Side-Effects following Oxford/AstraZeneca COVID-19 Vaccine in Tororo District, Eastern Uganda: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Study Population
2.4. Sample Size
2.5. Data Extraction
2.6. Data Collection Methods
2.7. Measurement of Variables
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Prevalence of Side-Effects to Oxford/AstraZeneca Vaccine in Tororo District
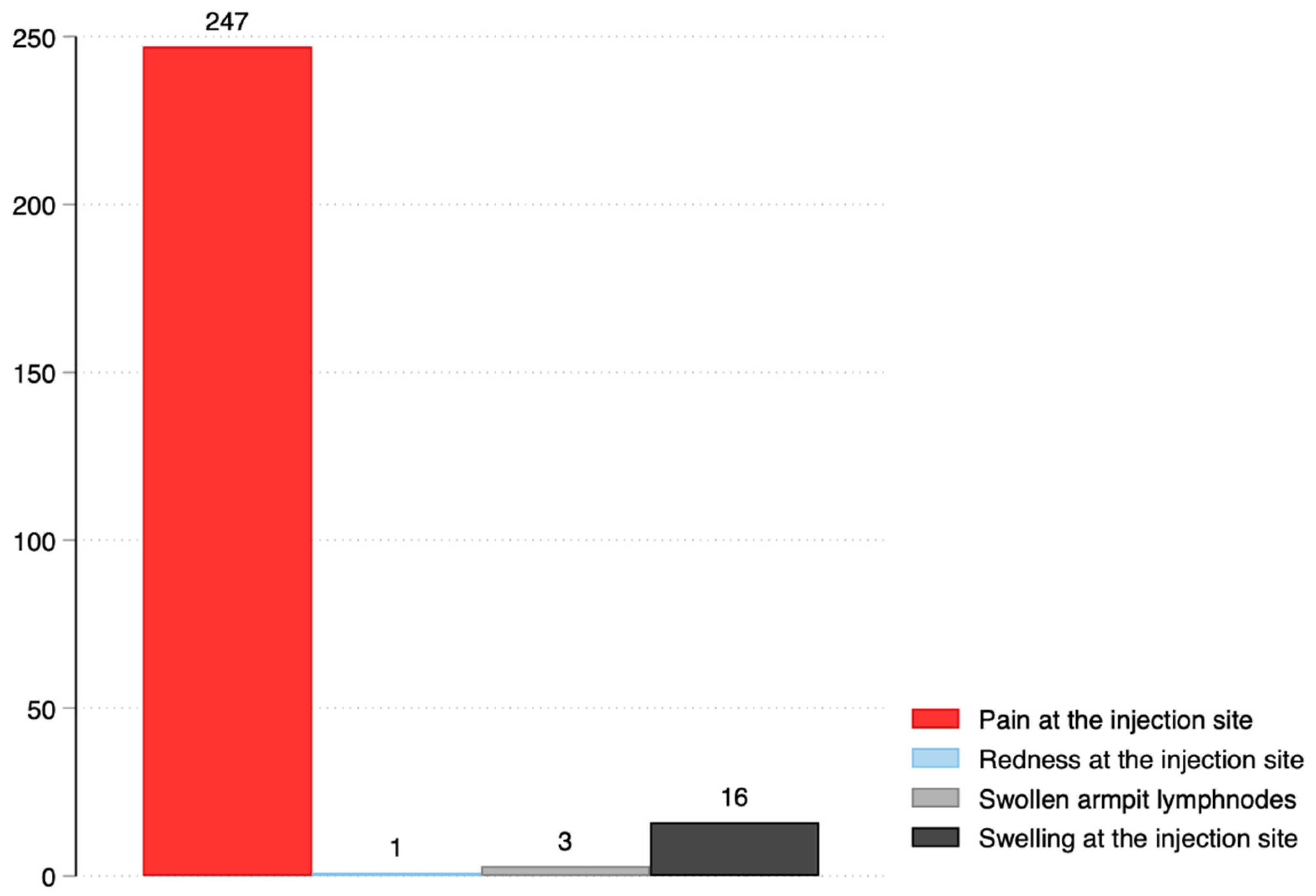
3.3. Side-Effect Profile
3.4. Health Care Seeking following Oxford/AstraZeneca Vaccine Side-Effects
3.5. Medications following Oxford/AstraZeneca Side-Effects
3.6. Deaths following Oxford/AstraZeneca Vaccination
3.7. Factors Associated with Experiencing Side-Effects to Oxford/AstraZeneca Vaccine
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucia, V.C.; Kelekar, A.; Afonso, N.M. COVID-19 vaccine hesitancy among medical students. J. Public Health 2021, 43, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.; King, C. COVID-19 vaccine side effects: The positives about feeling bad. Sci. Immunol. 2021, 6, eabj9256. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Shrestha, M.; Wagle, R.R.; Bhandari, G. Predictors of incompletion of immunization among children residing in the slums of Kathmandu valley, Nepal: A case-control study. BMC Public Health 2016, 16, 970. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Oryema, P.; Babirye, J.N.; Baguma, C.; Wasswa, P.; Guwatudde, D. Utilization of outreach immunization services among children in Hoima District, Uganda: A cluster survey. BMC Res. Notes 2017, 10, 111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robertson, E.; Reeve, K.S.; Niedzwiedz, C.L.; Moore, J.; Blake, M.; Green, M.; Katikireddi, S.V.; Benzeval, M.J. Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain Behav. Immun. 2021, 94, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Echoru, I.; Ajambo, P.D.; Keirania, E.; Bukenya, E.E.M. Sociodemographic factors associated with acceptance of COVID-19 vaccine and clinical trials in Uganda: A cross-sectional study in western Uganda. BMC Public Health 2021, 21, 1106. [Google Scholar] [CrossRef] [PubMed]
- Uganda Bureau of Statistics. 2020. Available online: https://www.citypopulation.de/en/uganda/admin/eastern/039__tororo/ (accessed on 20 September 2020).
- Adam, M.; Gameraddin, M.; Alelyani, M.; Alshahrani, M.Y.; Gareeballah, A.; Ahmad, I.; Azzawi, A.; Komit, B.; Musa, A. Evaluation of Post-Vaccination Symptoms of Two Common COVID-19 Vaccines Used in Abha, Aseer Region, Kingdom of Saudi Arabia. Patient Prefer. Adherence 2021, 15, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Omeish, H.; Najadat, A.; Al-Azzam, S.; Tarabin, N.; Abu Hameed, A.; Al-Gallab, N.; Abbas, H.; Rababah, L.; Rabadi, M.; Karasneh, R.; et al. Reported COVID-19 vaccines side effects among Jordanian population: A cross sectional study. Hum. Vaccines Immunother. 2022, 18, 1981086. [Google Scholar] [CrossRef] [PubMed]
- Desalegn, M.; Garoma, G.; Tamrat, H.; Desta, A.; Prakash, A. The prevalence of AstraZeneca COVID-19 vaccine side effects among Nigist Eleni Mohammed memorial comprehensive specialized hospital health workers. Cross sectional survey. PLoS ONE 2022, 17, e0265140. [Google Scholar] [CrossRef] [PubMed]
- Planès, S.; Villier, C.; Mallaret, M. The nocebo effect of drugs. Pharmacol. Res. Perspect. 2016, 4, e00208. [Google Scholar] [CrossRef] [PubMed]
- Solomon, Y.; Eshete, T.; Mekasha, B.; Assefa, W. COVID-19 Vaccine: Side Effects After the First Dose of the Oxford AstraZeneca Vaccine Among Health Professionals in Low-Income Country: Ethiopia. J. Multidiscip. Healthc. 2021, 14, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Saeed, B.Q.; Al-Shahrabi, R.; Alhaj, S.S.; Alkokhardi, Z.M.; Adrees, A.O. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int. J. Infect. Dis. 2021, 111, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rief, W. Fear of Adverse Effects and COVID-19 Vaccine Hesitancy: Recommendations of the Treatment Expectation Expert Group. JAMA Health Forum 2021, 2, e210804. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Wadman, M. Public needs to prep for vaccine side effects. Science 2020, 370, 1022. [Google Scholar] [CrossRef] [PubMed]
- Boekel, L.; Kummer, L.Y.; van Dam, K.P.J.; Hooijberg, F.; van Kempen, Z.; Vogelzang, E.H.; Wieske, L.; Eftimov, F.; van Vollenhoven, R.; Kuijpers, T.W.; et al. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021, 3, e542–e545. [Google Scholar] [CrossRef]
- Andrzejczak-Grządko, S.; Czudy, Z.; Donderska, M. Side effects after COVID-19 vaccinations among residents of Poland. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4418–4421. [Google Scholar] [PubMed]

| Characteristic, n = 2204 | Frequency (n) | Percentage |
|---|---|---|
| Age | ||
| <50 | 1515 | 68.7 |
| >50 | 689 | 31.3 |
| Sex | ||
| Male | 1310 | 59.4 |
| Female | 894 | 40.6 |
| Education level (n = 2203) | ||
| Primary | 398 | 18.1 |
| Secondary | 541 | 24.5 |
| Tertiary | 1264 | 57.4 |
| Religion (n = 2203) | ||
| Christian | 1953 | 88.7 |
| Moslem | 206 | 9.3 |
| Hindu | 44 | 2.0 |
| Marital status | ||
| Single | 304 | 13.8 |
| Married | 1900 | 86.2 |
| Occupation (n = 2203) | ||
| Teacher | 512 | 23.2 |
| Health worker | 377 | 17.1 |
| Security | 191 | 8.7 |
| Others * | 1123 | 51.0 |
| Previous COVID–19 Infection | ||
| Yes | 75 | 3.4 |
| No | 2129 | 96.6 |
| Place of Seeking Care (n = 424) | Frequency (n) | Percentage (%) |
|---|---|---|
| Did not seek health care | 269 | 63.4 |
| Consulted CHW | 21 | 5.0 |
| Consulted traditional healer | 3 | 0.7 |
| Visited health center | 18 | 4.3 |
| Visited hospital | 12 | 2.8 |
| Visited private clinic | 34 | 8.0 |
| Self-medication | 72 | 17.0 |
| Other * | 2 | 0.5 |
| Medication (n = 156) | Frequency (n) | Percentage (%) |
|---|---|---|
| Herbs | 5 | 3.2 |
| Paracetamol | 96 | 61.5 |
| Diclofenac | 33 | 21.2 |
| Amoxicillin | 11 | 7.1 |
| Azithromycin | 3 | 1.9 |
| Chloroquine | 2 | 1.3 |
| Ciprofloxacin | 6 | 3.9 |
| Vitamin C | 4 | 2.6 |
| Dexamethasone | 13 | 8.3 |
| Others * | 10 | 6.4 |
| Don’t know | 18 | 11.5 |
| Participant | Cause of Death | Period from the Date of the Second Dose of the Vaccine |
|---|---|---|
| 1 | Diabetes complication | Three weeks |
| 2 | Hypertension/stroke | Three weeks |
| 3 | Accident | Two weeks |
| 4 | Malaria | One month |
| 5 | Sudden death | Three months |
| 6 | Accident | Five months |
| 7 | Tuberculosis | Two months |
| Characteristic | Had Side Effects | COR | 95% CI | p-Value | AOR | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <50 | 415 (68.8) | 1 | 1 | ||||
| ≥50 | 188 (31.2) | 1.0 | 0.8–1.2 | 0.958 | 1.0 | 0.8–1.2 | 0.942 |
| Sex | |||||||
| Male | 322 (53.4) | 1 | 1 | ||||
| Female | 281 (46.6) | 1.4 | 1.2–1.7 | <0.001 | 1.3 | 1.1–1.6 | 0.004 * |
| Marital status | |||||||
| Single | 85 (14.1) | 1 | 1 | ||||
| Married | 518 (85.9) | 1.0 | 0.7–1.3 | 0.800 | 0.9 | 0.7–1.2 | 0.595 |
| Previous COVID-19 infection | |||||||
| No | 557 (92.4) | 1 | 1 | ||||
| Yes | 46 (7.6) | 4.5 | 2.8–7.2 | <0.001 | 4.3 | 2.7–7.0 | <0.001 * |
| Education level | |||||||
| Primary | 114 (18.9) | ||||||
| Secondary | 160 (26.5) | 1.0 | 0.8–1.4 | 0.756 | 1.1 | 0.8–1.5 | 0.423 |
| Tertiary | 329 (54.6) | 0.9 | 0.7–1.1 | 0.304 | 0.8 | 0.6–1.1 | 0.163 |
| Occupation | |||||||
| Teacher | 151 (25.04) | 1 | 1 | ||||
| Health worker | 110 (18.2) | 0.9 | 0.7–1.2 | 0.549 | 0.9 | 0.7–1.2 | 0.561 |
| Security | 27 (4.5) | 0.4 | 0.3–0.6 | <0.001 | 0.4 | 0.2–0.6 | <0.001 * |
| Others | 315 (52.2) | 1.0 | 0.7–1.3 | 0.919 | 0.8 | 0.6–1.1 | 0.152 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyango, J.; Mukunya, D.; Napyo, A.; Nantale, R.; Makoko, B.T.; Matovu, J.K.B.; Wanume, B.; Okia, D.; Okello, F.; Okware, S.; et al. Side-Effects following Oxford/AstraZeneca COVID-19 Vaccine in Tororo District, Eastern Uganda: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 15303. https://doi.org/10.3390/ijerph192215303
Onyango J, Mukunya D, Napyo A, Nantale R, Makoko BT, Matovu JKB, Wanume B, Okia D, Okello F, Okware S, et al. Side-Effects following Oxford/AstraZeneca COVID-19 Vaccine in Tororo District, Eastern Uganda: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(22):15303. https://doi.org/10.3390/ijerph192215303
Chicago/Turabian StyleOnyango, Jagire, David Mukunya, Agnes Napyo, Ritah Nantale, Brian T. Makoko, Joseph K. B. Matovu, Benon Wanume, David Okia, Francis Okello, Sam Okware, and et al. 2022. "Side-Effects following Oxford/AstraZeneca COVID-19 Vaccine in Tororo District, Eastern Uganda: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 22: 15303. https://doi.org/10.3390/ijerph192215303
APA StyleOnyango, J., Mukunya, D., Napyo, A., Nantale, R., Makoko, B. T., Matovu, J. K. B., Wanume, B., Okia, D., Okello, F., Okware, S., Olupot-Olupot, P., & Lubaale, Y. (2022). Side-Effects following Oxford/AstraZeneca COVID-19 Vaccine in Tororo District, Eastern Uganda: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(22), 15303. https://doi.org/10.3390/ijerph192215303







