Association between Perfluoroalkyl and Polyfluoroalkyl Substances and Women’s Infertility, NHANES 2013–2016
Abstract
:1. Introduction
2. Method
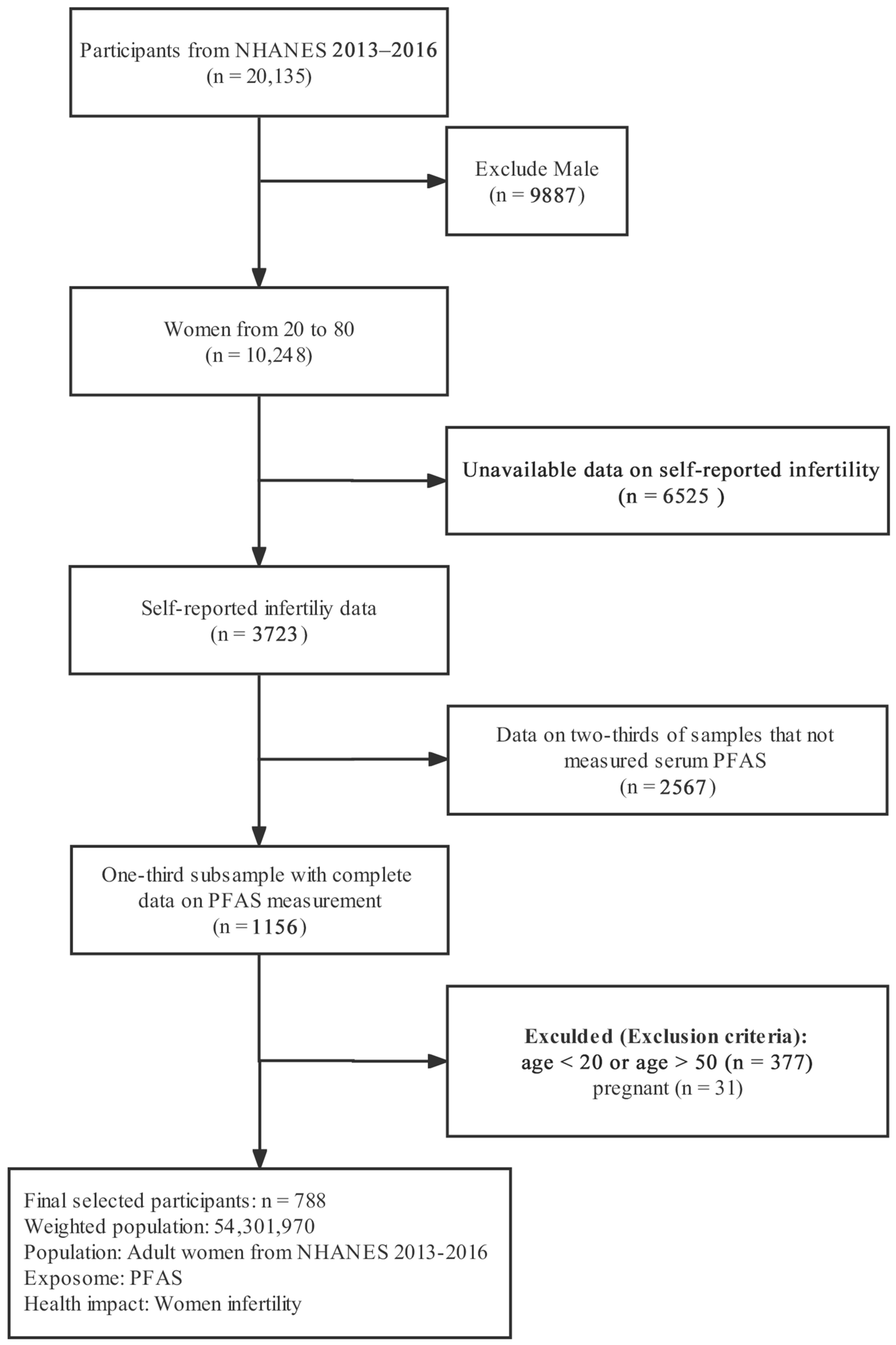
2.1. Study Design and Population
2.2. PFAS Measurement
2.3. Infertility Data
2.4. Covariates
2.5. Statistical Analysis
2.5.1. Statistical Method 1: Generalized Linear Regression Model (GLM)
2.5.2. Statistical Method 2: Generalized Additive Model (GAM)
2.5.3. Statistical Method 3: Bayesian Kernel Machine Regression (BKMR)
2.6. Stratified Analyses
3. Results
3.1. Population Characteristics
3.2. Distribution and Correlation of Serum PFAS
3.3. Using GLM to Evaluate Single PFAS Exposure
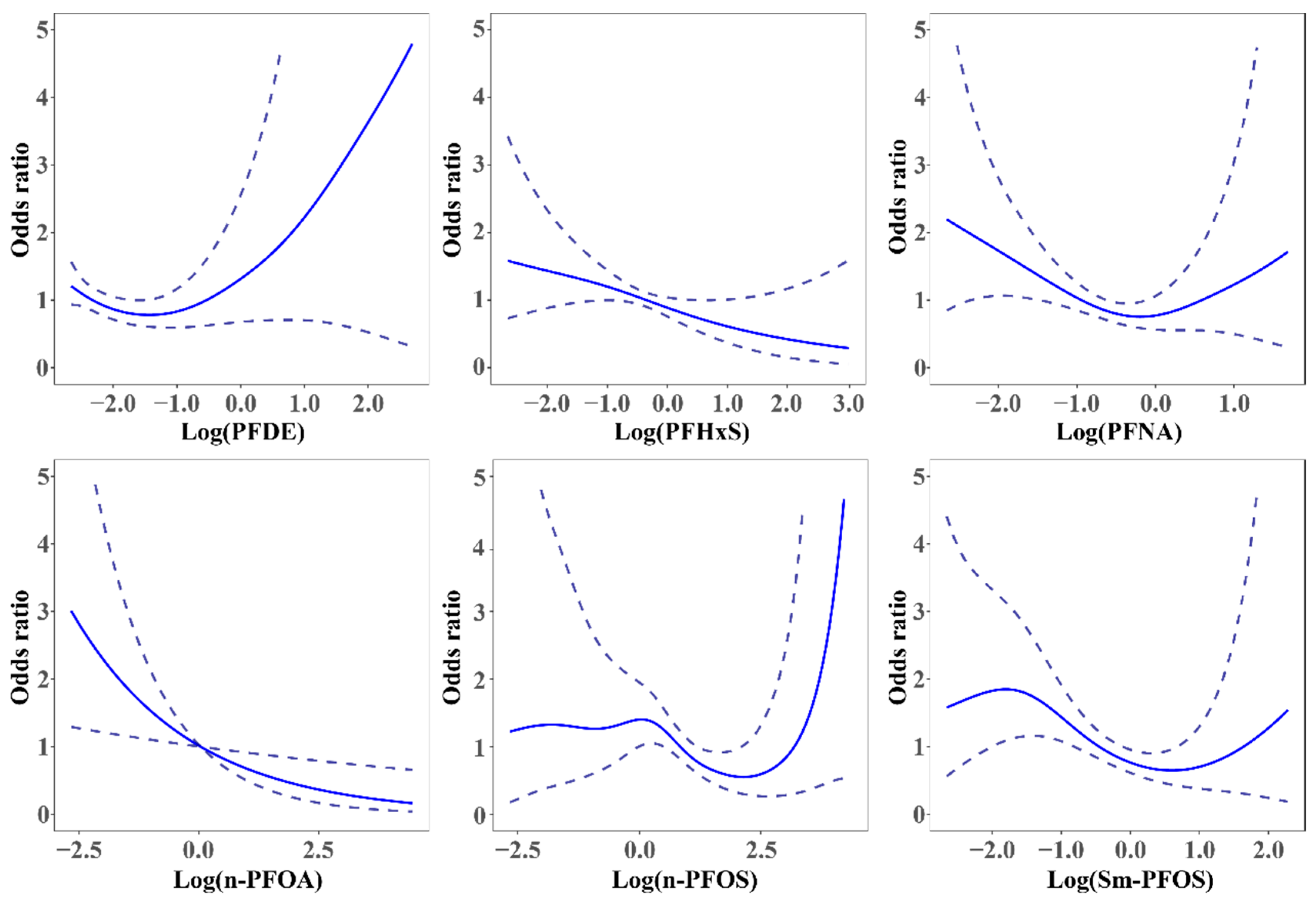
3.4. The Association between Serum PFAS and Self-Reported Infertility by the GAM
3.5. The Association between Serum PFAS and Self-Reported Infertility by the BKMR Model
3.6. Stratified Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sun, H.; Gong, T.-T.; Jiang, Y.-T.; Zhang, S.; Zhao, Y.-H.; Wu, Q.-J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging 2019, 11, 10952–10991. [Google Scholar] [CrossRef]
- Aghajanova, L.; Hoffman, J.; Mok-Lin, E.; Herndon, C.N. Obstetrics and gynecology residency and fertility needs: National survey results. Reprod. Sci. 2017, 24, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.L. Female infertility. In Yen and Jaffe’s Reproductive Endocrinology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 556–581.e7. [Google Scholar]
- Chandra, A.; Copen, C.E.; Stephen, E.H. Infertility and impaired fecundity in the United States, 1982–2010: Data from the National Survey of Family Growth. Natl. Health Stat Rep. 2013, 67, 1–18. [Google Scholar]
- Volgsten, H.; Svanberg, A.S.; Ekselius, L.; Lundkvist, Ö.; Poromaa, I.S. Risk factors for psychiatric disorders in infertile women and men undergoing in vitro fertilization treatment. Fertil. Steril. 2010, 93, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Volgsten, H.; Skoog Svanberg, A.; Ekselius, L.; Lundkvist, Ö.; Sundström Poromaa, I. Prevalence of psychiatric disorders in infertile women and men undergoing in vitro fertilization treatment. Hum. Reprod. 2008, 23, 2056–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valoriani, V.; Lotti, F.; Lari, D.; Miccinesi, G.; Vaiani, S.; Vanni, C.; Coccia, M.E.; Maggi, M.; Noci, I. Differences in psychophysical well-being and signs of depression in couples undergoing their first consultation for assisted reproduction technology (ART): An Italian pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Drosdzol, A.; Skrzypulec, V. Evaluation of marital and sexual interactions of Polish infertile couples. J. Sex. Med. 2009, 6, 3335–3346. [Google Scholar] [CrossRef]
- Iris, A.; Aydogan Kirmizi, D.; Taner, C.E. Effects of infertility and infertility duration on female sexual functions. Arch. Gynecol. Obstet. 2013, 287, 809–812. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per-and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; von Holst, H.E. Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 10900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, L.; Tong, C.; Fang, F.; Zhao, S.; Tian, Y.; Tao, Y.; Zhang, J.; Study, S.B.C. Plasma perfluoroalkyl and polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environ. Health Perspect. 2017, 125, 067012. [Google Scholar] [CrossRef]
- Zhang, S.; Tan, R.; Pan, R.; Xiong, J.; Tian, Y.; Wu, J.; Chen, L. Association of perfluoroalkyl and polyfluoroalkyl substances with premature ovarian insufficiency in Chinese women. J. Clin. Endocrinol. Metab. 2018, 103, 2543–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Ding, N.; Harlow, S.D.; Randolph, J.F.; Calafat, A.M.; Mukherjee, B.; Batterman, S.; Gold, E.B.; Park, S.K. Associations of perfluoroalkyl substances with incident natural menopause: The Study of Women’s Health Across the Nation. J. Clin. Endocrinol. Metab. 2020, 105, e3169–e3182. [Google Scholar] [CrossRef] [PubMed]
- Grønnestad, R.; Johanson, S.M.; Müller, M.H.; Schlenk, D.; Tanabe, P.; Krøkje, Å.; Jaspers, V.L.; Jenssen, B.M.; Ræder, E.M.; Lyche, J.L. Effects of an environmentally relevant PFAS mixture on dopamine and steroid hormone levels in exposed mice. Toxicol. Appl. Pharmacol. 2021, 428, 115670. [Google Scholar] [CrossRef]
- Xin, Y.; Wan, B.; Yu, B.; Fan, Y.; Chen, D.; Guo, L.H. Chlorinated Polyfluoroalkylether Sulfonic Acids Exhibit Stronger Estrogenic Effects than Perfluorooctane Sulfonate by Activating Nuclear Estrogen Receptor Pathways. Environ. Sci. Technol. 2020, 54, 3455–3464. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Jorquera, I.A.; Colli-Dula, R.C.; Kroll, K.; Jayasinghe, B.S.; Parachu Marco, M.V.; Silva-Sanchez, C.; Toor, G.S.; Denslow, N.D. Blood transcriptomics analysis of fish exposed to perfluoro alkyls substances: Assessment of a non-lethal sampling technique for advancing aquatic toxicology research. Environ. Sci. Technol. 2018, 53, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, X.; Cao, X.; Xia, Y.; Zhou, R.; Chen, L. Chronic exposure of female mice to an environmental level of perfluorooctane sulfonate suppresses estrogen synthesis through reduced histone H3K14 acetylation of the StAR promoter leading to deficits in follicular development and ovulation. Toxicol. Sci. 2015, 148, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhou, L.; Xu, J.; Zhang, L.; Li, M.; Xie, X.; Xie, Y.; Luo, D.; Zhang, D.; Yu, X. Maternal exposure to perfluorooctanoic acid inhibits luteal function via oxidative stress and apoptosis in pregnant mice. Reprod. Toxicol. 2017, 69, 159–166. [Google Scholar] [CrossRef]
- Domínguez, A.; Salazar, Z.; Arenas, E.; Betancourt, M.; Ducolomb, Y.; González-Márquez, H.; Casas, E.; Teteltitla, M.; Bonilla, E. Effect of perfluorooctane sulfonate on viability, maturation and gap junctional intercellular communication of porcine oocytes in vitro. Toxicol. In Vitro 2016, 35, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Chaparro-Ortega, A.; Betancourt, M.; Rosas, P.; Vázquez-Cuevas, F.G.; Chavira, R.; Bonilla, E.; Casas, E.; Ducolomb, Y. Endocrine disruptor effect of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on porcine ovarian cell steroidogenesis. Toxicol. In Vitro 2018, 46, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, I.; Kjellgren, J.; Persson, S.; Örn, S.; Sjunnesson, Y. Perfluorononanoic acid (PFNA) alters lipid accumulation in bovine blastocysts after oocyte exposure during in vitro maturation. Reprod. Toxicol. 2019, 84, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per-and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology 2022, 465, 153031. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Smurthwaite, K.; Aylward, L.L.; Kay, M.; Toms, L.M.; King, L.; Marrington, S.; Barnes, C.; Kirk, M.D.; Mueller, J.F.; et al. Serum concentration trends and apparent half-lives of per- and polyfluoroalkyl substances (PFAS) in Australian firefighters. Int. J. Hyg. Environ. Health 2022, 246, 114040. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Liang, H.; Tan, Y.; Chen, J.; Fei, Q.; Liu, S.; Guo, X.; Wen, L.; Wu, Y.; Jing, C. Mixed effects of perfluoroalkyl and polyfluoroalkyl substances exposure on cognitive function among people over 60 years old from NHANES. Environ. Sci. Pollut. Res. 2022, 29, 32093–32104. [Google Scholar] [CrossRef] [PubMed]
- Bulka, C.M.; Avula, V.; Fry, R.C. Associations of exposure to perfluoroalkyl substances individually and in mixtures with persistent infections: Recent findings from NHANES 1999–2016. Environ. Pollut. 2021, 275, 116619. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention; National Center for Health Statistics. NHANES 2005–2006 Public Data General Release File Documentation; U.S. Department of Health and Human Services: Hyattsville, MD, USA, 2007.
- Calafat, A.M.; Wong, L.-Y.; Kuklenyik, Z.; Reidy, J.A.; Needham, L.L. Polyfluoroalkyl chemicals in the US population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-S.; Wen, L.-L.; Chu, P.-L.; Lin, C.-Y. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013–2014. Environ. Pollut. 2018, 232, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Chen, C.; Zhang, Y.; Chen, S.; Huang, X.; Li, J.; Wang, Y.; Liu, X.; Deng, G.; Gao, J. Elevated blood mercury level has a non-linear association with infertility in US women: Data from the NHANES 2013–2016. Reprod. Toxicol. 2020, 91, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Trnka, B.; Polan, M.; Zigmont, V.A. Exposure to Di-2-ethylhexyl phthalate (DEHP) and infertility in women, NHANES 2013–2016. Reprod. Toxicol. 2021, 103, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Menken, J.; Trussell, J.; Larsen, U. Age and infertility. Science 1986, 233, 1389–1394. [Google Scholar] [CrossRef]
- Maheshwari, A.; Hamilton, M.; Bhattacharya, S. Effect of female age on the diagnostic categories of infertility. Hum. Reprod. 2008, 23, 538–542. [Google Scholar] [CrossRef] [Green Version]
- Greil, A.L.; McQuillan, J.; Shreffler, K.M.; Johnson, K.M.; Slauson-Blevins, K.S. Race-ethnicity and medical services for infertility: Stratified reproduction in a population-based sample of US women. J. Health Soc. Behav. 2011, 52, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Talmor, A.; Dunphy, B. Female obesity and infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 498–506. [Google Scholar] [CrossRef]
- Pasquali, R.; Patton, L.; Gambineri, A. Obesity and infertility. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Ryan, P.B.; Smarr, M.M.; Kannan, K.; Panuwet, P.; Dunlop, A.L.; Corwin, E.J.; Barr, D.B. Serum per-and polyfluoroalkyl substance (PFAS) concentrations and predictors of exposure among pregnant African American women in the Atlanta area, Georgia. Environ. Res. 2021, 198, 110445. [Google Scholar] [CrossRef] [PubMed]
- CDC. NHANES Tutorials—Module 3—Weighting. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx#:~:text=When%20a%20sample%20is%20weighted,represented%20by%20that%20sample%20person (accessed on 29 January 2022).
- Tao, C.; Li, Z.; Fan, Y.; Li, X.; Qian, H.; Yu, H.; Xu, Q.; Lu, C. Independent and combined associations of urinary heavy metals exposure and serum sex hormones among adults in NHANES 2013–2016. Environ. Pollut. 2021, 281, 117097. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.; Tibshirani, R. Generalized additive models for medical research. Stat. Methods Med. Res. 1995, 4, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Deng, W.; Small, R.; Schwartz, J.; Liu, J.; Shi, L. Health effects of air pollutant mixtures on overall mortality among the elderly population using Bayesian kernel machine regression (BKMR). Chemosphere 2022, 286, 131566. [Google Scholar] [CrossRef] [PubMed]
- Balasch, J. Ageing and infertility: An overview. Gynecol. Endocrinol. 2010, 26, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Dunson, D.B.; Baird, D.D.; Colombo, B. Increased infertility with age in men and women. Obstet. Gynecol. 2004, 103, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kirk, A.B.; Plasse, K.M.; Kirk, K.C.; Martin, C.F.; Ozsoy, G. Predicting Exposure to Perfluorinated Alkyl Substances (PFAS) among US Infants. Int. J. Environ. Res. Public Health 2022, 19, 8402. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, F.; Beausoleil, C.; Belcher, S.M.; Belzunces, L.P.; Emond, C.; Guerbet, M.; Rousselle, C. Non-monotonic dose-response relationships and endocrine disruptors: A qualitative method of assessment. Environ. Health 2015, 14, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, F.R.; Rajaobelina, K.; Praud, D.; Dow, C.; Antignac, J.P.; Kvaskoff, M.; Severi, G.; Bonnet, F.; Boutron-Ruault, M.-C.; Fagherazzi, G. Nonlinear associations between dietary exposures to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and type 2 diabetes risk in women: Findings from the E3N cohort study. Int. J. Hyg. Environ. Health 2018, 221, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, H.B.; Larose, T.L.; Øien, T.; Sandanger, T.M.; Odland, J.Ø.; Van De Bor, M.; Jacobsen, G.W. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: A prospective cohort study. Environ. Health 2018, 17, 9. [Google Scholar] [CrossRef] [Green Version]
- Park, S.K.; Ding, N.; Han, D. Perfluoroalkyl substances and cognitive function in older adults: Should we consider non-monotonic dose-responses and chronic kidney disease? Environ. Res. 2021, 192, 110346. [Google Scholar] [CrossRef]
- Jain, R.B.; Ducatman, A. Perfluoroalkyl substances follow inverted U-shaped distributions across various stages of glomerular function: Implications for future research. Environ. Res. 2019, 169, 476–482. [Google Scholar] [CrossRef]
- Barrett, E.S.; Chen, C.; Thurston, S.W.; Haug, L.S.; Sabaredzovic, A.; Fjeldheim, F.N.; Frydenberg, H.; Lipson, S.F.; Ellison, P.T.; Thune, I. Perfluoroalkyl substances and ovarian hormone concentrations in naturally cycling women. Fertil. Steril. 2015, 103, 1261–1270.e3. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Zhang, H.; Ding, L.; Feng, Y.; Xu, M.; Dai, J. The effect of perfluorododecanonic acid on endocrine status, sex hormones and expression of steroidogenic genes in pubertal female rats. Reprod. Toxicol. 2009, 27, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Weng, X.; Liu, S.; Chen, J.; Guo, X.; Gao, X.; Fei, Q.; Hao, G.; Jing, C.; Feng, L. Perfluoroalkyl and polyfluoroalkyl substance exposure and association with sex hormone concentrations: Results from the NHANES 2015–2016. Environ. Sci. Eur. 2021, 33, 69. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, Y.S.; Haslam, S.Z.; Yang, C. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol. Sci. 2010, 115, 214–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benninghoff, A.D.; Bisson, W.H.; Koch, D.C.; Ehresman, D.J.; Kolluri, S.K.; Williams, D.E. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol. Sci. 2011, 120, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Tilton, S.C.; Orner, G.A.; Benninghoff, A.D.; Carpenter, H.M.; Hendricks, J.D.; Pereira, C.B.; Williams, D.E. Genomic profiling reveals an alternate mechanism for hepatic tumor promotion by perfluorooctanoic acid in rainbow trout. Environ. Health Perspect. 2008, 116, 1047–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, N.; Harlow, S.D.; Randolph, J.F., Jr.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.S.; Jackson, T.; Javins, B.; Frisbee, S.J.; Shankar, A.; Ducatman, A.M. Implications of early menopause in women exposed to perfluorocarbons. J. Clin. Endocrinol. Metab. 2011, 96, 1747–1753. [Google Scholar] [CrossRef] [Green Version]
- Du, G.; Hu, J.; Huang, H.; Qin, Y.; Han, X.; Wu, D.; Song, L.; Xia, Y.; Wang, X. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ. Toxicol. Chem. 2013, 32, 353–360. [Google Scholar] [CrossRef]
- Gleicher, N.; Brown, T.; Dudkiewicz, A.; Karande, V.; Rao, R.; Balin, M.; Campbell, D.; Pratt, D. Estradiol/progesterone substitution in the luteal phase improves pregnancy rates in stimulated cycles—But only in younger women. Early Pregnancy 2000, 4, 64–73. [Google Scholar]
- Hutchinson-Williams, K.A.; Lunenfeld, B.; Diamond, M.P.; Lavy, G.; Boyers, S.P.; DeCherney, A.H. Human chorionic gonadotropin, estradiol, and progesterone profiles in conception and nonconception cycles in an in vitro fertilization program. Fertil. Steril. 1989, 52, 441–445. [Google Scholar] [CrossRef]
- Pizarro, B.M.; Cordeiro, A.; Reginatto, M.W.; Campos, S.P.; Mancebo, A.C.A.; Areas, P.C.; Antunes, R.A.; Souza, M.d.C.B.; Oliveira, K.J.; Bloise, F.F. Estradiol and progesterone levels are related to redox status in the follicular fluid during in vitro fertilization. J. Endocr. Soc. 2020, 4, bvaa064. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Suzuki, A.; Buchanan, D.L.; Katsu, Y.; Watanabe, H.; Iguchi, T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod. Toxicol. 2002, 16, 117–122. [Google Scholar] [CrossRef]
- Takai, Y.; Tsutsumi, O.; Ikezuki, Y.; Kamei, Y.; Osuga, Y.; Yano, T.; Taketan, Y. Preimplantation exposure to bisphenol A advances postnatal development. Reprod. Toxicol. 2000, 15, 71–74. [Google Scholar] [CrossRef]
- Cha, S.; Jung, K.; Lee, M.Y.; Hwang, Y.J.; Yang, E.; Lee, S.-H.; Jung, H.-i.; Cheon, Y.-P. Nonmonotonic effects of chronic low-dose di (2-ethylhexyl) phthalate on gonadal weight and reproductive. Dev. Reprod. 2018, 22, 85. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhou, W.; Wu, S.; Liang, F.; Li, Y.; Zhang, J.; Cui, L.; Feng, Y.; Wang, Y. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ. Pollut. 2019, 247, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.T.; Specht, I.O.; Lenters, V.; Bach, C.C.; Rylander, L.; Jönsson, B.A.; Lindh, C.H.; Giwercman, A.; Heederik, D.; Toft, G. Perfluoroalkyl substances and time to pregnancy in couples from Greenland, Poland and Ukraine. Environ. Health 2014, 13, 116. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhang, R.; Jin, F.; Lou, H.; Mao, Y.; Zhu, W.; Zhou, W.; Zhang, P.; Zhang, J. Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environ. Int. 2017, 102, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N. Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol a as a case study. Dose-Response 2014, 12, 259–276. [Google Scholar] [CrossRef]
- Lucier, G.W. Dose-response relationships for endocrine disruptors: What we know and what we don’t know. Regul. Toxicol. Pharmacol. RTP 1997, 26 Pt 1, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. The frequency of U-shaped dose responses in the toxicological literature. Toxicol. Sci. 2001, 62, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol. Sci. 2001, 22, 285–291. [Google Scholar] [CrossRef]
- Dunson, D.B.; Colombo, B.; Baird, D.D. Changes with age in the level and duration of fertility in the menstrual cycle. Hum. Reprod. 2002, 17, 1399–1403. [Google Scholar] [CrossRef]
- Liu, K.; Case, A.; Cheung, A.P.; Sierra, S.; AlAsiri, S.; Carranza-Mamane, B.; Dwyer, C.; Graham, J.; Havelock, J.; Hemmings, R. Advanced reproductive age and fertility. J. Obstet. Gynaecol. Can. 2011, 33, 1165–1175. [Google Scholar] [CrossRef]
- Prior, J.C. Ovarian aging and the perimenopausal transition. Endocrine 2005, 26, 297–300. [Google Scholar] [CrossRef]
- Tsai, M.-s.; Chang, S.-H.; Kuo, W.-H.; Kuo, C.-H.; Li, S.-Y.; Wang, M.-Y.; Chang, D.-Y.; Lu, Y.-S.; Huang, C.-S.; Cheng, A.-L. A case-control study of perfluoroalkyl substances and the risk of breast cancer in Taiwanese women. Environ. Int. 2020, 142, 105850. [Google Scholar] [CrossRef]
- Kim, Y.R.; White, N.; Bräunig, J.; Vijayasarathy, S.; Mueller, J.F.; Knox, C.L.; Harden, F.A.; Pacella, R.; Toms, L.-M.L. Per-and poly-fluoroalkyl substances (PFASs) in follicular fluid from women experiencing infertility in Australia. Environ. Res. 2020, 190, 109963. [Google Scholar] [CrossRef]
- Lum, K.J.; Sundaram, R.; Barr, D.B.; Louis, T.A.; Louis, G.M.B. Perfluoroalkyl chemicals, menstrual cycle length, and fecundity: Findings from a prospective pregnancy study. Epidemiology 2017, 28, 90. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; McLaughlin, J.K.; Lipworth, L.; Olsen, J. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod. 2009, 24, 1200–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.; Raza, M.; Pollack, A.Z. Perfluoroalkyl substances and endometriosis in US women in NHANES 2003–2006. Reprod. Toxicol. 2016, 65, 230–235. [Google Scholar] [CrossRef]



| Exposure | LOD (ng/mL) b | N (%) of Below LOD | Total | Infertility | p-Value | |
|---|---|---|---|---|---|---|
| No | Yes | |||||
| N | 788 | 682 | 106 | |||
| Individual PFAS (ng/mL) | ||||||
| PFDE, Median [IQR] | 0.10 | 32.73% | 0.10 [0.07, 0.20] | 0.10 [0.07, 0.20] | 0.10 [0.07, 0.20] | 0.620 |
| PFHxS, Median [IQR] | 0.10 | 1.92% | 0.60 [0.40, 1.02] | 0.60 [0.40, 1.10] | 0.60 [0.30, 0.80] | 0.078 |
| PFNA, Median [IQR] | 0.10 | 1.72% | 0.50 [0.30, 0.80] | 0.50 [0.30, 0.80] | 0.40 [0.30, 0.70] | 0.184 |
| n-PFOA, Median [IQR] | 0.10 | 0.79% | 1.10 [0.70, 1.60] | 1.10 [0.70, 1.60] | 0.90 [0.60, 1.50] | 0.083 |
| n-PFOS, Median [IQR] | 0.10 | 0.72% | 2.20 [1.30, 3.50] | 2.20 [1.30, 3.58] | 1.85 [1.30, 3.08] | 0.066 |
| Sm-PFOS, Median [IQR] | 0.10 | 1.37% | 0.70 [0.40, 1.10] | 0.70 [0.40, 1.20] | 0.55 [0.32, 1.10] | 0.041 * |
| Total PFAS (ng/mL) | ||||||
| ∑PFOS, Median [IQR] | - | - | 1.17 [0.77, 1.70] | 1.17 [0.77, 1.70] | 0.97 [0.67, 1.59] | 0.081 |
| PFAS | Quartile1 | Model 1 OR (95% CI) | p-Value | Model 2 OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Individual PFAS | |||||
| PFDE | Quartile1 | Ref. | Ref. | ||
| Quartile2 | 0.674 (0.297, 1.533) | 0.335 | 0.738 (0.292, 1.862) | 0.507 | |
| Quartile3 | 0.582 (0.283, 1.198) | 0.136 | 0.541 (0.232, 1.262) | 0.149 | |
| Quartile4 | 0.886 (0.463, 1.694) | 0.705 | 0.776 (0.414, 1.453) | 0.415 | |
| p-t | 0.429 | 0.236 | |||
| PFHxS | Quartile1 | Ref. | Ref. | ||
| Quartile2 | 0.496 (0.198, 1.242) | 0.129 | 0.442 (0.185, 1.054) | 0.065 | |
| Quartile3 | 1.151 (0.591, 2.241) | 0.670 | 0.987 (0.481, 2.025) | 0.97 | |
| Quartile4 | 0.532 (0.253, 1.118) | 0.093 | 0.532 (0.236, 1.199) | 0.123 | |
| p-t | 0.295 | 0.337 | |||
| PFNA | Quartile1 | Ref. | Ref. | ||
| Quartile2 | 0.682 (0.316, 1.474) | 0.318 | 0.660 (0.297, 1.467) | 0.297 | |
| Quartile3 | 0.537 (0.252, 1.144) | 0.104 | 0.430 (0.214, 0.860) | 0.019 * | |
| Quartile4 | 0.650 (0.278, 1.520) | 0.309 | 0.580 (0.252, 1.331) | 0.190 | |
| p-t | 0.218 | 0.098 | |||
| n-PFOA | Quartile1 | Ref. | Ref. | ||
| Quartile2 | 0.785 (0.471, 1.310) | 0.342 | 0.664 (0.390, 1.131) | 0.127 | |
| Quartile3 | 0.509 (0.296, 0.877) | 0.017 * | 0.396 (0.199, 0.788) | 0.010 * | |
| Quartile4 | 0.502 (0.240, 1.046) | 0.065 | 0.380 (0.172, 0.842) | 0.019 * | |
| p-t | 0.035 * | 0.013 * | |||
| n-PFOS | Quartile1 | Ref. | Ref. | ||
| Quartile2 | 1.440 (0.669, 3.102) | 0.339 | 1.819 (0.930, 3.557) | 0.079 | |
| Quartile3 | 0.791 (0.367, 1.704) | 0.537 | 0.773 (0.358, 1.670) | 0.500 | |
| Quartile4 | 0.660 (0.330 1.323) | 0.232 | 0.589 (0.288, 1.204) | 0.141 | |
| p-t | 0.111 | 0.032 * | |||
| Sm-PFOS | Quartile1 | Ref. | Ref. | ||
| Quartile2 | 0.816 (0.391, 1.699) | 0.575 | 0.780 (0.342, 1.778) | 0.543 | |
| Quartile3 | 1.006 (0.502, 2.015) | 0.986 | 0.861 (0.426, 1.739) | 0.667 | |
| Quartile4 | 0.648 (0.317, 1.325) | 0.225 | 0.461 (0.200, 1.062) | 0.068 | |
| p-t | 0.301 | 0.076 | |||
| Total PFAS | |||||
| ∑PFOS | Quartile1 | Ref. | Ref. | ||
| Quartile2 | 1.06 (0.59, 1.902) | 0.841 | 1.303 (0.762, 2.229) | 0.321 | |
| Quartile3 | 0.563 (0.279, 1.138) | 0.106 | 0.539 (0.261, 1.113) | 0.092 | |
| Quartile4 | 0.677 (0.348, 1.317) | 0.240 | 0.557 (0.281, 1.104) | 0.091 | |
| p-t | 0.127 | 0.032 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Zeng, Z.; Liang, H.; Weng, X.; Yao, H.; Fu, Y.; Li, Y.; Chen, J.; Wei, X.; Jing, C. Association between Perfluoroalkyl and Polyfluoroalkyl Substances and Women’s Infertility, NHANES 2013–2016. Int. J. Environ. Res. Public Health 2022, 19, 15348. https://doi.org/10.3390/ijerph192215348
Tan Y, Zeng Z, Liang H, Weng X, Yao H, Fu Y, Li Y, Chen J, Wei X, Jing C. Association between Perfluoroalkyl and Polyfluoroalkyl Substances and Women’s Infertility, NHANES 2013–2016. International Journal of Environmental Research and Public Health. 2022; 19(22):15348. https://doi.org/10.3390/ijerph192215348
Chicago/Turabian StyleTan, Yuxuan, Zurui Zeng, Huanzhu Liang, Xueqiong Weng, Huojie Yao, Yingyin Fu, Yexin Li, Jingmin Chen, Xiangcai Wei, and Chunxia Jing. 2022. "Association between Perfluoroalkyl and Polyfluoroalkyl Substances and Women’s Infertility, NHANES 2013–2016" International Journal of Environmental Research and Public Health 19, no. 22: 15348. https://doi.org/10.3390/ijerph192215348
APA StyleTan, Y., Zeng, Z., Liang, H., Weng, X., Yao, H., Fu, Y., Li, Y., Chen, J., Wei, X., & Jing, C. (2022). Association between Perfluoroalkyl and Polyfluoroalkyl Substances and Women’s Infertility, NHANES 2013–2016. International Journal of Environmental Research and Public Health, 19(22), 15348. https://doi.org/10.3390/ijerph192215348





