Impact of Voltage Application on Degradation of Biorefractory Pharmaceuticals in an Anaerobic–Aerobic Coupled Upflow Bioelectrochemical Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactor Configurations
2.2. Bacterial Inoculate and Reactors Operation
2.3. Chemicals and Analytical Methods
2.4. Bacterial Community Analysis
2.5. Statistical Analysis
3. Results
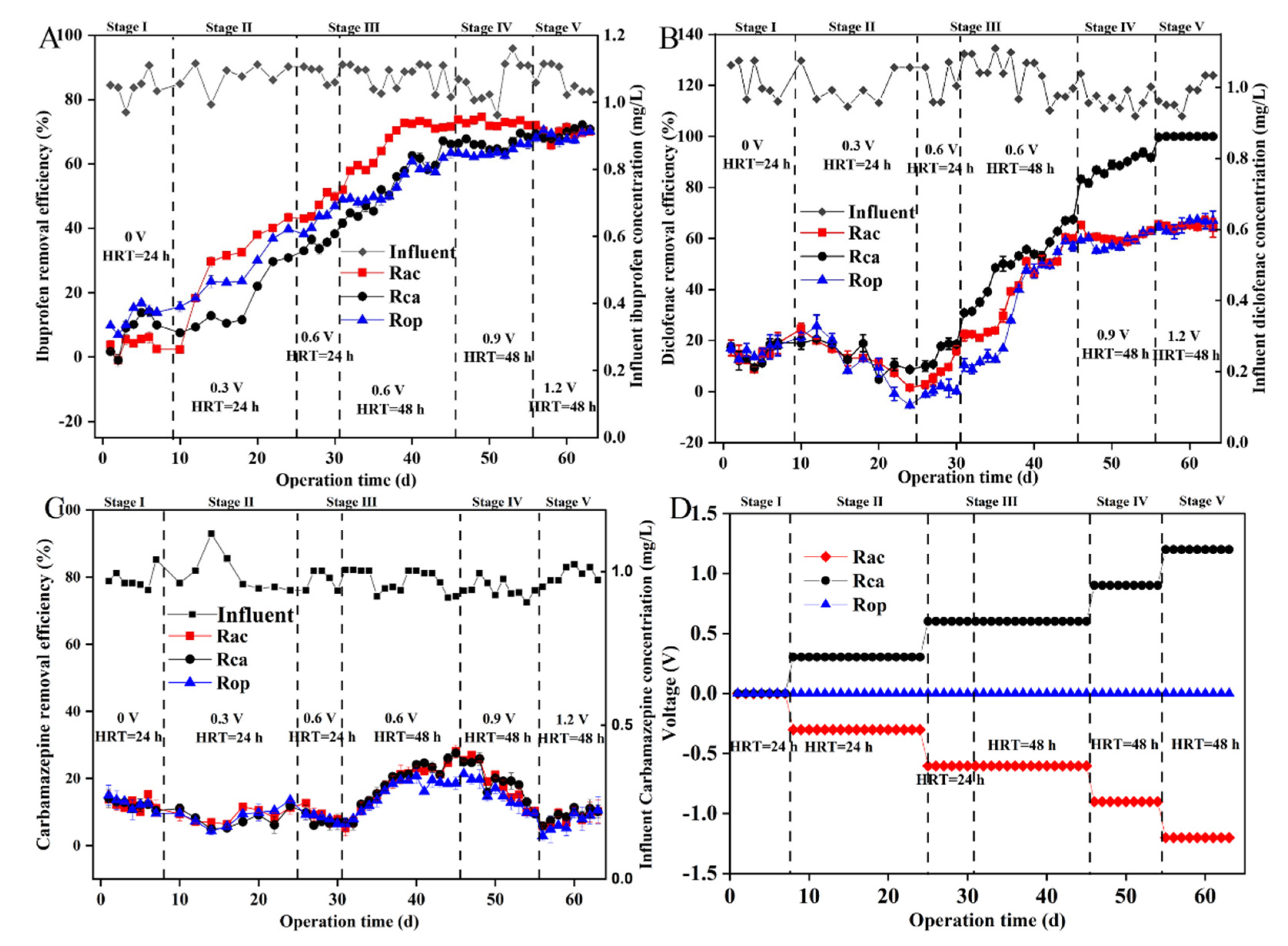
3.1. The Overall Elimination Efficiency of the Coupled System
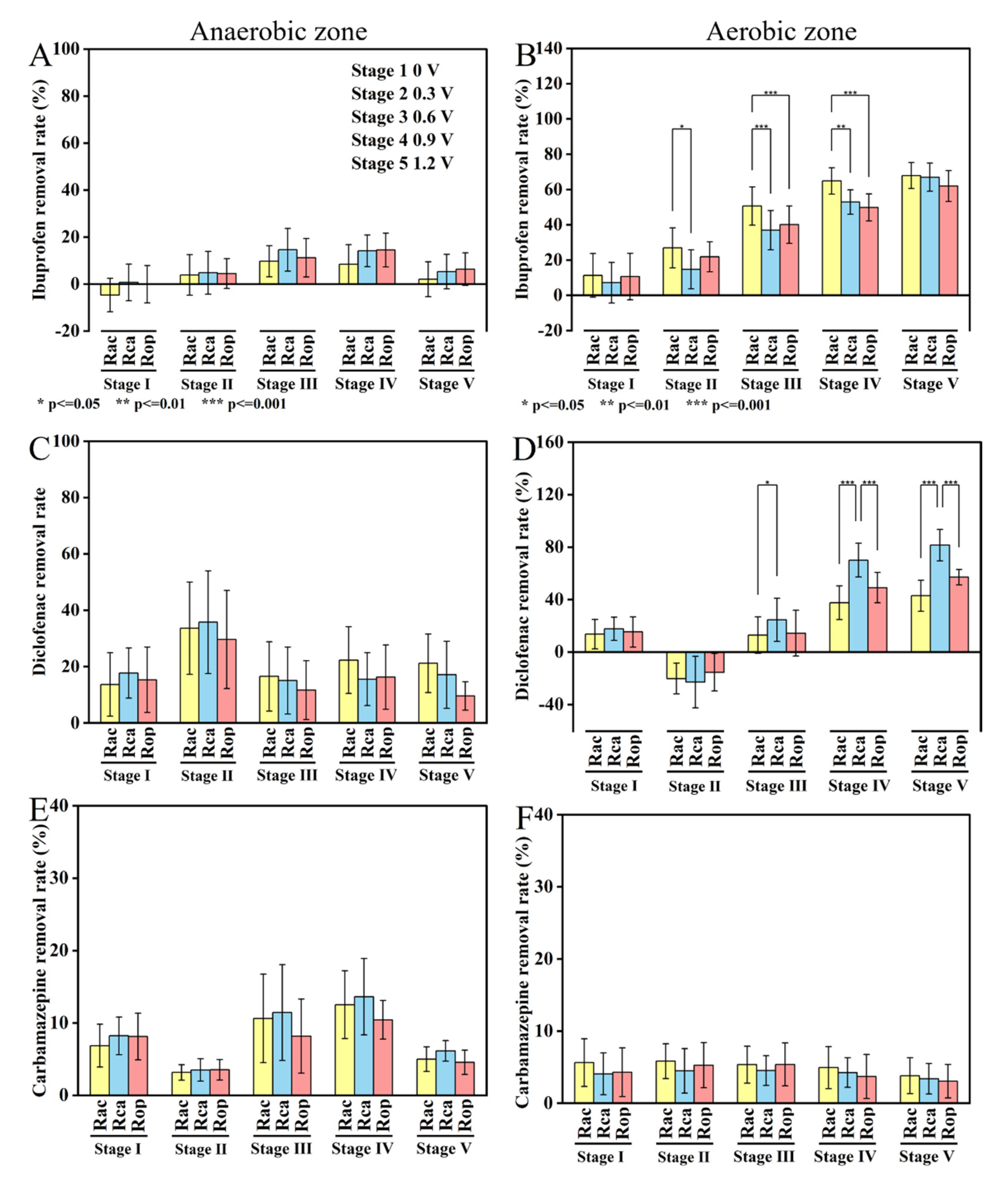
3.2. Pharmaceutical Removal in Cathodic and Anodic Chambers
3.3. Effect of Intermittent Supply on Bacterial Communities
3.3.1. Total Bacteria Quantity
3.3.2. Analysis of Bacterial Diversity
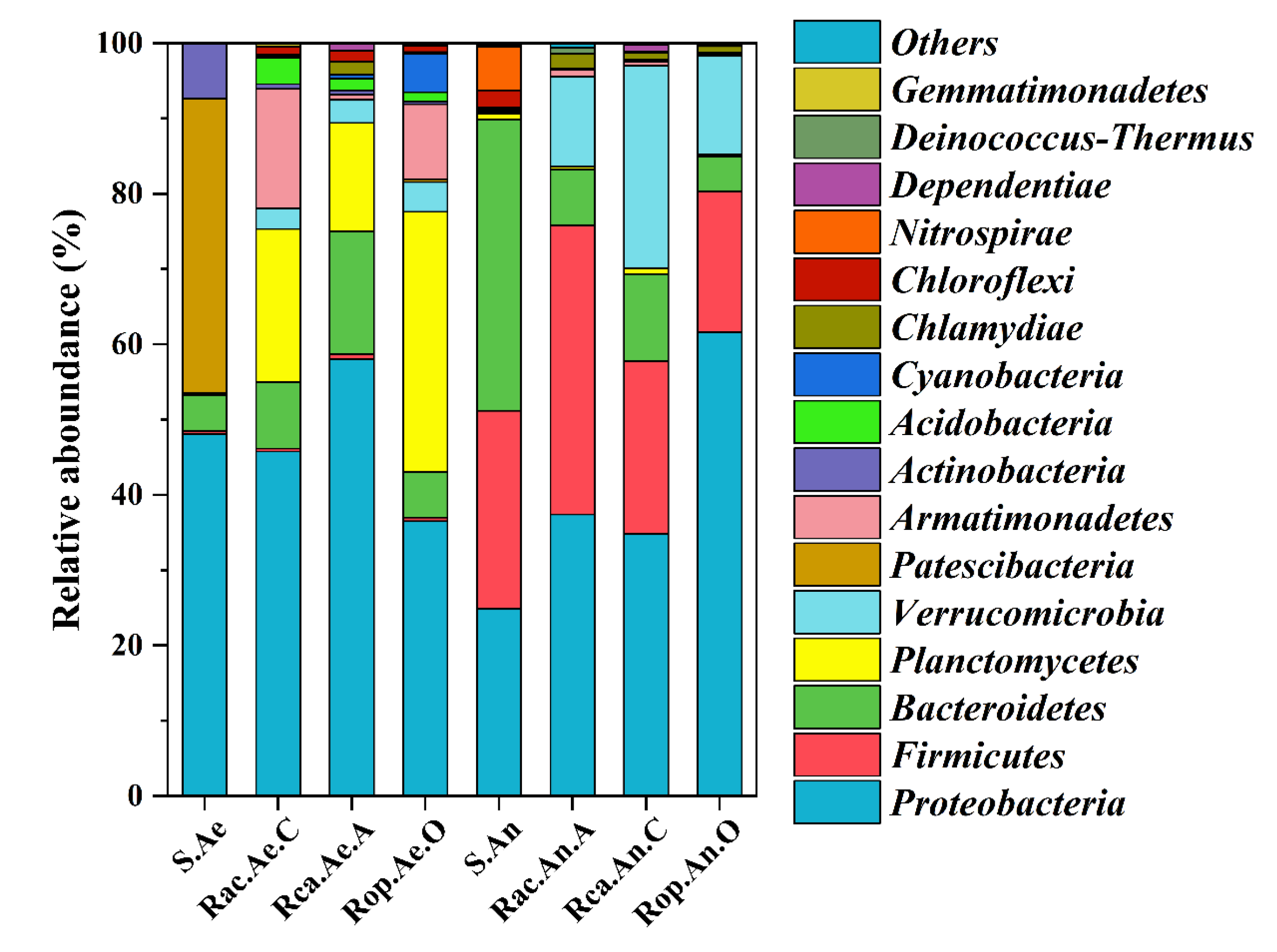
3.3.3. Bacterial Community Structure Analysis
4. Discussion
4.1. Operational Performance of the AO-UBERs
4.2. Effect of Electrical Stimulation on Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajibola, A.S.; Fawole, S.T.; Ajibola, F.O.; Adewuyi, G.O. Diclofenac and Ibuprofen Determination in Sewage Sludge Using a QuEChERS Approach: Occurrence and Ecological Risk Assessment in Three Nigerian Wastewater Treatment Plants. Bull. Environ. Contam. Toxicol. 2021, 106, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Batucan, N.S.P.; Tremblay, L.A.; Northcott, G.L.; Matthaei, C.D. Medicating the environment? A critical review on the risks of carbamazepine, diclofenac and ibuprofen to aquatic organisms. Environ. Adv. 2022, 7, 100164. [Google Scholar] [CrossRef]
- Valcárcel, Y.; Alonso, S.G.; Rodríguez-Gil, J.; Maroto, R.R.; Gil, A.; Catalá, M. Analysis of the presence of cardiovascular and analgesic/anti-inflammatory/antipyretic pharmaceuticals in river-and drinking-water of the Madrid Region in Spain. Chemosphere 2010, 82, 1062–1071. [Google Scholar] [CrossRef]
- Wu, J.; Shi, D.; Wang, S.; Yang, X.; Zhang, H.; Zhang, T.; Zheng, L.; Zhang, Y. Derivation of Water Quality Criteria for Carbamazepine and Ecological Risk Assessment in the Nansi Lake Basin. Int. J. Environ. Res. Public Health 2022, 19, 10875. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Li, Y.; Wang, Y.; Chen, W.; Wang, H.; Chen, X.; Wu, W.; Huang, Z.; Yu, C.-P.; Sun, Q. Occurrence, fate, and mass balance of different classes of pharmaceuticals and personal care products in an anaerobic-anoxic-oxic wastewater treatment plant in Xiamen, China. Water Res. 2017, 123, 655–667. [Google Scholar] [CrossRef]
- Zhang, K.; Yuan, G.; Werdich, A.A.; Zhao, Y. Ibuprofen and diclofenac impair the cardiovascular development of zebrafish (Danio rerio) at low concentrations. Environ. Pollut. 2020, 258, 113613. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef]
- Sharma, J.; Joshi, M.; Bhatnagar, A.; Chaurasia, A.K.; Nigam, S. Pharmaceutical residues: One of the significant problems in achieving ‘clean water for all’ and its solution. Environ. Res. 2022, 215, 114219. [Google Scholar] [CrossRef]
- Mansour, F.; Al-Hindi, M.; Yahfoufi, R.; Ayoub, G.M.; Ahmad, M.N. The use of activated carbon for the removal of pharmaceuticals from aqueous solutions: A review. Rev. Environ. Sci. Bio/Technol. 2018, 17, 109–145. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Yan, W.; Xiao, Y.; Yan, W.; Ding, R.; Wang, S.; Zhao, F. The effect of bioelectrochemical systems on antibiotics removal and antibiotic resistance genes: A review. Chem. Eng. J. 2018, 358, 1421–1437. [Google Scholar] [CrossRef]
- Yang, X.-L.; Zhang, S.; Li, H.; Zhang, L.-M.; Song, H.-L.; Wang, Y.-W. Effects of voltage on sulfadiazine degradation and the response of sul genes and microbial communities in biofilm-electrode reactors. Ecotoxicol. Environ. Saf. 2018, 151, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yun, H.; Cui, D.; Qi, M.; Shao, C.; Cui, D.; Ren, N.; Liang, B.; Wang, A. Response of antimicrobial nitrofurazone-degrading biocathode communities to different cathode potentials. Bioresour. Technol. 2017, 241, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, J.; Zhang, Y.; Liu, W.; Wang, A. Exerting applied voltage promotes microbial activity of marine anammox bacteria for nitrogen removal in saline wastewater treatment. Water Res. 2022, 215, 118285. [Google Scholar] [CrossRef]
- Chen, M.; Ren, L.; Qi, K.; Li, Q.; Lai, M.; Li, Y.; Li, X.; Wang, Z. Enhanced removal of pharmaceuticals and personal care products from real municipal wastewater using an electrochemical membrane bioreactor. Bioresour. Technol. 2020, 311, 123579. [Google Scholar] [CrossRef]
- De Vrieze, J.; Gildemyn, S.; Arends, J.B.; Vanwonterghem, I.; Verbeken, K.; Boon, N.; Verstraete, W.; Tyson, G.W.; Hennebel, T.; Rabaey, K. Biomass retention on electrodes rather than electrical current enhances stability in anaerobic digestion. Water Res. 2014, 54, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Mishra, S. Complete biodegradation of azo dye in an integrated microbial fuel cell-aerobic system using novel bacterial consortium. Int. J. Environ. Sci. Technol. 2018, 16, 1069–1078. [Google Scholar] [CrossRef]
- Jiang, X.; Shen, J.; Han, Y.; Lou, S.; Han, W.; Sun, X.; Li, J.; Mu, Y.; Wang, L. Efficient nitro reduction and dechlorination of 2,4-dinitrochlorobenzene through the integration of bioelectrochemical system into upflow anaerobic sludge blanket: A comprehensive study. Water Res. 2016, 88, 257–265. [Google Scholar] [CrossRef]
- Ailijiang, N.; Chang, J.; Liang, P.; Zhang, X.; Huang, X. Electrical stimulation on biodegradation of phenolics in a novel anaerobic–aerobic-coupled upflow bioelectrochemical reactor. Chem. Eng. J. 2021, 421, 127840. [Google Scholar] [CrossRef]
- Wang, W.; Han, H.; Yuan, M.; Li, H.; Fang, F.; Wang, K. Treatment of coal gasification wastewater by a two-continuous UASB system with step-feed for COD and phenols removal. Bioresour. Technol. 2011, 102, 5454–5460. [Google Scholar] [CrossRef]
- Martínez-Alcalá, I.; Guillén-Navarro, J.M.; Lahora, A. Occurrence and fate of pharmaceuticals in a wastewater treatment plant from southeast of Spain and risk assessment. J. Environ. Manag. 2020, 279, 111565. [Google Scholar] [CrossRef] [PubMed]
- Ailijiang, N.; Chang, J.; Liang, P.; Li, P.; Wu, Q.; Zhang, X.; Huang, X. Electrical stimulation on biodegradation of phenol and responses of microbial communities in conductive carriers supported biofilms of the bioelectrochemical reactor. Bioresour. Technol. 2016, 201, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Q.; Sidike, A.; Ailijiang, N.; Mamat, A.; Zhang, G.; Pu, M.; Cheng, W.; Pang, Z. The impact of different voltage application modes on biodegradation of chloramphenicol and shift of microbial community structure. Front. Environ. Sci. Eng. 2022, 16, 141. [Google Scholar] [CrossRef]
- Ailijiang, N.; Chang, J.; Liang, P.; Zhang, X.; Huang, X. Impact of electrical stimulation modes on the degradation of refractory phenolics and the analysis of microbial communities in an anaerobic-aerobic-coupled upflow bioelectrochemical reactor. Bioresour. Technol. 2020, 320, 124371. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Su, C.; Chen, Z.; Gong, T.; Lu, X.; Chen, Z.; Lin, X. Effect of hydraulic retention time on the denitrification performance and metabolic mechanism of a multi-chambered bio-electrochemical system. J. Environ. Manag. 2021, 299, 113575. [Google Scholar] [CrossRef] [PubMed]
- Escapa, A.; Gil-Carrera, L.; García, V.; Morán, A. Performance of a continuous flow microbial electrolysis cell (MEC) fed with domestic wastewater. Bioresour. Technol. 2012, 117, 55–62. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, H.; Sun, S.; Li, Z. Cooperative denitrification in biocathodes under low carbon to nitrogen ratio conditions coupled with simultaneous degradation of ibuprofen in photoanodes. Bioresour. Technol. 2022, 351, 126988. [Google Scholar] [CrossRef]
- Feng, Y.; Long, Y.; Wang, Z.; Wang, X.; Shi, N.; Suo, N.; Shi, Y.; Yu, Y. Performance and microbial community of an electric biological integration reactor (EBIR) for treatment of wastewater containing ibuprofen. Bioresour. Technol. 2018, 274, 447–458. [Google Scholar] [CrossRef]
- Fatehifar, M.; Borghei, S.M.; Nia, A.E. Application of moving bed biofilm reactor in the removal of pharmaceutical compounds (diclofenac and ibuprofen). J. Environ. Chem. Eng. 2018, 6, 5530–5535. [Google Scholar] [CrossRef]
- Rago, L.; Cristiani, P.; Villa, F.; Zecchin, S.; Colombo, A.; Cavalca, L.; Schievano, A. Influences of dissolved oxygen concentration on biocathodic microbial communities in microbial fuel cells. Bioelectrochemistry 2017, 116, 39–51. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, M.; Yuan, Y.; Zhang, P.; Du, S.; Ya, T.; Chen, D.; Wang, X.; Zhang, T. Responses of microbial communities and their interactions to ibuprofen in a bio-electrochemical system. J. Environ. Manag. 2021, 289, 112473. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Kang, J.; Xia, S.; Zhang, Z.; Price, W.E. Removal and fate of micropollutants in a sponge-based moving bed bioreactor. Bioresour. Technol. 2014, 159, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Hartl, M.; García-Galán, M.J.; Matamoros, V.; Fernández-Gatell, M.; Rousseau, D.P.; Du Laing, G.; Garfí, M.; Puigagut, J. Constructed wetlands operated as bioelectrochemical systems for the removal of organic micropollutants. Chemosphere 2021, 271, 129593. [Google Scholar] [CrossRef] [PubMed]
- Daghio, M.; Tofalos, A.E.; Leoni, B.; Cristiani, P.; Papacchini, M.; Jalilnejad, E.; Bestetti, G.; Franzetti, A. Bioelectrochemical BTEX removal at different voltages: Assessment of the degradation and characterization of the microbial communities. J. Hazard. Mater. 2018, 341, 120–127. [Google Scholar] [CrossRef]
- Liu, X.; Liang, C.; Liu, X.; Lu, S.; Xi, B. Intensified pharmaceutical and personal care products removal in an electrolysis-integrated tidal flow constructed wetland. Chem. Eng. J. 2020, 394, 124860. [Google Scholar] [CrossRef]
- Qiu, B.; Hu, Y.; Tang, C.; Chen, Y.; Cheng, J. Degradation of diclofenac via sequential reduction-oxidation by Ru/Fe modified biocathode dual-chamber bioelectrochemical system: Performance, pathways and degradation mechanisms. Chemosphere 2021, 291, 132881. [Google Scholar] [CrossRef]
- Qiu, B.; Hu, Y.; Liang, C.; Wang, L.; Shu, Y.; Chen, Y.; Cheng, J. Enhanced degradation of diclofenac with Ru/Fe modified anode microbial fuel cell: Kinetics, pathways and mechanisms. Bioresour. Technol. 2019, 300, 122703. [Google Scholar] [CrossRef]
- Chtourou, M.; Mallek, M.; Dalmau, M.; Mamo, J.; Santos-Clotas, E.; Ben Salah, A.; Walha, K.; Salvado, V.; Monclús, H. Triclosan, carbamazepine and caffeine removal by activated sludge system focusing on membrane bioreactor. Process Saf. Environ. Prot. 2018, 118, 1–9. [Google Scholar] [CrossRef]
- Hai, F.I.; Li, X. Removal of carbamazepine and sulfamethoxazole by MBR under anoxic and aerobic conditions. Bioresour. Technol. 2011, 102, 10386–10390. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, B.S.; Dhar, B.R. An intermittent power supply scheme to minimize electrical energy input in a microbial electrolysis cell assisted anaerobic digester. Bioresour. Technol. 2020, 319, 124109. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Jiang, X.; Su, G.; Han, W.; Sun, X.; Li, J.; Mu, Y.; Wang, L. Simultaneous debromination and mineralization of bromophenol in an up-flow electricity-stimulated anaerobic system. Water Res. 2019, 157, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.; Li, K.; Zhou, H. Effects of increasing organic loading rate on performance and microbial community shift of an up-flow anaerobic sludge blanket reactor treating diluted pharmaceutical wastewater. J. Biosci. Bioeng. 2014, 118, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.; Sellamuthu, B.; Piché-Choquette, S.; Drogui, P.; Tyagi, R.D.; Vaudreuil, M.A.; Sauvé, S.; Buelna, G.; Dubé, R. The bacterial community structure of submerged membrane bioreactor treating synthetic hospital wastewater. Bioresour. Technol. 2019, 286, 121362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Courtois, S.; Gitungo, S.; Raczko, R.F.; Dyksen, J.E.; Li, M.; Axe, L. Microbial community analysis in biologically active filters exhibiting efficient removal of emerging contaminants and impact of operational conditions. Sci. Total Environ. 2018, 640, 1455–1464. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Piché-Choquette, S.; Drogui, P.; Tyagi, R.D.; Vaudreuil, M.A.; Sauvé, S.; Buelna, G.; Dubé, R. Acclimatization of microbial community of submerged membrane bioreactor treating hospital wastewater. Bioresour. Technol. 2021, 319, 124223. [Google Scholar] [CrossRef]
- Arriaga, S.; de Jonge, N.; Nielsen, M.L.; Andersen, H.R.; Borregaard, V.; Jewel, K.; Ternes, T.A.; Nielsen, J.L. Evaluation of a membrane bioreactor system as post-treatment in waste water treatment for better removal of micropollutants. Water Res. 2016, 107, 37–46. [Google Scholar] [CrossRef]
- Wang, L.; Deng, S.; Wang, S.; Su, H. Analysis of aerobic granules under the toxic effect of ampicillin in sequencing batch reactors: Performance and microbial community. J. Environ. Manag. 2017, 204, 152–159. [Google Scholar] [CrossRef]
- Yu, J.; Widyaningsih, E.; Park, Y.; Lee, T. Nitrogen removal and microbial community diversity in single-chamber electroactive biofilm reactors with different ratios of the cathode surface area to reactor volume. Sci. Total Environ. 2020, 758, 143677. [Google Scholar] [CrossRef]
- Han, T.; Zheng, J.; Han, Y.; Xu, X.; Li, M.; Schwarz, C.; Zhu, L. Comprehensive insights into core microbial assemblages in activated sludge exposed to textile-dyeing wastewater stress. Sci. Total Environ. 2021, 791, 148145. [Google Scholar] [CrossRef]
- Huang, J.; Yan, Y.; Ou, Y.; Jia, Y.; Sun, L.; Zhao, Q.; Lu, H. Unraveling pharmaceuticals removal in a sulfur-driven autotrophic denitrification process: Performance, kinetics and mechanisms. Chin. Chem. Lett. 2022, 4, 31. [Google Scholar] [CrossRef]
- Lee, J.; Koo, T.; Yulisa, A.; Hwang, S. Magnetite as an enhancer in methanogenic degradation of volatile fatty acids under ammonia-stressed condition. J. Environ. Manag. 2019, 241, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Chen, Y.; He, J.; Guo, D.; Pan, X.; Ma, Y.; Qu, F.; Nan, J. Cation exchange resin-induced hydrolysis for improving biodegradability of waste activated sludge: Characterization of dissolved organic matters and microbial community. Bioresour. Technol. 2020, 302, 122870. [Google Scholar] [CrossRef] [PubMed]
- Thelusmond, J.-R.; Strathmann, T.J.; Cupples, A.M. The identification of carbamazepine biodegrading phylotypes and phylotypes sensitive to carbamazepine exposure in two soil microbial communities. Sci. Total Environ. 2016, 571, 1241–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Rene, E.R.; Han, B.; Ma, W. Enhanced fluoroglucocorticoid removal from groundwater in a bio-electrochemical system with polyaniline-loaded activated carbon three-dimensional electrodes: Performance and mechanisms. J. Hazard. Mater. 2021, 416, 126197. [Google Scholar] [CrossRef]
- Cao, Q.; Li, X.; Xie, Z.; Li, C.; Huang, S.; Zhu, B.; Li, D.; Liu, X. Compartmentation of microbial communities in structure and function for methane oxidation coupled to nitrification–denitrification. Bioresour. Technol. 2021, 341, 125761. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yao, H.; Deng, S.; Jia, F.; Cai, W.; Hu, Z.; Guo, J.; Li, H. Performance and microbial community dynamics relationship within a step-feed anoxic/oxic/anoxic/oxic process (SF-A/O/A/O) for coking wastewater treatment. Sci. Total Environ. 2021, 792, 148263. [Google Scholar] [CrossRef]
- Yao, M.; Duan, L.; Wei, J.; Qian, F.; Hermanowicz, S.W. Carbamazepine removal from wastewater and the degradation mechanism in a submerged forward osmotic membrane bioreactor. Bioresour. Technol. 2020, 314, 123732. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, G.; Zhang, Z. Long-term stable and energy-neutral mixed biofilm electrode for complete nitrogen removal from high-salinity wastewater: Mechanism and microbial community. Bioresour. Technol. 2020, 313, 123660. [Google Scholar] [CrossRef]
- Chen, H.; Yu, Y.; Yu, Y.; Ye, J.; Zhang, S.; Chen, J. Exogenous electron transfer mediator enhancing gaseous toluene degradation in a microbial fuel cell: Performance and electron transfer mechanism. Chemosphere 2021, 282, 131028. [Google Scholar] [CrossRef]
- Liang, D.; Li, C.; He, W.; Li, Z.; Feng, Y. Response of exoelectrogens centered consortium to nitrate on collaborative metabolism, microbial community, and spatial structure. Chem. Eng. J. 2021, 426, 130975. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Benabdelkamel, H.; Al-Humaid, L. Degradation of sulfadiazine and electricity generation from wastewater using Bacillus subtilis EL06 integrated with an open circuit system. Chemosphere 2021, 276, 130145. [Google Scholar] [CrossRef] [PubMed]
- Quéméner, E.D.-L.; Bridier, A.; Tian, J.-H.; Madigou, C.; Bureau, C.; Qi, Y.; Bouchez, T. Biorefinery for heterogeneous organic waste using microbial electrochemical technology. Bioresour. Technol. 2019, 292, 121943. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zheng, X.; Li, M.; Han, L.; Liu, X.; Zhang, F.; Hou, X. A three chamber bioelectrochemical system appropriate for in-situ remediation of nitrate-contaminated groundwater and its reaction mechanisms. Water Res. 2019, 158, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Lu, S.; Liu, X.; Wang, G.; Zhang, Y.; Li, D.; Wan, Z. Removal of tri-(2-chloroisopropyl) phosphate (TCPP) by three types of constructed wetlands. Sci. Total Environ. 2020, 749, 141668. [Google Scholar] [CrossRef]
- Wu, H.; Cui, M.; Yang, N.; Liu, Y.; Wang, X.; Zhang, L.; Zhan, G. Aerobic biocathodes with potential regulation for ammonia oxidation with concomitant cathodic oxygen reduction and their microbial communities. Bioelectrochemistry 2021, 144, 107997. [Google Scholar] [CrossRef]
- Salgado, R.; Brito, D.; Noronha, J.P.; Almeida, B.; Bronze, M.R.; Oehmen, A.; Carvalho, G.; Crespo, M.T.B. Metabolite identification of ibuprofen biodegradation by Patulibacter medicamentivorans under aerobic conditions. Environ. Technol. 2018, 41, 450–465. [Google Scholar] [CrossRef]
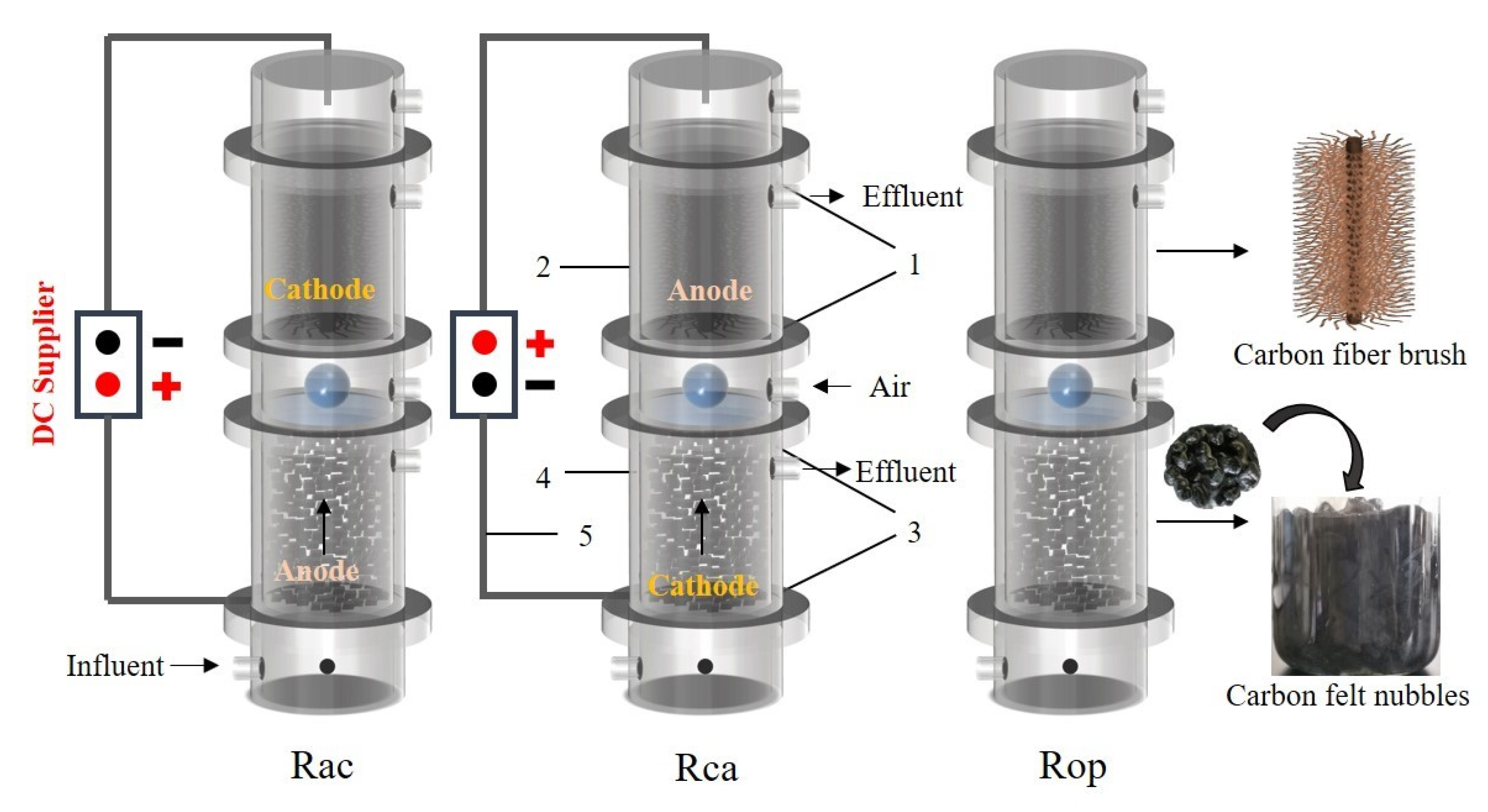

| Reactor | Anaerobic Zone | Aerobic Zone | Voltage Applied Modes | Notes |
|---|---|---|---|---|
| Rac | Anodic chamber | Cathodic chamber | 0, 0.3, 0.6, 0.9, 1.2 V | R1, R2 |
| Rca | Cathodic chamber | Anodic chamber | 0, 0.3, 0.6, 0.9, 1.2 V | R3, R4 |
| Rop | Anaerobic zone | Aerobic zone | No applied voltage | R5, R6 |
| Sample ID | Sampling Site | log10 (Copies/g CFN or g CFB) |
|---|---|---|
| Rac.Ae.C | Rac_ aerobic cathode | 10.4 |
| Rca.Ae.A | Rca_ aerobic anode | 10.3 |
| Rop.Ae.O | Rop_ aerobic open circuit | 10.2 |
| Rac.An.A | Rac_ anaerobic anode | 10.1 |
| Rca.An.C | Rca_ anaerobic cathode | 10.2 |
| Rop.An.O | Rop_ anaerobic open circuit | 10.2 |
| Sample ID | Sampling Site | OTUs | Evenness |
|---|---|---|---|
| S.Ae | Sludge_aerobic | 271 | 0.66 |
| Rac.Ae.C | Rac_aerobic cathode | 274 | 0.94 |
| Rca.Ae.A | Rca_aerobic anode | 289 | 0.95 |
| Rop.Ae.O | Rop_aerobic open circuit | 275 | 0.92 |
| S.An | Sludge_anaerobic | 235 | 0.70 |
| Rac.An.A | Rac_anaerobic anode | 312 | 0.89 |
| Rca.An.C | Rca_anaerobic cathode | 340 | 0.97 |
| Rop.An.O | Rop_anaerobic open circuit | 295 | 0.86 |
| Dominant Zone | Genera | MAIN Function | Highlights | References |
|---|---|---|---|---|
| Aerobic | 1. Hydrogenophaga | 1. Potential for diclofenac and ibuprofen removal. 2. Enhancing dehydrogenase activity and secretion of EPS. 3. Found in electroactive biofilm and uses hydrogen as an electron. | Enriched in the anodic and cathodic chambers | [46,47,48] |
| 2. Methyloversatilis | 1. Essential for the oxidation of aromatic hydrocarbons. 2. Has the ability to degrade pharmaceuticals. | [49,50] | ||
| 3. SWB02 | 1. Utilizing volatile fatty acids from the anaerobic chamber. 2. May enhance electron transfer. | [51,52] | ||
| 4. Emticicia | Correlated with pharmaceutical removal | [53] | ||
| 5. Methylophilus | 1. Pharmaceutical biodegradation. 2. Has the ability of dechlorination. | Enriched in the anodic chamber | [54,55] | |
| 6. SM1A02 | 1. Electrotrophic bacteria and enhanced electron transfer. 2. Anammox genus and may be correlated with pharmaceutical removal. | Enriched in the cathodic chamber | [56,57,58] | |
| Anaerobic | 1. Acidovorax | 1. Could grow using inorganics as electron donors. 2. Closely related to pharmaceutical removal. | Enriched in the anodic and cathodic chamber | [35,59,60] |
| 2. Sporomusa | Generating and conducting electrons. | Enriched in the anodic chamber | [61,62] | |
| 3. Terrimicrobium | Degrading substances containing -Cl. | Enriched in the cathodic chamber | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wu, M.; Ailijiang, N.; Mamat, A.; Chang, J.; Pu, M.; He, C. Impact of Voltage Application on Degradation of Biorefractory Pharmaceuticals in an Anaerobic–Aerobic Coupled Upflow Bioelectrochemical Reactor. Int. J. Environ. Res. Public Health 2022, 19, 15364. https://doi.org/10.3390/ijerph192215364
Zhang Q, Wu M, Ailijiang N, Mamat A, Chang J, Pu M, He C. Impact of Voltage Application on Degradation of Biorefractory Pharmaceuticals in an Anaerobic–Aerobic Coupled Upflow Bioelectrochemical Reactor. International Journal of Environmental Research and Public Health. 2022; 19(22):15364. https://doi.org/10.3390/ijerph192215364
Chicago/Turabian StyleZhang, Qiongfang, Mei Wu, Nuerla Ailijiang, Anwar Mamat, Jiali Chang, Miao Pu, and Chaoyue He. 2022. "Impact of Voltage Application on Degradation of Biorefractory Pharmaceuticals in an Anaerobic–Aerobic Coupled Upflow Bioelectrochemical Reactor" International Journal of Environmental Research and Public Health 19, no. 22: 15364. https://doi.org/10.3390/ijerph192215364





