If Smoking Were Eliminated, Which US Counties Would Still Have High Rates of Smoking-Related Cancers?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cancer Data
- Trachea, bronchus, and lung (ICD-O-3 codes C33.9–34.9);
- Larynx (C32.0–32.9);
- Oral cavity and pharyngeal (C00–14.8);
- Esophagus (C15.0–15.9);
- Stomach (C16.0–16.9);
- Colon and rectum (C18.0–20.9);
- Liver (C22.0);
- Pancreas (C25.0–25.9);
- Kidney and renal pelvis (C64.9–65.9);
- Urinary bladder (C67.0–67.9);
- Cervix (C53.0–53.9);
- Acute myeloid leukemia (ICD-O-3 histology codes 9840, 9861, 9865–9867, 9869, 9871–9874, 9895–9898, 9910–9911, and 9920).
2.2. Smoking, Environmental, and Demographic Data
2.3. Modeling Actual and Simulated Cancer Rates
2.4. Analyzing Environmental Predictors of Low-Benefit Counties
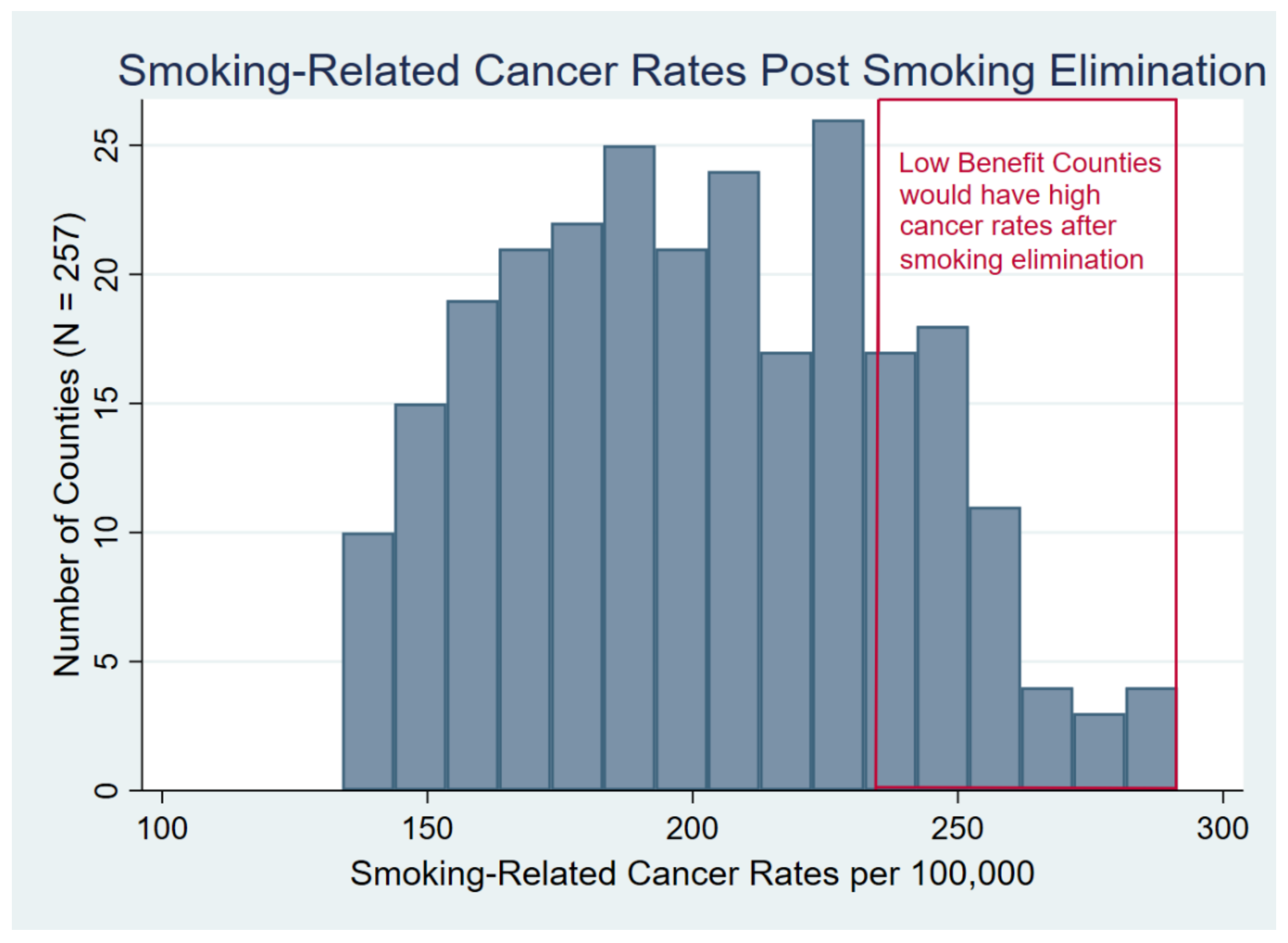
3. Results
3.1. Descriptive Statistics for All Metropolitan Counties
3.2. Comparing Low-Benefit and High-Benefit Counties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Sensitivity Analysis
| All Metropolitan Counties | Counties with Post-Smoking Elimination Incidence Rates above and below the 20th Percentile * | |||
|---|---|---|---|---|
| Above | Above | t-Test | ||
| Mean Cancer Incidence and Covariates | (n = 133) | (n = 36) | (n = 97) | p-Value |
| Cancer Rate—Observed | 279.3 | 291.4 | 274.8 | <0.01 |
| Cancer Rate—Smoking Eliminated | 205.9 | 252 | 188.8 | <0.01 |
| Cancer Rate—Percent Reduction | 26.10% | 13.60% | 30.80% | <0.01 |
| Smoking Prevalence (lagged) | 22 | 21.7 | 22.1 | 0.22 |
| PM2.5 (μg/m3) | 14.12 | 13.4 | 14.4 | 0.17 |
| County Population | 128,814 | 267,355 | 77,397 | <0.01 |
| Environmental Quality Index | 0.34 | 0.95 | 0.11 | <0.01 |
| Air Quality Index | 0.68 | 1.19 | 0.49 | <0.01 |
| Water Quality Index | −0.28 | −0.17 | −0.32 | 0.42 |
| Land Quality Index | −0.04 | 0.2 | −0.13 | 0.04 |
| Built Environment Quality Index ** | 0.06 | 0.63 | −0.15 | <0.01 |
| Sociodemographic Quality Index ** | 0.37 | 0.75 | 0.23 | <0.01 |
References
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, A.B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, D.J.; Hoppin, P.; Jacobs, M.; Clapp, R.; Kriebel, D. Cancer rates not explained by smoking: A county-level analysis. Environ. Health. 2020, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.N.A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; et al. SEER Cancer Statistics Review (CSR), 1975–2016 Bethesda, MD2019. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 30 May 2019).
- Economic Research Service. US Department of Agriculture. 2013 Rural-Urban Continuum Codes. Available online: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation/ (accessed on 17 November 2021).
- Gallaway, M.S.; Henley, S.J.; Steele, C.B.; Momin, B.; Thomas, C.C.; Jamal, A.; Trivers, K.F.; Singh, S.D.; Stewart, S.L. Surveillance for cancers associated with tobacco use—United States, 2010–2014. MMWR Surveill. Summ. 2018, 67, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Dwyer-Lindgren, L.; Mokdad, A.H.; Srebotnjak, T.; Flaxman, A.D.; Hansen, G.M.; Murray, C.J.L. Cigarette smoking prevalence in US counties: 1996–2012. Popul. Health Metr. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Bechle, M.; Kim, S.-Y.; Adams, P.J.; Pandis, S.N.; Pope, C.A., III; Robinson, A.L.; Sheppard, L.; Szpiro, A.A.; Marshall, J.D. Spatial decomposition analysis of NO2 and PM2.5 air pollution in the United States. Atmos. Environ. 2020, 241, 117470. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Environmental Quality Index (EQI). Available online: https://www.epa.gov/healthresearch/environmental-quality-index-eqi (accessed on 18 August 2021).
- U.S. Environmental Protection Agency. Creating an Overall Environmental Quality Index—Technical Report, 2000–2005; U.S. Environmental Protection Agency: Washington, DC, USA, 2014; EPA/600/R-14/304.
- Outdoor Air Pollution/IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; WHO: Geneva, Switzerland, 2016; Volume 109, Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Outdoor-Air-Pollution-2015 (accessed on 14 November 2022).
- President’s Cancer Panel. President’s Cancer Panel 2008–2009: Reducing Environmental Cancer Risk, Appendix F; US Department of Health and Human Services: Washington, DC, USA, 2010. Available online: http://deainfo.nci.nih.gov/advisory/pcp/annualReports/pcp08-09rpt/PCP_Report_08-09_508.pdf (accessed on 14 November 2022).
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Swanton, C.; Hill, W.; Lim, E.; Lee, C.; Weeden, C.; Augustine, M.; Chen, K.; Kuan, F.C.; Marongiu, F.; Evans, E.; et al. Non-Small-Cell Lung Cancer Promotion by Air Pollutants; Research Square: Durham, NC, USA, 2022. [Google Scholar] [CrossRef]
- Editorial. Cancer risk paradox: Grand plans fall short? Lancet Oncol. 2017, 18, 555. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Colditz, G.A.; Hiatt, R.A. Combating environmental causes of cancer. N. Engl. J. Med. 2011, 364, 2266, Author reply 2267–2268. [Google Scholar] [PubMed]
- Doll, R.; Peto, R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981, 66, 1191–1308. [Google Scholar] [CrossRef] [PubMed]
| IRR † | 95% CI ‡ | ||
|---|---|---|---|
| Age Group | |||
| 20–24 | 1 | (Ref.) | |
| 25–29 | 1.85 | 1.62 | 2.10 |
| 30–34 | 3.42 | 3.03 | 3.85 |
| 35–39 | 5.78 | 5.15 | 6.48 |
| 40–44 | 9.28 | 8.30 | 10.38 |
| 45–49 | 15.82 | 14.18 | 17.64 |
| 50–54 | 30.69 | 27.57 | 34.17 |
| 55–59 | 46.94 | 42.19 | 52.22 |
| 60–64 | 67.21 | 60.43 | 74.75 |
| 65–69 | 93.44 | 84.02 | 103.90 |
| 70–74 | 124.34 | 111.81 | 138.28 |
| 75–79 | 152.63 | 137.22 | 169.77 |
| 80–84 | 173.00 | 155.48 | 192.50 |
| Sex | |||
| Female | 1 | (Ref.) | |
| Male | 1.42 | 1.39 | 1.46 |
| Smoking Prevalence | |||
| 4.0%<10.1% | 1 | (Ref.) | |
| 10.1%<12.2% | 1.14 | 1.02 | 1.28 |
| 12.2%<15.7% | 1.21 | 1.09 | 1.34 |
| 15.7%<19.2% | 1.28 | 1.15 | 1.43 |
| 19.2%<22.9% | 1.39 | 1.24 | 1.56 |
| 22.9%<38.3% | 1.66 | 1.47 | 1.88 |
| Mean Cancer Incidence and Covariates | All Metropolitan Counties | Counties with Post-Smoking Elimination Incidence Rates above and below the 20th Percentile * | ||
|---|---|---|---|---|
| Above | Below | t-Test | ||
| (Low benefit) | (High Benefit) | |||
| (n = 257) | (n = 51) | (n = 206) | p-Value | |
| Cancer Rate per 100,000—Observed | 275.1 | 301.5 | 268.6 | <0.01 |
| Cancer Rate—Smoking Eliminated | 201.9 | 252.6 | 189.3 | <0.01 |
| Cancer Rate—Percent Reduction | 25.0% | 15.6% | 27.3% | <0.01 |
| County Population | 226,186 | 250,342 | 220,206 | 0.73 |
| PM2.5 (μg/m3) | 14.1 | 13.7 | 14.3 | 0.32 |
| Environmental Quality Index | 0.56 | 0.98 | 0.45 | <0.01 |
| Air Quality Index | 0.74 | 1.24 | 0.62 | <0.01 |
| Water Quality Index | 0.08 | −0.05 | 0.12 | 0.28 |
| Land Quality Index | 0.18 | 0.26 | 0.16 | 0.46 |
| Built Environment Quality Index ** | 0.13 | 0.66 | 0.01 | <0.01 |
| Sociodemographic Quality Index ** | 0.47 | 0.65 | 0.43 | 0.17 |
| Smoking Prevalence (%, lagged 20 years) | 21.8 | 23.1% | 21.5% | 0.03 |
| (a) | ||||
|---|---|---|---|---|
| Crude | ||||
| Exposures | OR † | 95% CI ‡ | AIC § | |
| Environmental Quality Index | 1.96 | 1.34 | 2.86 | 246.2 |
| Air Quality Index | 5.99 | 3.20 | 11.22 | 219.4 |
| Water Quality Index | 0.85 | 0.63 | 1.15 | 259.0 |
| Land Quality Index | 1.15 | 0.79 | 1.67 | 259.5 |
| Built Environment Quality Index * | 2.70 | 1.68 | 4.32 | 237.2 |
| Sociodemographic Quality Index * | 1.23 | 0.91 | 1.64 | 258.3 |
| PM2.5 (μg/m3) | 0.96 | 0.88 | 1.04 | 259.1 |
| Smoking Prevalence | 1.08 | 1.01 | 1.15 | 255.4 |
| (b) | ||||
| Adjusted | ||||
| EPA Exposure Indices | OR † | 95% CI ‡ | AIC § | |
| Air Quality Index | 4.43 | 2.14 | 9.19 | 223.4 |
| Water Quality Index | 0.80 | 0.56 | 1.14 | |
| Land Quality Index | 1.00 | 0.62 | 1.61 | |
| Built Environment Quality Index * | 1.59 | 0.90 | 2.82 | |
| Sociodemographic Quality Index * | 0.96 | 0.67 | 1.37 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myers, D.J.; Kriebel, D. If Smoking Were Eliminated, Which US Counties Would Still Have High Rates of Smoking-Related Cancers? Int. J. Environ. Res. Public Health 2022, 19, 15292. https://doi.org/10.3390/ijerph192215292
Myers DJ, Kriebel D. If Smoking Were Eliminated, Which US Counties Would Still Have High Rates of Smoking-Related Cancers? International Journal of Environmental Research and Public Health. 2022; 19(22):15292. https://doi.org/10.3390/ijerph192215292
Chicago/Turabian StyleMyers, Douglas J., and David Kriebel. 2022. "If Smoking Were Eliminated, Which US Counties Would Still Have High Rates of Smoking-Related Cancers?" International Journal of Environmental Research and Public Health 19, no. 22: 15292. https://doi.org/10.3390/ijerph192215292




