SARS-CoV-2 in Pregnancy—A Retrospective Analysis of Selected Maternal and Fetal Laboratory Parameters
Abstract
:1. Introduction
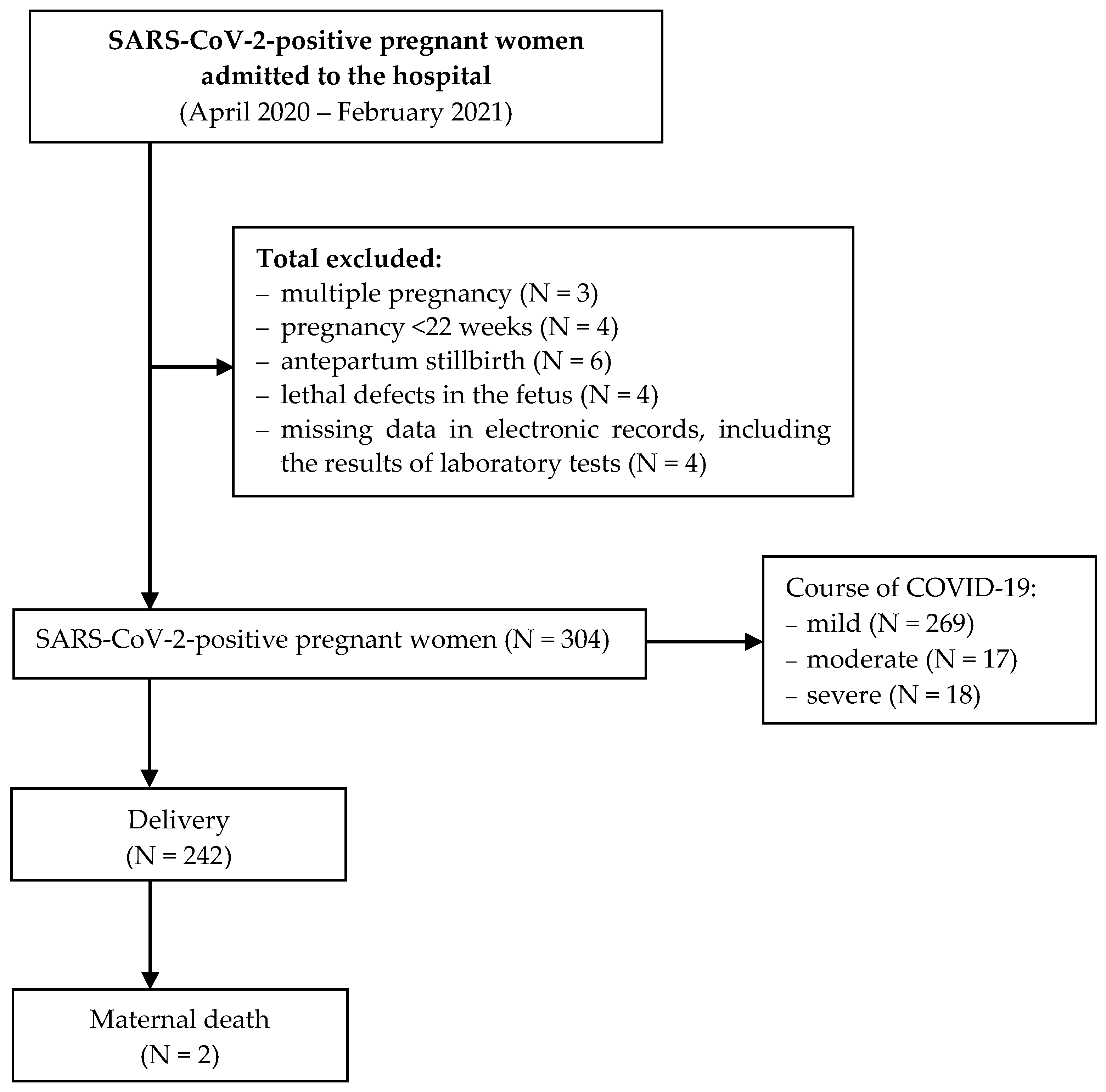
2. Materials and Methods
Statistical Analysis
3. Results
3.1. The Effect of SARS-CoV-2 Infection on Selected Maternal Blood Parameters
3.2. The Effect of COVID-19 Severity on Selected Blood Parameters
3.3. The Effect of Maternal SARS-CoV-2 Infection on Neonatal Condition at Birth
4. Discussion
Strengths and Weaknesses of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABG | Arterial Blood Gas |
| APTT | activated partial thromboplastin |
| ARDS | acute respiratory distress syndrome |
| BMI | body mass index |
| COVID-19 | Corona Virus Disease 2019 |
| CRP | C-Reactive Protein |
| ECS | elective cesarean section |
| EMCS | emergency cesarean section |
| RT-PCR | reverse transcription polymerase chain reaction |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SpO2 | oxygen saturation |
| SVD | spontaneous vaginal delivery |
| VACCUM | vacuum extraction |
References
- Chen, L.; Li, Q.; Zheng, D.; Jiang, H.; Wei, Y.; Zou, L.; Dong, X.; Qu, J.; Gong, F. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China. N. Engl. J. Med. 2020, 382, e100. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Li, Q.; Zheng, D.; Jiang, H. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Li, T.; Han, M. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Infection and Pregnancy. 2020. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/coronavirus-covid-19-infection-in-pregnancy-v3-20-03-18.pdf (accessed on 29 September 2022).
- Salem, D.; Katranji, F.; Bakdash, T. COVID-19 infection in pregnant women: Review of maternal and fetal outcomes. Int. J. Gynaecol. Obstet. 2021, 152, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. CMAJ 2021, 193, e540–e548. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Tang, K.; Guo, Y. Withdrawn: Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, A.; Kalafat, E.; Benlioglu, C.; O’Brien, P.; Morris, E.; Draycott, T. SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis of clinical features and pregnancy outcomes. E-Clin. Med. 2020, 25, 100446. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Heath, P.T.; Ladhani, S.N.; Le Doare, K. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 1, m3320. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Bernstein, P.S.; Debolt, C.; Stone, J.; Sutton, D.M.; Simpson, L.L.; Whiting, L.; Fowler, C. Characteristics and outcomes of 241 Births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York City Medical Centers. Obstet. Gynecol. 2020, 136, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.J.; Carruthers, J.; Calvert, C.; Denny, C.; Donaghy, J.; Goulding, A.; Hopcroft, L.E.M.; Hopkins, L.; McLaughlin, T.; Pan, J.; et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med. 2022, 28, 504–512. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Multifetal Gestations: Twin, Triplet, and Higher-Order Multifetal Pregnancies ACOG Practice Bulletin Summary, Number 231. Obstet. Gynecol. 2021, 137, 1140–1143. [CrossRef]
- Tucker, F.D.; Morris, J.K.; JRC Management Committee; Neville, A.; Garne, E.; Kinsner-Ovaskainen, A.; Lanzoni, M.; Loane, M.A.; Martin, S.; Nicholl, C.; et al. EUROCAT: An update on its functions and activities. J. Community Genet. 2018, 4, 407–410. [Google Scholar] [CrossRef] [Green Version]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 31 October 2022).
- American Academy of Pediatrics Committee on Fetus and Newborn; American College of Obstetricians and Gynecologists Committee on Obstetric Practice. The Apgar Score. Pediatrics 2015, 136, 819–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malin, G.L.; Morris, R.K.; Khan, K.S. Strength of association between umbilical cord pH and perinatal and long term outcomes: Systematic review and meta-analysis. BMJ 2010, 340, c1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinka, J.; Wielgos, M.; Leszczynska-Gorzelak, B.; Piekarska, A.; Huras, H.; Sieroszewski, P.; Czajkowski, K.; Wysocki, J.; Lauterbach, R.; Helwich, E.; et al. COVID-19 impact on perinatal care: Risk factors, clinical manifestation and prophylaxis. Polish experts’ opinion for December 2020. Ginekol. Pol. 2021, 92, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hedermann, G.; Hedley, P.L.; Bækvad-Hansen, M.; Hjalgrim, H.; Rostgaard, K.; Poorisrisak, P.; Breindahl, M.; Melbye, M.; Hougaard, D.M.; Christiansen, M.; et al. Danish premature birth rates during the COVID-19 lockdown. Arch. Dis. Child Fetal Neonatal Ed. 2021, 106, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Pormohammad, A.; Sheikh Neshin, S.A.; Ghorbani, S.; Bose, D.; Alimohammadi, S.; Basirjafari, S.; Mohammadi, M.; Rasmussen-Ivey, C.; Razizadeh, M.H.; et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis. Rev. Med. Virol. 2021, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Petty, J.; Whiting, L.; Fowler, C. Exploring modifiable risk-factors for premature birth in the context of COVID-19 mitigation measures: A discussion paper. J. Neonatal. Nurs. 2021, 27, 172–179. [Google Scholar] [CrossRef]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, L.D.; Saad, A.F.; Saade, G. Early Acute Respiratory Support for Pregnant Patients with Coronavirus Disease 2019 (COVID-19) Infection. Obstet. Gynecol. 2020, 136, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Boushra, M.; Koyfman, A.; Long, B. COVID-19 in pregnancy and the puer- perium: A review for emergency physicians. Am. J. Emerg. Med. 2020, 5, 505. [Google Scholar]
- Turan, O.; Hakim, A.; Dashraath, P.; Jeslyn, W.J.L.; Wright, A.; Abdul-Kadir, R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: A systematic review. Int. J. Gynecol. Obstet. 2020, 4, 151. [Google Scholar] [CrossRef] [PubMed]
- Lokken, E.M.; Huebner, E.M.; Taylor, G.G.; Hendrickson, S.; Vanderhoeven, J.; Kachikis, A.; Heath, P.T.; Ladhani, S.N.; Le Doare, K. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am. J. Obstet. Gynecol. 2021, 225, e1–e77. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.S.; Khan, N.A.; Haider Kazmi, S.J.; Ahmed, A.; Hassan, M.; Jawed, R.; Lio, T.; Hanzmi, M. Hematological parameters predicting severity and mortality in COVID-19 patients of Pakistan: A retrospective comparative analysis. J Community Hosp. Intern. Med. Perspect. 2020, 29, 514–520. [Google Scholar] [CrossRef]
- Blakeway, H.; Prasad, S.; Kalafat, E.; Heath, P.T.; Ladhani, S.N.; Le Doare, K.; Magee, L.A.; O’Brien, P.; Rezvani, A.; von Dadelszen, P.; et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am. J. Obstet. Gynecol. 2022, 226.e1, 236.e14. [Google Scholar] [CrossRef]
- de Medeiros, K.S.; Sarmento, A.C.A.; Costa, A.P.F.; Macêdo, L.T.A.; da Silva, L.A.S.; de Freitas, C.L.; Simões, A.C.Z.; Gonçalves, A.K. Consequences and implications of the coronavirus disease (COVID-19) on pregnancy and newborns: A comprehensive systematic review and meta-analysis. Int. J. Gynaecol. Obstet. 2022, 156, 394–405. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Dong, X.; Qu, J.; Gong, F. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef]
- Zumla, A.; Hui, D.; Perlman, S. Middle East respiratory syndrome. Lancet 2015, 386, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Stukalov, A.; Girault, V.; Grass, V.; Karayel, O.; Bergant, V.; Urban, C.; Haas, D.A.; Huang, Y.; Oubraham, L.; Wang, A. Multilevel proteomics reveals hosts perturbations by SARS-CoV-2 and SARS CoV. Nature 2021, 594, 246–252. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, Y.; Liao, Q.; Cheng, Y. Blood Test Results of Pregnant COVID-19 Patients: An Updated Case-Control Study. Front. Cell Infect. Microbiol. 2020, 7, 560899. [Google Scholar] [CrossRef]
- Benhamou, D.; Keita, H.; Ducloy-Bouthors, A.S.; Obstetric Anaesthesia and Critical Care Club Working Group. Coagulation changes and thromboembolic risk in COVID-19 obstetric patients. Anaesth. Crit. Care Pain Med. 2020, 39, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, V.; Pedersen, L.; Grijota, M.; Nielsen, G.L.; Rothman, K.J.; Sørensen, H.T. Association of Apgar score at five minutes with long-term neurologic disability and cognitive function in a prevalence study of Danish conscripts. BMC Pregnancy Childbirth 2009, 2, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, A.; Otterblad Olausson, P.; Källen, K. Apgar scores at 5 minutes after birth in relation to school performance at 16 years of age. Obstet. Gynecol. 2011, 118, 201–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hekimoğlu, B.; Aktürk Acar, F. Effects of COVID-19 pandemic period on neonatal mortality and morbidity. Pediatr. Neonatol. 2022, 63, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.; Quigley, M.A.; Placzek, A.; Knight, M.; Ladhani, S.; Draper, E.S.; Sharkey, D.; Doherty, C.; Mactier, H.; Kurinczuk, J.J. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: A prospective national cohort study using active surveillance. Lancet Child Adolesc. Health 2021, 5, 113–121. [Google Scholar] [CrossRef]




| Parameters | Study Group (N = 304) | Control Group (N = 329) | p-Value | ||
|---|---|---|---|---|---|
| M ± SD | Me (Min–Max) | M ± SD | Me (Min–Max) | ||
| Age (years) | 30.9 ± 5.0 | 31 (18–45) | 29.9 ± 5.1 | 30 (17–44) | 0.016 |
| BMI (kg/m2) | 28.8 ± 5.2 | 28 (18.3–48) | 29.2 ± 4.4 | 28 (19–45.2) | 0.170 |
| Weight (kg) | 80.7 ± 16.5 | 79 (50–139) | 80.7 ± 13.0 | 75 (53–120) | 0.832 |
| Gestational week at hospital admission (weeks) | 35.5 ± 7.1 | 38 (23–41) | 39.0 ± 1.0 | 39 (33–42) | <0.0001 |
| Comorbidities—n (%) | (N = 304) | (N = 329) | |||
| Gestational diabetes grade G1 | 38 (12.50) | 30 (9.11) | 0.019 | ||
| Gestational diabetes grade G2 | 6 (1.97) | 7 (2.13) | |||
| Hypertension | 8 (2.63) | 20 (6.08) | |||
| Hypothyroidism | 48 (15.79) | 29 (8.81) | |||
| Course of COVID-19—n (%) | (N = 304) | --- | --- | ||
| Mild | 269 (88.49) | --- | |||
| Moderate | 17 (5.59) | --- | |||
| Severe | 18 (5.92) | --- | |||
| Oxygen therapy—n (%) | (N = 304) | --- | |||
| No | 273 (89.80) | --- | |||
| Yes | 31 (10.20) | --- | |||
| Mode of delivery—n (%) | (N = 242) | (N = 329) | 0.011 | ||
| Spontaneous vaginal delivery (SVD) | 44 (18.18) | 257 (78.12) | |||
| Elective cesarean section (ECS) | 188 (77.69) | 30 (9.12) | |||
| Emergency cesarean section (EMCS) | 6 (2.48) | 29 (8.81) | |||
| Vacuum extraction (VACCUM) | 4 (1.65) | 13 (3.95) | |||
| Death during the perinatal period—n (%) | (N = 242) | (N = 329) | |||
| No | 240 (99.17) | 329 (100.00) | 0.098 | ||
| Yes | 2 (0.83) | 0 (0.00) | |||
| Parameter | Study Group (N = 304) | Control Group (N = 329) | p-Value | ||
|---|---|---|---|---|---|
| M ± SD | Me (Min–Max) | M ± SD | Me (Min–Max) | ||
| Hemoglobin (mmol/L) | 7.5 ± 0.8 | 7.6 (5.4–10.5) | 6.7 ± 0.9 | 6.8 (3.5–8.8) | 0.000 |
| Erythrocyte(T/L) | 4.1 ± 0.4 | 4.1 (2.8–5.8) | 3.7 ± 1.9 | 3.6 (1.7–4.8) | 0.000 |
| Leukocyte (G/L) | 9.7 ± 4.0 | 9.1 (3.6–34.6) | 13.3 ± 3.9 | 12.9 (4.4–42.2) | 0.000 |
| Neutrophil (%) | 74.1 ± 7.6 | 74.2 (46.9–92.6) | 75.2 ± 7.6 | 75.9 (10.1–96.3) | 0.025 |
| Fibrinogen (G/L) | 4.3 ± 1.0 | 4.2 (0.6–6.7) | 3.7 ± 0.8 | 3.7 (0.5–6.8) | 0.000 |
| APTT (seconds) | 29.1 ± 3.4 | 28.6 (22.6–44.5) | 30.8 ± 3.4 | 30.1 (20.8–53.4) | 0.000 |
| CRP (mg/L) | 22.1 ± 32.5 | 7.6 (0.6–194.5) | 9.8 ± 21.6 | 4.0 (0.4–142.3) | 0.000 |
| Procalcitonin (ng/mL) | 1.2 ± 3.7 | 0.1 (0.02–20.2) | 0.3 ± 0.5 | 0.1 (0.002–1.4) | 0.988 |
| D-dimer (µg/L) | 3244 ± 5856 | 1655 (418–41,922) | --- | --- | --- |
| Parameter | Mild Course (N = 269) | Moderate Course (N = 17) | Severe Course (N = 18) | p-Value | |||
|---|---|---|---|---|---|---|---|
| M ±SD | Me (Min–Max) | M ± SD | Me (Min–Max) | M ± SD | Me (Min–Max) | ||
| APTT | 28.18 ± 2.7 | 28 (22.6–36.4) | 32 ± 4.2 | 31 (26.1–39.9) | 32.8 ± 10 | 27.6 (26.3–44.5) | 0.020 |
| CRP | 16.0 ± 27.4 | 5.4 (0.6–194.5) | 50.2 ±30.0 | 45.5 (17.1–110.1) | 66.78 ± 42.1 | 66.78 (0.6–136) | 0.000 |
| Procalcitonin | 0.07 ± 0.08 | 0.03 (0.02–0.32) | 0.47 ± 0.4 | 0.22 (0.07–1.01) | 1.5 ± 1.8 | 1.5 (0.24–3.65) | 0.032 |
| Parameters | Study Group (N = 242) | Control Group (N = 329) | p-Value | ||
|---|---|---|---|---|---|
| M ± SD | Me (Min–Max) | M ± SD | Me (Min–Max) | ||
| Apgar 1′ | 9.3 ± 1.73 | 10 (0–10) | 9.8 ± 0.67 | 10 (5–10) | 0.000 |
| Apgar 5′ | 9.5 ± 1.74 | 10 (0–10) | 9.9 ± 0.36 | 10 (7–10) | 0.000 |
| ABG test (pH) | 7.3 ± 0.06 | 7.320 (6.68–7.45) | 7.32 ± 0.23 | 7.350 (3.370–7.500) | 0.016 |
| Pairs of Variables (N = 242) | R Spearman | t(N − 2) | p-Value |
|---|---|---|---|
| D-dimer & Apgar 1′ | 0.234294 | 1.80349 | 0.076 |
| D-dimer & Apgar 5′ | 0.174482 | 1.31415 | 0.194 |
| Fibrinogen & Apgar 1′ | 0.171710 | 1.30434 | 0.197 |
| Fibrinogen & Apgar 5′ | 0.101214 | 0.75449 | 0.453 |
| APTT & Apgar 1′ | −0.214552 | −1.64384 | 0.105 |
| APTT & Apgar 5′ | −0.229382 | −1.74774 | 0.086 |
| CRP & Apgar 1′ | −0.129546 | −1.64739 | 0.101 |
| CRP & Apgar 5′ | −0.086987 | −1.07653 | 0.283 |
| Procalcitonin & Apgar 1′ | −0.444997 | −2.16597 | 0.043 |
| Procalcitonin & Apgar 5′ | −0.344115 | −1.59753 | 0.126 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobkowski, M.; Pięta, B.; Sowińska, A.; Grabowska, M.; Koch-Brzozowska, K.; Wilczak, M.; Bień, A. SARS-CoV-2 in Pregnancy—A Retrospective Analysis of Selected Maternal and Fetal Laboratory Parameters. Int. J. Environ. Res. Public Health 2022, 19, 15307. https://doi.org/10.3390/ijerph192215307
Sobkowski M, Pięta B, Sowińska A, Grabowska M, Koch-Brzozowska K, Wilczak M, Bień A. SARS-CoV-2 in Pregnancy—A Retrospective Analysis of Selected Maternal and Fetal Laboratory Parameters. International Journal of Environmental Research and Public Health. 2022; 19(22):15307. https://doi.org/10.3390/ijerph192215307
Chicago/Turabian StyleSobkowski, Maciej, Beata Pięta, Anna Sowińska, Marlena Grabowska, Katarzyna Koch-Brzozowska, Maciej Wilczak, and Agnieszka Bień. 2022. "SARS-CoV-2 in Pregnancy—A Retrospective Analysis of Selected Maternal and Fetal Laboratory Parameters" International Journal of Environmental Research and Public Health 19, no. 22: 15307. https://doi.org/10.3390/ijerph192215307
APA StyleSobkowski, M., Pięta, B., Sowińska, A., Grabowska, M., Koch-Brzozowska, K., Wilczak, M., & Bień, A. (2022). SARS-CoV-2 in Pregnancy—A Retrospective Analysis of Selected Maternal and Fetal Laboratory Parameters. International Journal of Environmental Research and Public Health, 19(22), 15307. https://doi.org/10.3390/ijerph192215307







