Psychophysiological Assessment of Children with Cerebral Palsy during Robotic-Assisted Gait Training through Infrared Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Particpants
2.2. Clinical Evaluation
2.3. Experimental Design
2.4. IRT Measurements and Data Analysis
- Mean value (MeanTemp)—average value of the thermal signal T over time (i.e., N samples) defined as:
- Standard deviation (STD)—standard deviation of the thermal signal T overtime (i.e., N samples) defined as:
- Sample Entropy (SampEn): defined as the negative natural logarithm of the conditional probability that signals that the subseries of length m (pattern length) that match pointwise within a tolerance r (similarity factor) also match at the m + 1 point. SampEn of a time series {t1,……,tN} of length N is computed employing the following set of equations:
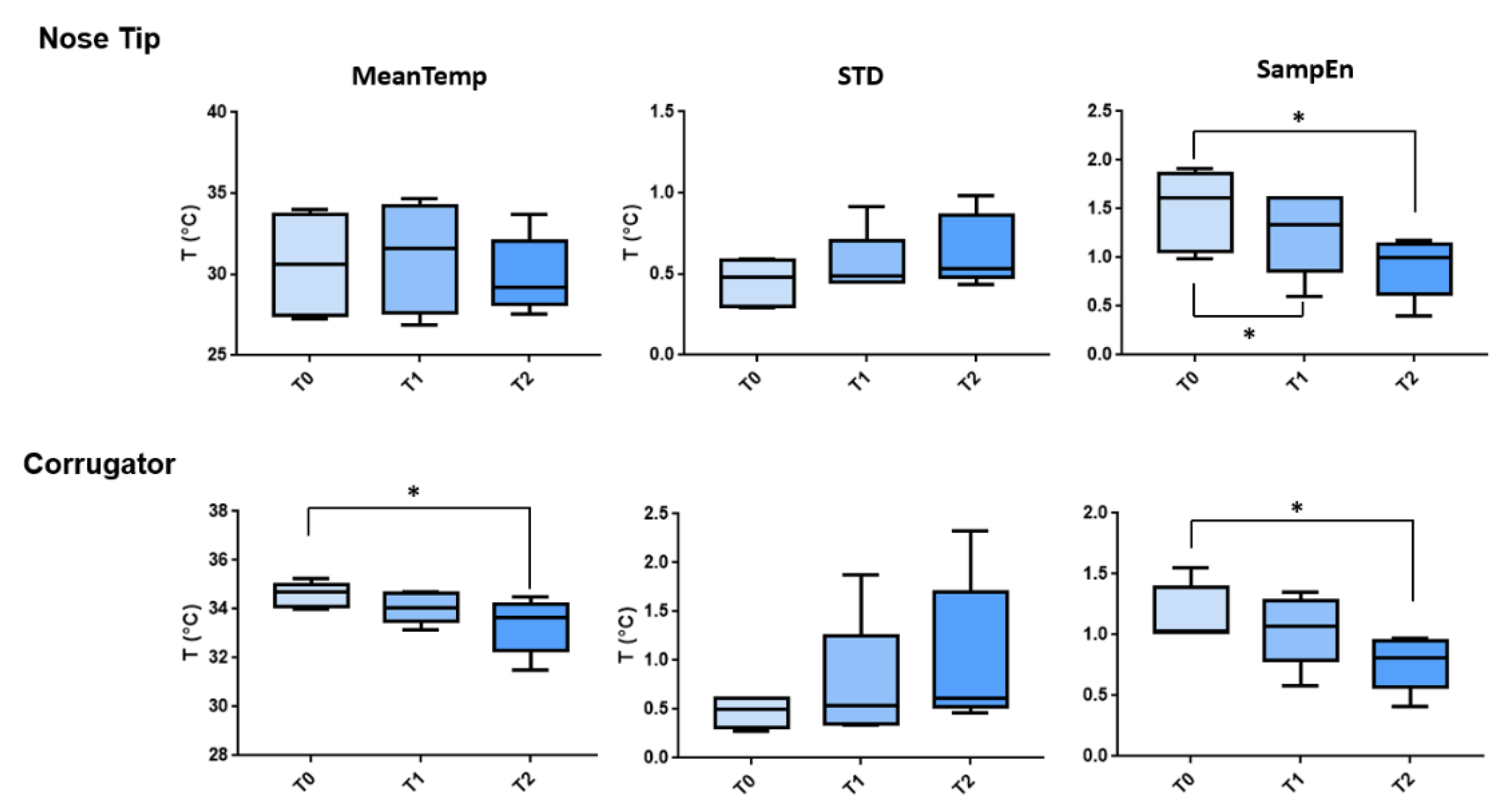
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cherni, Y.; Ballaz, L.; Lemaire, J.; Dal Maso, F.; Begon, M. Effect of Low Dose Robotic-Gait Training on Walking Capacity in Children and Adolescents with Cerebral Palsy. Neurophysiol. Clin. 2020, 50, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, M.; Sarecka-Hujar, B.; Kopyta, I. Cerebral Palsy: Current Opinions on Definition, Epidemiology, Risk Factors, Classification and Treatment Options. Neuropsychiatr. Dis. Treat. 2020, 16, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Cameron, D.; Rosenbaum, P.L.; Walter, S.D.; Russell, D. Stability of the Gross Motor Function Classification System. Dev. Med. Child Neurol. 2006, 48, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Blackman, J.A.; Svensson, C.I.; Marchand, S. Pathophysiology of Chronic Pain in Cerebral Palsy: Implications for Pharmacological Treatment and Research. Dev. Med. Child Neurol. 2018, 60, 861–865. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.-C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef]
- Schwartz, I.; Meiner, Z. Robotic-Assisted Gait Training in Neurological Patients: Who May Benefit? Ann. Biomed. Eng. 2015, 43, 1260–1269. [Google Scholar] [CrossRef]
- Rossignol, S.; Dubuc, R.; Gossard, J.-P. Dynamic Sensorimotor Interactions in Locomotion. Physiol. Rev. 2006, 86, 89–154. [Google Scholar] [CrossRef]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; MacKay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical Activity and Exercise Recommendations for Stroke Survivors. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef]
- Riener, R.; Lünenburger, L.; Maier, I.C.; Colombo, G.; Dietz, V. Locomotor Training in Subjects with Sensori-Motor Deficits: An Overview of the Robotic Gait Orthosis Lokomat. J. Healthc. Eng. 2010, 1, 197–216. [Google Scholar] [CrossRef]
- Schmartz, A.C.; Meyer-Heim, A.D.; Müller, R.; Bolliger, M. Measurement of Muscle Stiffness Using Robotic Assisted Gait Orthosis in Children with Cerebral Palsy: A Proof of Concept. Disabil. Rehabil. Assist. Technol. 2011, 6, 29–37. [Google Scholar] [CrossRef]
- Grafman, J. Conceptualizing Functional Neuroplasticity. J. Commun. Disord. 2000, 33, 345–356. [Google Scholar] [CrossRef]
- Granild-Jensen, J.B.; Rackauskaite, G.; Flachs, E.M.; Uldall, P. Predictors for Early Diagnosis of Cerebral Palsy from National Registry Data. Dev. Med. Child Neurol. 2015, 57, 931–935. [Google Scholar] [CrossRef]
- Forcione, M.; Chiarelli, A.M.; Davies, D.J.; Perpetuini, D.; Sawosz, P.; Merla, A.; Belli, A. Cerebral Perfusion and Blood–Brain Barrier Assessment in Brain Trauma Using Contrast-Enhanced near-Infrared Spectroscopy with Indocyanine Green: A Review. J. Cereb. Blood Flow Metab. 2020, 40, 1586–1598. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-infrared Spectroscopy (FNIRS) for Cognitive Neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5. [Google Scholar] [CrossRef] [PubMed]
- Lequerica, A.H.; Kortte, K. Therapeutic Engagement: A Proposed Model of Engagement in Medical Rehabilitation. Am. J. Phys. Med. Rehabil. 2010, 89, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Saul, J.P. Beat-to-Beat Variations of Heart Rate Reflect Modulation of Cardiac Autonomic Outflow. Physiology 1990, 5, 32–37. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Di Credico, A.; Perpetuini, D.; Izzicupo, P.; Gaggi, G.; Cardone, D.; Filippini, C.; Merla, A.; Ghinassi, B.; Di Baldassarre, A. Estimation of Heart Rate Variability Parameters by Machine Learning Approaches Applied to Facial Infrared Thermal Imaging. Front. Cardiovasc. Med. 2022, 9, 893374. [Google Scholar] [CrossRef]
- Acharya, U.R.; Joseph, K.P.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart Rate Variability: A Review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Gąsior, J.S.; Zamunér, A.R.; Silva, L.E.V.; Williams, C.A.; Baranowski, R.; Sacha, J.; Machura, P.; Kochman, W.; Werner, B. Heart Rate Variability in Children and Adolescents with Cerebral Palsy—A Systematic Literature Review. J. Clin. Med. 2020, 9, 1141. [Google Scholar] [CrossRef]
- Uno, H. Sympathetic Innervation Of The Sweat Glands And Pilorrector Muscles Of Macaques And Human Beings. J. Investig. Dermatol. 1977, 69, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kirshner, S.; Weiss, P.L.; Tirosh, E. Differences in Autonomic Functions as Related to Induced Stress between Children with and without Cerebral Palsy While Performing a Virtual Meal-Making Task. Res. Dev. Disabil. 2016, 49–50, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.L.; Tirosh, E.; Fehlings, D. Role of Virtual Reality for Cerebral Palsy Management. J. Child Neurol. 2014, 29, 1119–1124. [Google Scholar] [CrossRef]
- Kushki, A.; Fairley, J.; Merja, S.; King, G.; Chau, T. Comparison of Blood Volume Pulse and Skin Conductance Responses to Mental and Affective Stimuli at Different Anatomical Sites. Physiol. Meas. 2011, 32, 1529. [Google Scholar] [CrossRef]
- Salazar-López, E.; Domínguez, E.; Ramos, V.J.; de la Fuente, J.; Meins, A.; Iborra, O.; Gálvez, G.; Rodríguez-Artacho, M.A.; Gómez-Milán, E. The Mental and Subjective Skin: Emotion, Empathy, Feelings and Thermography. Conscious. Cogn. 2015, 34, 149–162. [Google Scholar] [CrossRef]
- Bernardi, L.; Berardesca, E. Measurement of Skin Blood Flow by Laser-Doppler Flowmetry. In Bioengineering of the Skin: Methods and Instrumentation; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-00-306896-9. [Google Scholar]
- Perpetuini, D.; Chiarelli, A.M.; Maddiona, L.; Rinella, S.; Bianco, F.; Bucciarelli, V.; Gallina, S.; Perciavalle, V.; Vinciguerra, V.; Merla, A. Multi-Site Photoplethysmographic and Electrocardiographic System for Arterial Stiffness and Cardiovascular Status Assessment. Sensors 2019, 19, 5570. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and Its Application in Clinical Physiological Measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, D.; Chiarelli, A.M.; Cardone, D.; Rinella, S.; Massimino, S.; Bianco, F.; Bucciarelli, V.; Vinciguerra, V.; Fallica, G.; Perciavalle, V.; et al. Photoplethysmographic Prediction of the Ankle-Brachial Pressure Index through a Machine Learning Approach. Appl. Sci. 2020, 10, 2137. [Google Scholar] [CrossRef]
- Cardone, D.; Perpetuini, D.; Filippini, C.; Mancini, L.; Nocco, S.; Tritto, M.; Rinella, S.; Giacobbe, A.; Fallica, G.; Ricci, F.; et al. Classification of Drivers’ Mental Workload Levels: Comparison of Machine Learning Methods Based on ECG and Infrared Thermal Signals. Sensors 2022, 22, 7300. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Derakshan, N.; Santos, R.; Calvo, M.G. Anxiety and Cognitive Performance: Attentional Control Theory. Emotion 2007, 7, 336. [Google Scholar] [CrossRef]
- Clay-Warner, J.; Robinson, D.T. Infrared Thermography as a Measure of Emotion Response. Emot. Rev. 2015, 7, 157–162. [Google Scholar] [CrossRef]
- Filippini, C.; Di Crosta, A.; Palumbo, R.; Perpetuini, D.; Cardone, D.; Ceccato, I.; Di Domenico, A.; Merla, A. Automated Affective Computing Based on Bio-Signals Analysis and Deep Learning Approach. Sensors 2022, 22, 1789. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, D.; Filippini, C.; Zito, M.; Cardone, D.; Merla, A. Altered Microcirculation in Alzheimer’s Disease Assessed by Machine Learning Applied to Functional Thermal Imaging Data. Bioengineering 2022, 9, 492. [Google Scholar] [CrossRef]
- Rodríguez Medina, D.A.; Domínguez Trejo, B.; Cortés Esteban, P.; Cruz Albarrán, I.A.; Morales Hernández, L.A.; Leija Alva, G. Biopsychosocial Assessment of Pain with Thermal Imaging of Emotional Facial Expression in Breast Cancer Survivors. Medicines 2018, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Bartlett, D. Gross Motor Function Classification System: Impact and Utility. Dev. Med. Child Neurol. 2004, 46, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Salavati, M.; Krijnen, W.P.; Rameckers, E.A.A.; Looijestijn, P.L.; Maathuis, C.G.B.; van der Schans, C.P.; Steenbergen, B. Reliability of the Modified Gross Motor Function Measure-88 (GMFM-88) for Children with Both Spastic Cerebral Palsy and Cerebral Visual Impairment: A Preliminary Study. Res. Dev. Disabil. 2015, 45, 32–48. [Google Scholar] [CrossRef]
- Ring, E.F.J.; Ammer, K. Infrared Thermal Imaging in Medicine. Physiol. Meas. 2012, 33, R33. [Google Scholar] [CrossRef]
- Diakides, M.; Bronzino, J.D.; Peterson, D.R. Medical Infrared Imaging: Principles and Practices; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-7250-5. [Google Scholar]
- Merla, A.; Romani, G.L. Biomedical Applications of Functional Infrared Imaging. In Proceedings of the Engineering in Medicine and Biology Society, 27th Annual International Conference of the IEEE-EMBS, Shanghai, China, 31 August–3 September 2005; IEEE: Piscataway, NJ, USA, 2006; pp. 690–693. [Google Scholar]
- Perpetuini, D.; Cardone, D.; Filippini, C.; Chiarelli, A.M.; Merla, A. A Motion Artifact Correction Procedure for FNIRS Signals Based on Wavelet Transform and Infrared Thermography Video Tracking. Sensors 2021, 21, 5117. [Google Scholar] [CrossRef]
- Perpetuini, D.; Chiarelli, A.M.; Filippini, C.; Cardone, D.; Croce, P.; Rotunno, L.; Anzoletti, N.; Zito, M.; Zappasodi, F.; Merla, A. Working Memory Decline in Alzheimer’s Disease is Detected by Complexity Analysis of Multimodal EEG-FNIRS. Entropy 2020, 22, 1380. [Google Scholar] [CrossRef]
- Perpetuini, D.; Cardone, D.; Chiarelli, A.M.; Filippini, C.; Croce, P.; Zappasodi, F.; Rotunno, L.; Anzoletti, N.; Zito, M.; Merla, A. Autonomic Impairment in Alzheimer’s Disease Is Revealed by Complexity Analysis of Functional Thermal Imaging Signals during Cognitive Tasks. Physiol. Meas. 2019, 40, 034002. [Google Scholar] [CrossRef]
- Gopalan, G.; Goldstein, L.; Klingenstein, K.; Sicher, C.; Blake, C.; McKay, M.M. Engaging Families into Child Mental Health Treatment: Updates and Special Considerations. J. Can. Acad. Child Adolesc. Psychiatry 2010, 19, 182–196. [Google Scholar] [PubMed]
- King, G.; Currie, M.; Petersen, P. Child and Parent Engagement in the Mental Health Intervention Process: A Motivational Framework. Child Adolesc. Ment. Health 2014, 19, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, M.C. The Particularities of Engagement: Intersubjectivity in Occupational Therapy Practice. OTJR Occup. Particip. Health 2012, 32, 151–159. [Google Scholar] [CrossRef]
- Poulsen, A.A.; Rodger, S.; Ziviani, J.M. Understanding Children’s Motivation from a Self-Determination Theoretical Perspective: Implications for Practice. Aust. Occup. Ther. J. 2006, 53, 78–86. [Google Scholar] [CrossRef]
- Majnemer, A.; Shevell, M.; Law, M.; Poulin, C.; Rosenbaum, P. Level of Motivation in Mastering Challenging Tasks in Children with Cerebral Palsy. Dev. Med. Child Neurol. 2010, 52, 1120–1126. [Google Scholar] [CrossRef]
- Majnemer, A.; Shikako-Thomas, K.; Lach, L.; Shevell, M.; Law, M.; Schmitz, N. Mastery Motivation in Adolescents with Cerebral Palsy. Res. Dev. Disabil. 2013, 34, 3384–3392. [Google Scholar] [CrossRef]
- Miller, L.; Ziviani, J.; Ware, R.S.; Boyd, R.N. Mastery Motivation as a Predictor of Occupational Performance Following Upper Limb Intervention for School-Aged Children with Congenital Hemiplegia. Dev. Med. Child Neurol. 2014, 56, 976–983. [Google Scholar] [CrossRef]
- Miller, L.; Ziviani, J.; Ware, R.S.; Boyd, R.N. Mastery Motivation in Children with Congenital Hemiplegia: Individual and Environmental Associations. Dev. Med. Child Neurol. 2014, 56, 267–274. [Google Scholar] [CrossRef]
- Harniess, P.A.; Gibbs, D.; Bezemer, J.; Purna Basu, A. Parental Engagement in Early Intervention for Infants with Cerebral Palsy—A Realist Synthesis. Child Care Health Dev. 2022, 48, 359–377. [Google Scholar] [CrossRef]
- Kox, M.; Stoffels, M.; Smeekens, S.P.; van Alfen, N.; Gomes, M.; Eijsvogels, T.M.H.; Hopman, M.T.E.; van der Hoeven, J.G.; Netea, M.G.; Pickkers, P. The Influence of Concentration/Meditation on Autonomic Nervous System Activity and the Innate Immune Response: A Case Study. Psychosom. Med. 2012, 74, 489–494. [Google Scholar] [CrossRef]
- Aboy, M.; Cuesta-Frau, D.; Austin, D.; Mico-Tormos, P. Characterization of Sample Entropy in the Context of Biomedical Signal Analysis. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 5942–5945. [Google Scholar]
- Malliani, A.; Lombardi, F.; Pagani, M. Power Spectrum Analysis of Heart Rate Variability: A Tool to Explore Neural Regulatory Mechanisms. Br. Heart J. 1994, 71, 1. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological Time-Series Analysis Using Approximate Entropy and Sample Entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M.; Goldberger, A.L. Physiological Time-Series Analysis: What Does Regularity Quantify? Am. J. Physiol. Heart Circ. Physiol. 1994, 266, H1643–H1656. [Google Scholar] [CrossRef] [PubMed]
- Wallard, L.; Dietrich, G.; Kerlirzin, Y.; Bredin, J. Robotic-Assisted Gait Training Improves Walking Abilities in Diplegic Children with Cerebral Palsy. Eur. J. Paediatr. Neurol. 2017, 21, 557–564. [Google Scholar] [CrossRef]
- van Kammen, K.; Reinders-Messelink, H.A.; Elsinghorst, A.L.; Wesselink, C.F.; Meeuwisse-de Vries, B.; van der Woude, L.H.; Boonstra, A.M.; den Otter, R. Amplitude and Stride-to-Stride Variability of Muscle Activity during Lokomat Guided Walking and Treadmill Walking in Children with Cerebral Palsy. Eur. J. Paediatr. Neurol. 2020, 29, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Meseguer-Henarejos, A.-B.; Sánchez-Meca, J.; López-Pina, J.-A.; Carles-Hernández, R. Inter- and Intra-Rater Reliability of the Modified Ashworth Scale: A Systematic Review and Meta-Analysis. Eur. J. Phys. Rehabil. Med. 2018, 54, 576–590. [Google Scholar] [CrossRef]
- Lefmann, S.; Russo, R.; Hillier, S. The Effectiveness of Robotic-Assisted Gait Training for Paediatric Gait Disorders: Systematic Review. J. Neuroeng. Rehabil. 2017, 14, 1. [Google Scholar] [CrossRef]
- Carvalho, I.; Pinto, S.M.; das Chagas, D.V.; Praxedes dos Santos, J.L.; de Sousa Oliveira, T.; Batista, L.A. Robotic Gait Training for Individuals with Cerebral Palsy: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 2332–2344. [Google Scholar] [CrossRef]
- Sarhan, R.S.M.; Chevidikunnan, M.F.; Gaowgzeh, R.A.M. Locomotor Treadmill Training Program Using Driven Gait Orthosis Versus Manual Treadmill Therapy on Motor Output In Spastic Diplegic Cerebral Palsy Children. J. Health Allied Sci. NU 2014, 4, 10–17. [Google Scholar] [CrossRef]


| Subj | GMFCS Level (T0) | GMFCS Level (T2) | GMFM-88 (T0) | GMFM-88 (T2) | Delta | Delta (%) |
|---|---|---|---|---|---|---|
| 1 | III | III | 32.10 | 42.00 | 9.9 | 30.8 |
| 2 | IV | IV | 11.10 | 16.10 | 3 | 45.0 |
| 3 | I | I | 47.20 | 49.20 | 2 | 4.1 |
| 4 | IV | III | 17.20 | 25.50 | 8.3 | 48.3 |
| 5 | IV | III | 30.00 | 36.30 | 6.3 | 21.0 |
| 6 | IV | III | 16.00 | 24.30 | 8.3 | 51.9 |
| 7 | V | IV | 15.50 | 22.40 | 6.9 | 44.5 |
| 8 | IV | III | 12.40 | 16.30 | 3.9 | 31.4 |
| ROI | Metric | Comparison | t-Stat | Adjusted p-Value |
|---|---|---|---|---|
| Nose Tip | SampEn | T0 vs. T1 | 3.751 | 0.0426 |
| SampEn | T0 vs. T2 | 3.582 | 0.0492 | |
| Corrugator | MeanTemp | T0 vs. T2 | 3.672 | 0.0455 |
| SampEn | T0 vs T2 | 4.506 | 0.0234 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perpetuini, D.; Russo, E.F.; Cardone, D.; Palmieri, R.; Filippini, C.; Tritto, M.; Pellicano, F.; De Santis, G.P.; Pellegrino, R.; Calabrò, R.S.; et al. Psychophysiological Assessment of Children with Cerebral Palsy during Robotic-Assisted Gait Training through Infrared Imaging. Int. J. Environ. Res. Public Health 2022, 19, 15224. https://doi.org/10.3390/ijerph192215224
Perpetuini D, Russo EF, Cardone D, Palmieri R, Filippini C, Tritto M, Pellicano F, De Santis GP, Pellegrino R, Calabrò RS, et al. Psychophysiological Assessment of Children with Cerebral Palsy during Robotic-Assisted Gait Training through Infrared Imaging. International Journal of Environmental Research and Public Health. 2022; 19(22):15224. https://doi.org/10.3390/ijerph192215224
Chicago/Turabian StylePerpetuini, David, Emanuele Francesco Russo, Daniela Cardone, Roberta Palmieri, Chiara Filippini, Michele Tritto, Federica Pellicano, Grazia Pia De Santis, Raffaello Pellegrino, Rocco Salvatore Calabrò, and et al. 2022. "Psychophysiological Assessment of Children with Cerebral Palsy during Robotic-Assisted Gait Training through Infrared Imaging" International Journal of Environmental Research and Public Health 19, no. 22: 15224. https://doi.org/10.3390/ijerph192215224
APA StylePerpetuini, D., Russo, E. F., Cardone, D., Palmieri, R., Filippini, C., Tritto, M., Pellicano, F., De Santis, G. P., Pellegrino, R., Calabrò, R. S., Filoni, S., & Merla, A. (2022). Psychophysiological Assessment of Children with Cerebral Palsy during Robotic-Assisted Gait Training through Infrared Imaging. International Journal of Environmental Research and Public Health, 19(22), 15224. https://doi.org/10.3390/ijerph192215224











