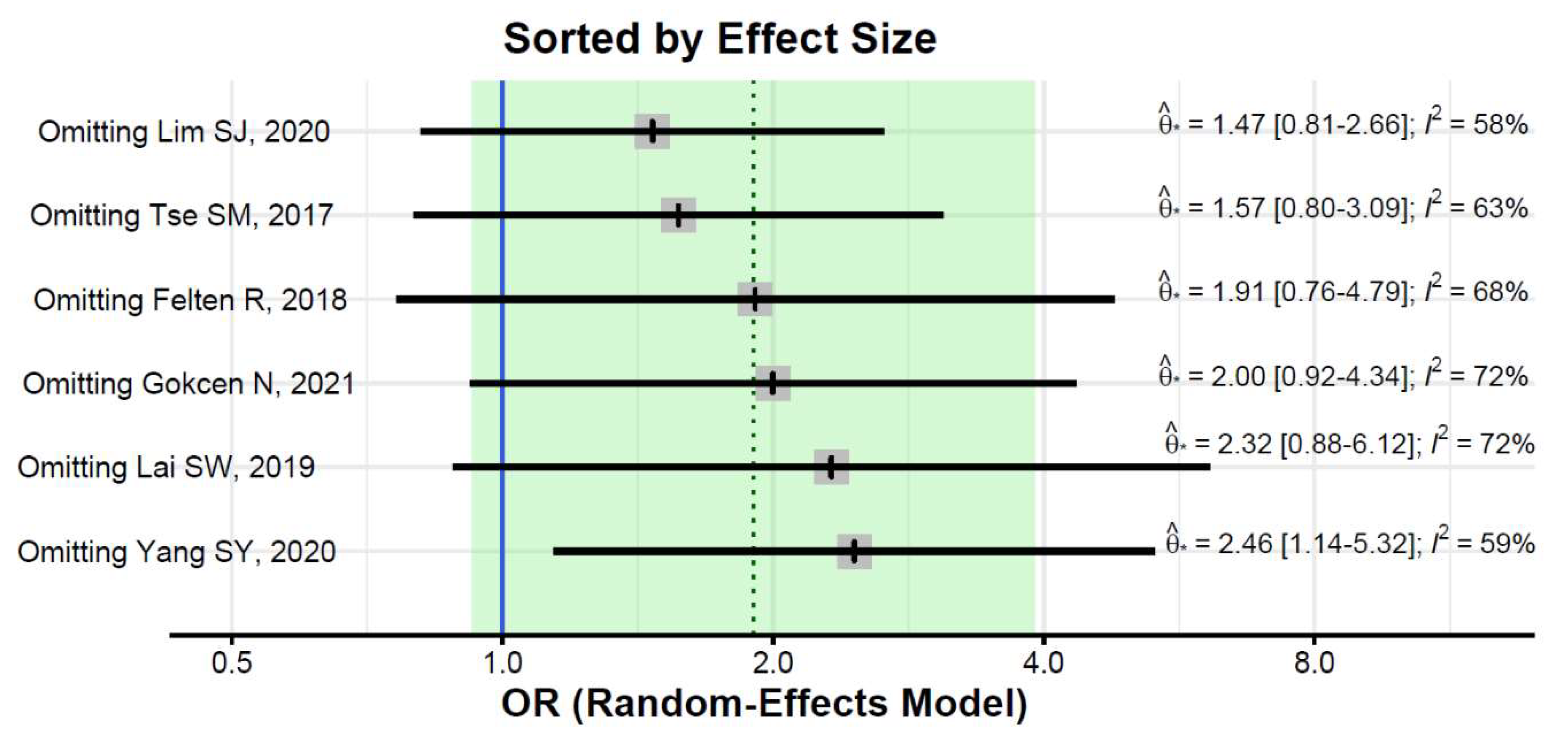
The ORs for AVN risk among patients with diabetes in individual studies ranged from 0.81 to 8.02. The ORs among patients with diabetes were significantly increased in three studies: Lai et al. [33], Tse et al. [35], and Lim et al. [36], whereas the relationship between diabetes and the risk of osteonecrosis was non-significant in the remaining studies (see Figure 3 for details).
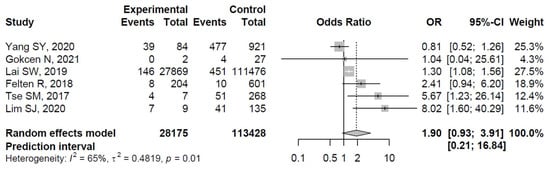
Figure 3.
Forest plot showing the association between diabetes and risk of osteonecrosis in sites other than the jaw (odds ratios > 1 indicate increased risk of AVN in sites other than the jaw among patients with diabetes) [31,32,33,34,35,36].
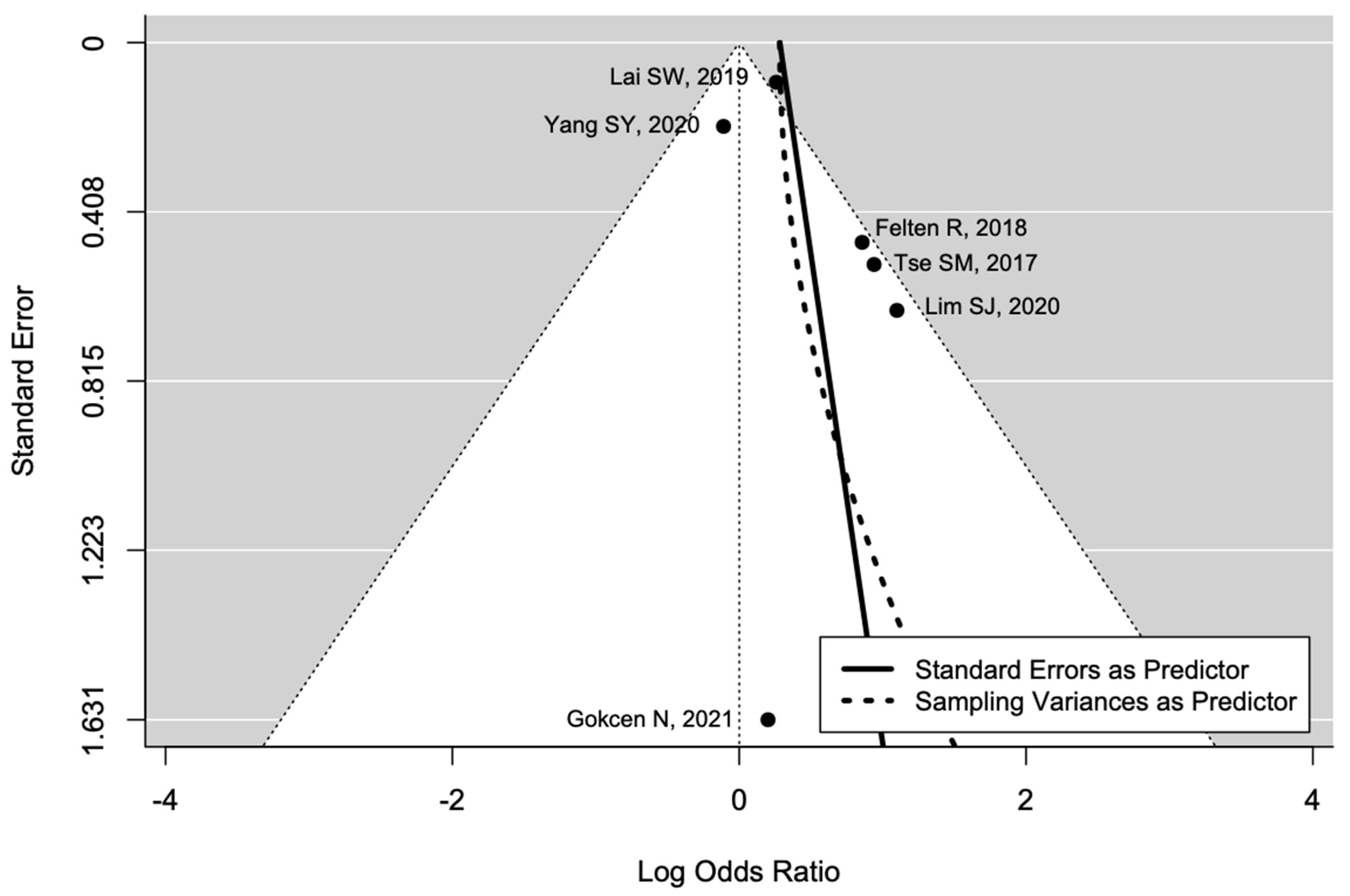
The pooled estimate indicated a non-significantly increased risk of AVN in patients with diabetes (OR = 1.90, 95%CI: 0.93–3.91; Figure 3). There was significant heterogeneity (I2 = 65%, tau2 = 0.48, and p = 0.01; prediction interval, 0.21–16.84; Figure 3). There was no asymmetry in the funnel plot (Figure 4). There was no significant publication bias (Egger’s regression: t = 1.0807, df = 4, and p = 0.34). Based on Rosenthal’s analysis, the number of fail-safe (n = 15) was greater than the number of included observations (n = 6) (p = 0.001), which means there is no risk of publication bias.
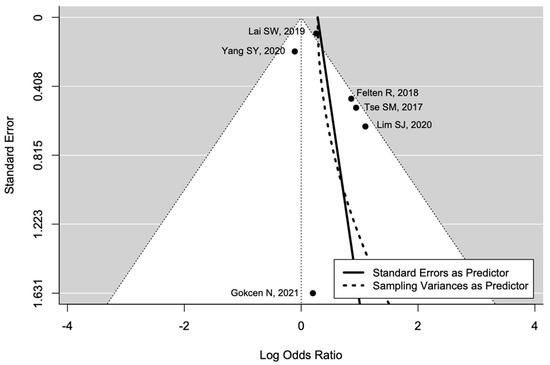
Figure 4.
Funnel plot for publication bias [31,32,33,34,35,36].
The pooled estimate did not change substantially in leave-one-out analyses, but the estimate was significant after excluding the Yang et al. study (OR = 2.46, 95%CI: 1.14–5.32; Figure 4). Heterogeneity remained substantial after the exclusion of any of the studies (I2 of 58% or greater, Figure 5).
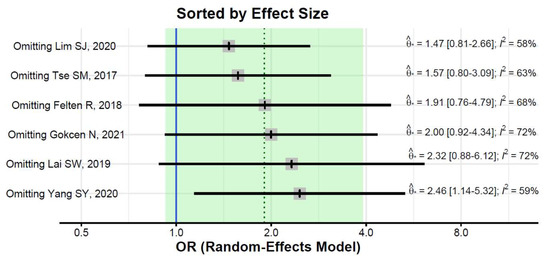
Figure 5.
Leave-one-out sensitivity analyses [31,32,33,34,35,36].
4. Discussion
This systematic review and meta-analysis summarized the results from observational studies carried out among patients with AVN in bone sites other than the jaw depending on the diagnosis of diabetes. Overall, the available evidence is scarce, with four of the sic studies reporting eight or fewer AVN cases among patients with diabetes. Of the two large studies, the population-based study by Lai et al., designed specifically to analyze diabetes as a risk factor of AVN, reported a significantly increased risk of femoral head AVN in patients with diabetes [33]. The other large study (Yang et al. 2020 [31]), carried out in a single orthopedic hospital found a non-significantly lower risk of AVN in patients with diabetes. The pooled estimate in our meta-analysis indicated an increased risk of AVN in bone sties other than the jaw among patients with diabetes, but the effect was non-significant. Moreover, a substantial heterogeneity resulted in a very wide prediction interval. We found no significant publication bias.
MRI was used in all studies to diagnose AVN. Only the study based on the Taiwanese registry diagnosis was made based on plain film, nuclear scan, or MRI. MRI is useful in the early diagnosis of AVN and can identify patients at risk of femoral head fracture [15].
Lai et al. published the largest study to date, with 146 cases of non-traumatic AVN of the femoral head among patients with diabetes [33]. In that nationwide study from Taiwan, a cohort with diabetes was compared with a cohort without diabetes matched for sex, age, corticosteroid use, and several comorbidities; the risk of avascular osteonecrosis was significantly greater in patients with diabetes (hazard ratio = 1.16; 95%CI, 1.11–1.21) [33]. The second-largest study in our systematic review was the case-control study by Yang et al. [31], who compared patients with femoral head osteonecrosis (n = 755) and those with fractures of extremities (n = 489). These investigators reported that diabetes was associated with a non-significantly lower risk of femoral head AVN (OR = 0.81; 95%CI, 0.52–1.26). However, the study was carried out in a single hospital, and the control group was older than the patients with AVN (65 vs. 57 years). This age imbalance could partly account for the largest prevalence of diabetes in the control group than the AVN group (9.2% vs. 7.6%), which resulted in a lower risk of AVN associated with diabetes. After the exclusion of the study by Yang et al., the pooled estimate increased and was significant (OR = 2.46, 95%CI, 1.14–5.32). Felten et al. studied the risk of AVN in 805 kidney transplant recipients, with a total of 18 cases of new-onset avascular necrosis (8 in patients with diabetes); patients with or without new-onset AVN after transplant were of a similar age (51 vs. 55 years) [34]. The significant risk factors of AVN included pre-transplant diabetes, high corticosteroid doses, obesity, and hyperparathyroidism [34]. Tse and Mok reported 55 cases of AVN among 277 patients with systemic lupus erythematosus; although the risk for AVN was significantly increased in those with diabetes, there were only 4 cases of AVN in these patients [35]. Similarly, in the study by Lim et al., among 144 patients with primary brain tumors who received corticosteroids, the risk of AVN was increased significantly, but there were only 9 patients with diabetes [36]. The risk of AVN was not related to diabetes in the study by Gokcen et al. among 29 patients with Takayasu arteritis, but all of the patients in that study used corticosteroids [32].
A potential relationship between the risk of AVN and diabetes was first noticed among patients treated with bisphosphonates who developed AVN of the jaw. Bisphosphonates, which inhibit osteoclastic bone resorption, are used commonly to treat bone diseases such as osteoporosis, multiple myeloma, and bone metastases [37]. A systematic review of 27 studies estimated that 2.7% of bisphosphonate users develop AVN of the jaw [38]. Although it is unclear how bisphosphonates cause jaw necrosis, various mechanisms are suspected, including decreased bone remodeling, impaired wound healing, and local anti-angiogenic effects [39]. Several studies reported that the risk of jaw AVN might be increased by diabetes, which may inhibit bone formation, increase apoptosis of bone cells, and impair angiogenesis [28]. However, there was no significant relationship between diabetes and the risk of AVN of the jaw in a population-based study among 900 patients (largest to date) [40]. Our systematic review and meta-analysis suggest that the association between diabetes and the risk of AVN in bones other that the jaw is also debatable.
Our study had several limitations. We included only six studies, most of which were not designed specifically to study diabetes as a risk factor for AVN in sites other than the jaw. Moreover, the generalizability of the meta-analysis results is limited by substantial heterogeneity, which could be due to the inclusion of studies carried out in very heterogenous populations. The heterogeneity did not decrease in the leave-one-out sensitivity analyses, and there were too few studies to carry out subgroup analyses for different populations. Moreover, corticosteroid use, an established risk factor for AVN, was prevalent in the studies included.
5. Conclusions
In conclusion, although the pooled estimate from this meta-analysis suggests that diabetes might increase the risk of AVN in sites other than the jaw, the existing evidence is limited. The relationship between diabetes and the risk AVN should be further investigated in well-designed, population-based studies with well-matched control groups.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijerph192215219/s1, PRISMA checklist (Supplementary File S1), systematic review protocol (Supplementary File S2). Reference [41] is cited in the supplementary materials.
Author Contributions
Conceptualization, W.K., T.P., A.K., A.Ś., I.K. and J.K.; methodology, W.K.; formal analysis, W.K., T.P., A.K., A.Ś., I.K., M.H. and J.K.; investigation, W.K.; data curation, T.P., W.K. and A.K.; writing—original draft preparation, W.K.; writing—review and editing, W.K., T.P. and A.K.; visualization, W.K.; and supervision, W.K., T.P., A.K., A.Ś., I.K., M.H. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
Publication was funded by the Medical University of Lodz, Department of Social Medicine (project No. 503/6-029-01/503-61-001-19-00).
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barney, J.; Piuzzi, N.S.; Akhondi, H. Femoral Head Avascular Necrosis; StatPearls: Treasure Island, FL, USA, 2022.
- Narayanan, A.; Khanchandani, P.; Borkar, R.M.; Ambati, C.R.; Roy, A.; Han, X.; Bhoskar, R.N.; Ragampeta, S.; Gannon, F.; Mysorekar, V.; et al. Avascular Necrosis of Femoral Head: A Metabolomic, Biophysical, Biochemical, Electron Microscopic and Histopathological Characterization. Sci. Rep. 2017, 7, 10721. [Google Scholar] [CrossRef] [PubMed]
- Petek, D.; Hannouche, D.; Suva, D. Osteonecrosis of the femoral head: Pathophysiology and current concepts of treatment. EFORT Open Rev. 2019, 4, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.N.; Racine, J.; Jones, L.C.; Aaron, R.K. Pathophysiology and risk factors for osteonecrosis. Curr. Rev. Musculoskelet. Med. 2015, 8, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Konarski, W.; Poboży, T.; Hordowicz, M.; Śliwczyński, A.; Kotela, I.; Krakowiak, J.; Kotela, A. Bone Infarcts and Tumorigenesis—Is There a Connection? A Mini-Mapping Review. Int. J. Environ. Res. Public Health 2022, 19, 9282. [Google Scholar] [CrossRef]
- Matthews, A.H.; Davis, D.D.; Fish, M.J.; Stitson, D. Avascular Necrosis; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Chen, S.; Kavanagh, A.; Zarick, C. Steroid-Induced Avascular Necrosis in the Foot and Ankle—Pathophysiology, Surgical, and Nonsurgical Therapies: Case Study and Literature Review. Foot Ankle Spec. 2021, 193864002110173. [Google Scholar] [CrossRef]
- Large, T.M.; Adams, M.R.; Loeffler, B.J.; Gardner, M.J. Posttraumatic Avascular Necrosis after Proximal Femur, Proximal Humerus, Talar Neck, and Scaphoid Fractures. J. Am. Acad. Orthop. Surg. 2019, 27, 794–805. [Google Scholar] [CrossRef]
- Lavernia, C.J.; Sierra, R.J.; Grieco, F.R. Osteonecrosis of the Femoral Head. J. Am. Acad. Orthop. Surg. 1999, 7, 250–261. [Google Scholar] [CrossRef]
- Mont, M.A.; Salem, H.S.; Piuzzi, N.S.; Goodman, S.B.; Jones, L.C. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? J. Bone Jt. Surg. 2020, 102, 1084–1099. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, F.; Wang, B.; Liu, B.; Li, L.; Kim, S.-Y.; Goodman, S.B.; Hernigou, P.; Cui, Q.; Lineaweaver, W.C.; et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version). J. Orthop. Transl. 2020, 21, 100–110. [Google Scholar] [CrossRef]
- Malizos, K.N.; Karantanas, A.H.; Varitimidis, S.E.; Dailiana, Z.H.; Bargiotas, K.; Maris, T. Osteonecrosis of the femoral head: Etiology, imaging and treatment. Eur. J. Radiol. 2007, 63, 16–28. [Google Scholar] [CrossRef]
- Coskun Benlidayi, I.; Guzel, R. Oral Bisphosphonate Related Osteonecrosis of the Jaw: A Challenging Adverse Effect. ISRN Rheumatol. 2013, 2013, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, Z.; Camacho, P.M. Rare adverse effects of bisphosphonate therapy. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Konarski, W.; Poboży, T.; Śliwczyński, A.; Kotela, I.; Krakowiak, J.; Hordowicz, M.; Kotela, A. Avascular Necrosis of Femoral Head—Overview and Current State of the Art. Int. J. Environ. Res. Public Health 2022, 19, 7348. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, M.; Miranda, L.; Oliva, F.; Aletto, C.; Maffulli, N. Osteotomies for avascular necrosis of the femoral head. Br. Med. Bull. 2021, 137, 98–111. [Google Scholar] [CrossRef]
- Lamb, J.N.; Holton, C.; O’Connor, P.; Giannoudis, P.V. Avascular necrosis of the hip. BMJ 2019, l2178. [Google Scholar] [CrossRef]
- Konarski, W.; Poboży, T.; Kotela, A.; Śliwczyński, A.; Kotela, I.; Hordowicz, M.; Krakowiak, J. The Risk of Avascular Necrosis Following the Stabilization of Femoral Neck Fractures: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 10050. [Google Scholar] [CrossRef]
- Grond, S.E.; Little, R.E.; Campbell, D.A.; Loehrl, T.A.; Poetker, D.M. Oral corticosteroid use and the risk of developing avascular necrosis: A large retrospective review. Int. Forum Allergy Rhinol. 2022, 12, 903–909. [Google Scholar] [CrossRef]
- Yoon, B.-H.; Jones, L.C.; Chen, C.-H.; Cheng, E.Y.; Cui, Q.; Drescher, W.; Fukushima, W.; Gangji, V.; Goodman, S.B.; Ha, Y.-C.; et al. Etiologic Classification Criteria of ARCO on Femoral Head Osteonecrosis Part 2: Alcohol-Associated Osteonecrosis. J. Arthroplasty 2019, 34, 169–174.e1. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Kim, D.; Cho, S.-K.; Kim, Y.; Bae, S.-C.; Sung, Y.-K. Clinical characteristics of multifocal osteonecrosis in Korean patients with rheumatic disease. Int. J. Rheum. Dis. 2018, 21, 1301–1308. [Google Scholar] [CrossRef]
- Li, X.; Brazauskas, R.; Wang, Z.; Al-Seraihy, A.; Baker, K.S.; Cahn, J.-Y.; Frangoul, H.A.; Gajewski, J.L.; Hale, G.A.; Hsu, J.W.; et al. Avascular Necrosis of Bone after Allogeneic Hematopoietic Cell Transplantation in Children and Adolescents. Biol. Blood Marrow Transplant. 2014, 20, 587–592. [Google Scholar] [CrossRef]
- Bayard, C.; Ledergerber, B.; Flepp, M.; Lecompte, T.; Moulin, E.; Hoffmann, M.; Weber, R.; Staehelin, C.; Di Benedetto, C.; Fux, C.A.; et al. Associations Between Antiretroviral Treatment and Avascular Bone Necrosis: The Swiss HIV Cohort Study. Open Forum Infect. Dis. 2017, 4, ofx177. [Google Scholar] [CrossRef]
- Paul, S.; Ali, A.; Katare, R. Molecular complexities underlying the vascular complications of diabetes mellitus—A comprehensive review. J. Diabetes Complicat. 2020, 34, 107613. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546. [Google Scholar] [CrossRef]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; Al Suwaidi, J.; Abi Khalil, C. Macrovascular Complications in Patients with Diabetes and Prediabetes. Biomed Res. Int. 2017, 2017, 7839101. [Google Scholar] [CrossRef]
- Khalil, H. Diabetes microvascular complications—A clinical update. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S133–S139. [Google Scholar] [CrossRef] [PubMed]
- Peer, A.; Khamaisi, M. Diabetes as a Risk Factor for Medication-Related Osteonecrosis of the Jaw. J. Dent. Res. 2015, 94, 252–260. [Google Scholar] [CrossRef]
- Rahimi-Nedjat, R.; Sagheb, K.; Pabst, A.; Olk, L.; Walter, C. Diabetes Mellitus and Its Association to the Occurrence of Medication-Related Osteonecrosis of the Jaw. Dent. J. 2016, 4, 17. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Yang, S.Y.; Zeng, L.Y.; Li, C.; Yan, H. Correlation between an ABO Blood Group and Primary Femoral Head Necrosis: A Case–Control Study. Orthop. Surg. 2020, 12, 450–456. [Google Scholar] [CrossRef]
- Gokcen, N.; Komac, A.; Tuncer, F.; Kocak Buyuksutcu, G.; Ozdemir Isik, O.; Yazici, A.; Cefle, A. Risk factors of avascular necrosis in Takayasu arteritis: A cross sectional study. Rheumatol. Int. 2022, 42, 529–534. [Google Scholar] [CrossRef]
- Lai, S.W.; Lin, C.L.; Liao, K.F. Real-world database examining the association between avascular necrosis of the femoral head and diabetes in Taiwan. Diabetes Care 2019, 42, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Felten, R.; Perrin, P.; Caillard, S.; Moulin, B.; Javier, R.M. Avascular osteonecrosis in kidney transplant recipients: Risk factors in a recent cohort study and evaluation of the role of secondary hyperparathyroidism. PLoS ONE 2019, 14, e0212931. [Google Scholar] [CrossRef] [PubMed]
- Tse, S.M.; Mok, C.C. Time trend and risk factors of avascular bone necrosis in patients with systemic lupus erythematosus. Lupus 2017, 26, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Yeo, I.; Park, C.W.; Lee, H.; Park, Y.S.; Lee, J., II. Risk factors for osteonecrosis of the femoral head in brain tumor patients receiving corticosteroid after surgery. PLoS ONE 2020, 15, e0238368. [Google Scholar] [CrossRef]
- Vannala, V.; Palaian, S.; Shankar, P.R. Therapeutic Dimensions of Bisphosphonates: A Clinical Update. Int. J. Prev. Med. 2020, 11, 166. [Google Scholar] [CrossRef]
- Martins, L.H.I.; Ferreira, D.C.; Silva, M.T.; Motta, R.H.L.; Franquez, R.T.; de Cássia Bergamaschi Bergamaschi, C. Frequency of osteonecrosis in bisphosphonate users submitted to dental procedures: A systematic review. Oral Dis. 2021. [Google Scholar] [CrossRef]
- Payne, K.F.; Goodson, A.M.; Tahim, A.S.; Rafi, I.; Brennan, P.A. Why worry about bisphosphonate-related osteonecrosis of the jaw? A guide to diagnosis, initial management, and referral of patients. Br. J. Gen. Pract. 2017, 67, 330–331. [Google Scholar] [CrossRef]
- Wilkinson, G.S.; Kuo, Y.-F.; Freeman, J.L.; Goodwin, J.S. Intravenous Bisphosphonate Therapy and Inflammatory Conditions or Surgery of the Jaw: A Population-Based Analysis. JNCI J. Natl. Cancer Inst. 2007, 99, 1016–1024. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).