Lipid Biomarker Investigation of the Delivery and Preservation of Autochthonous Organic Carbon in the Pearl River and Its Contribution to the Carbon Sink: Evidence from the Water and Surface Sediment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Sites
2.1.1. Study Area
2.1.2. Sample Collection
2.2. Chemical Analysis
2.3. Biomarker Analysis
2.4. Grain Size of Suspended Sediment and TSS Analysis
3. Results
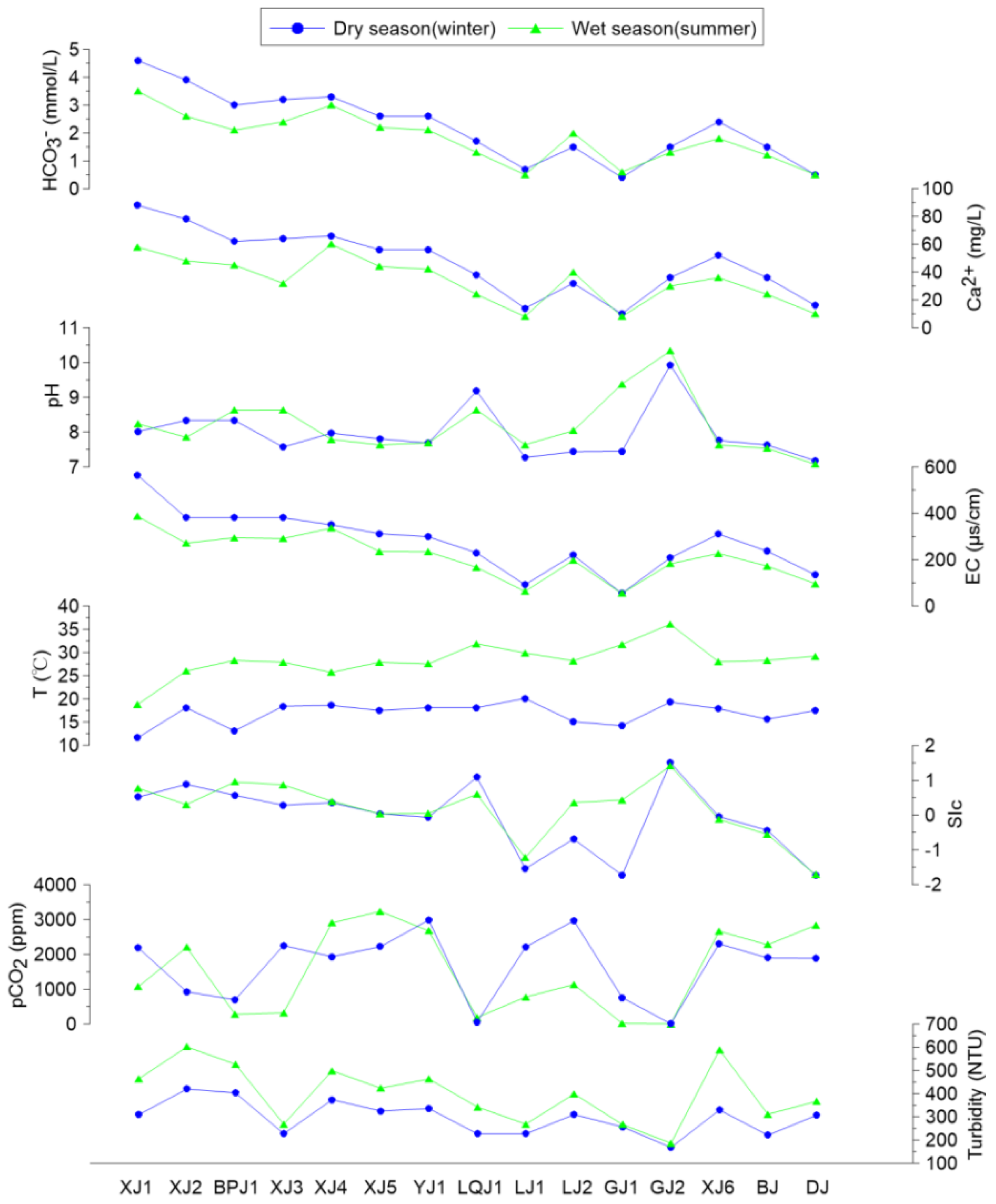
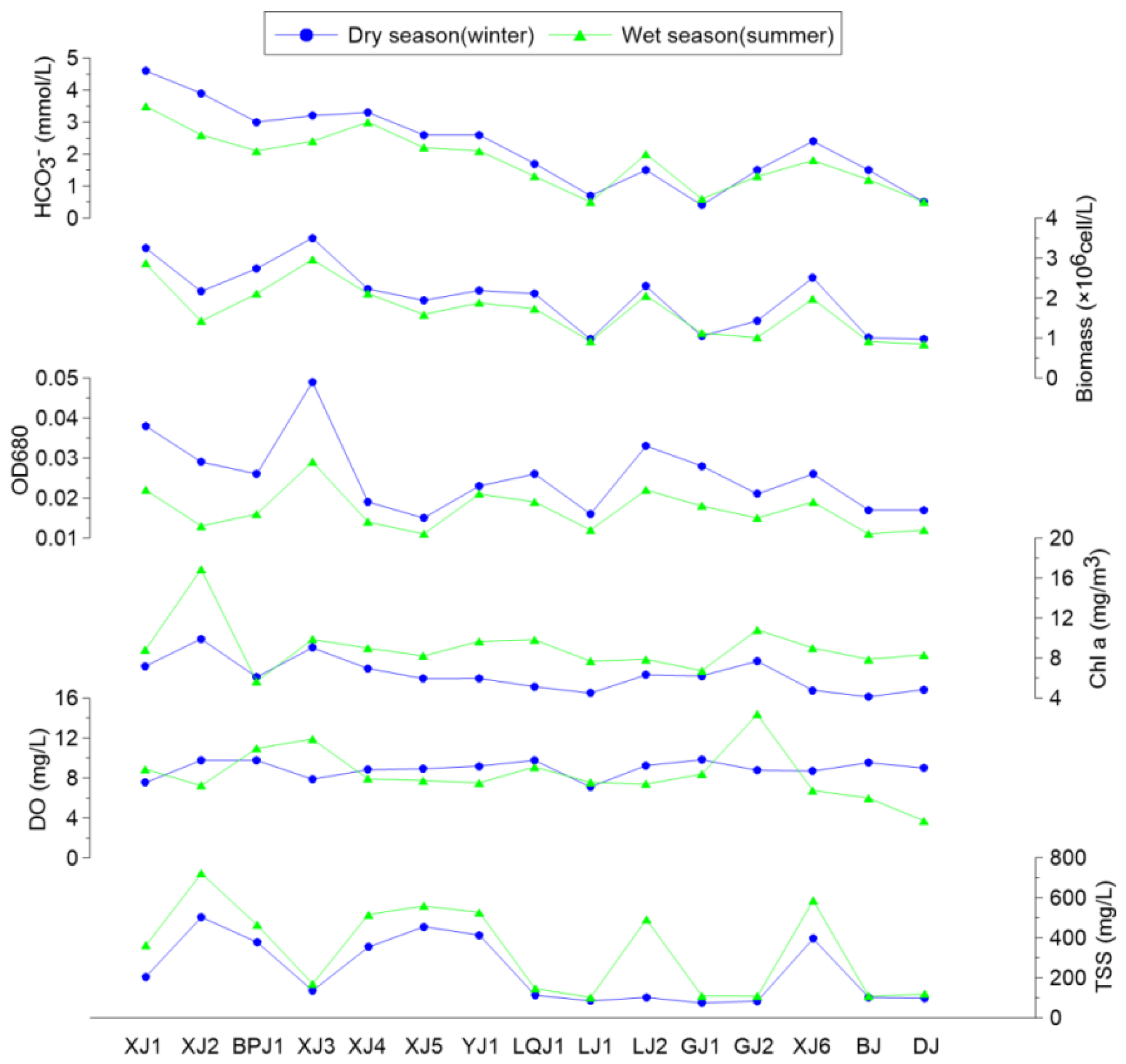
3.1. Seasonal Variation in Chemical, Phytoplankton, and Biomarker Compositions and Their Implications for Carbonate Weathering Coupled with Aquatic Photosynthesis
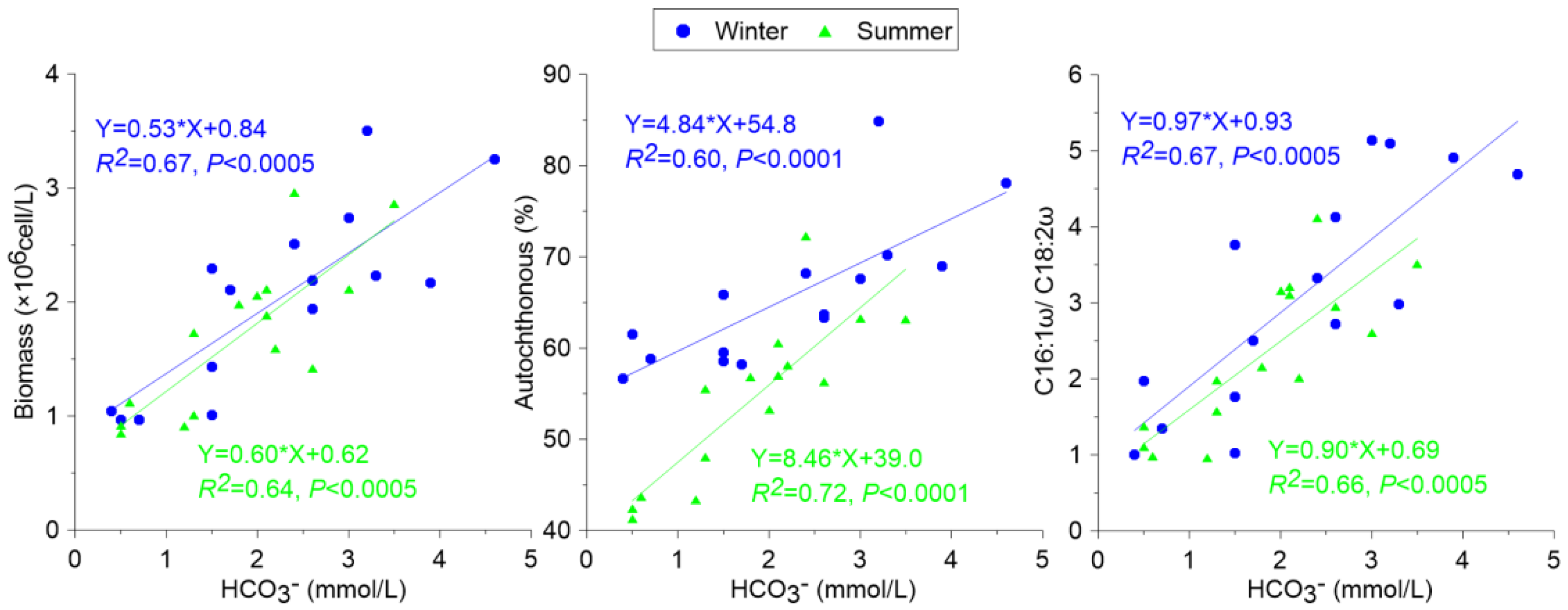
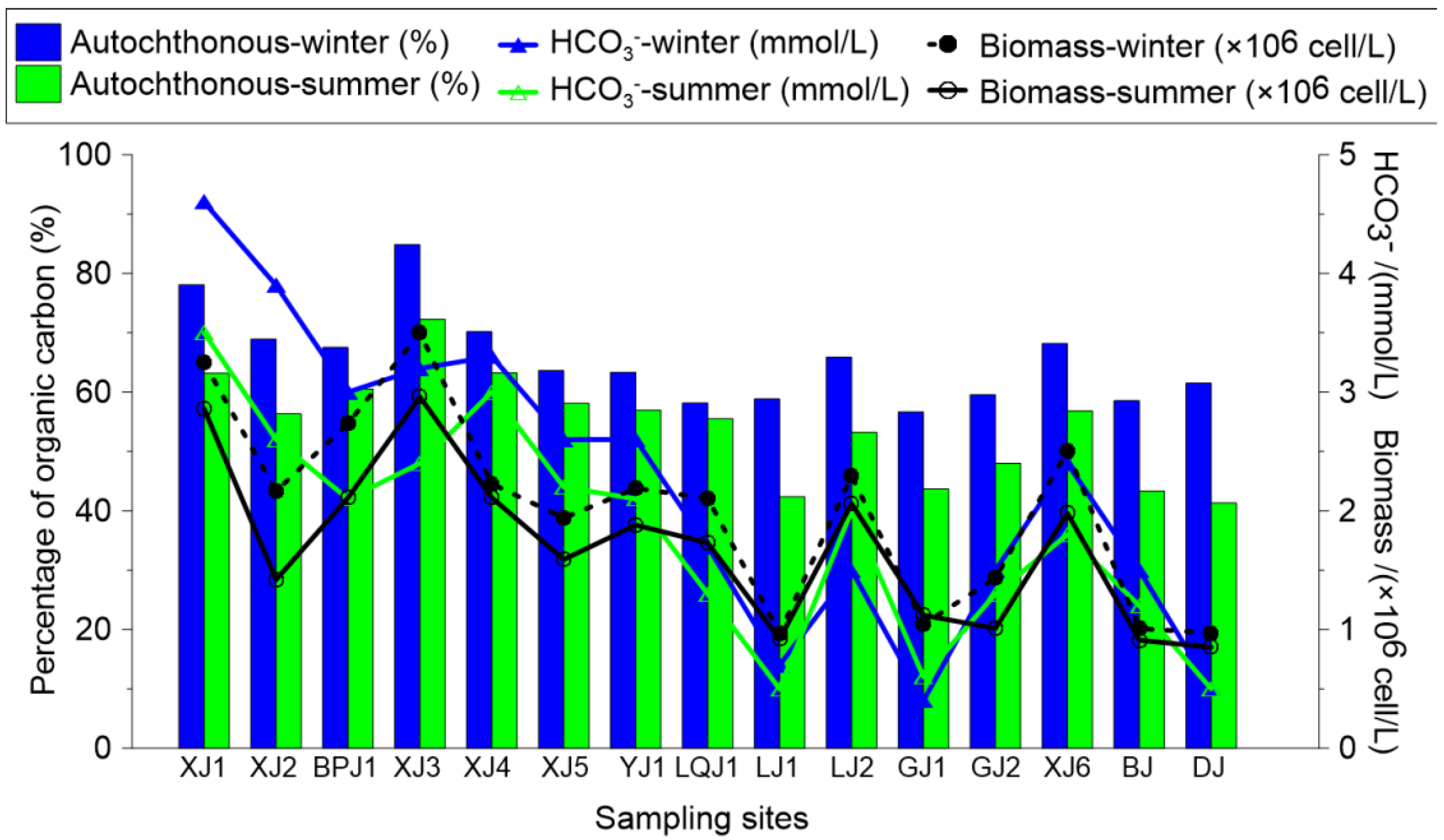
3.1.1. Seasonal Distribution of Biochemical Compositions in Surface Water and the DIC Fertilization Effect
3.1.2. Biomarker Distribution in Surface Water—Implications for Carbonate Weathering Coupled with Aquatic Photosynthesis
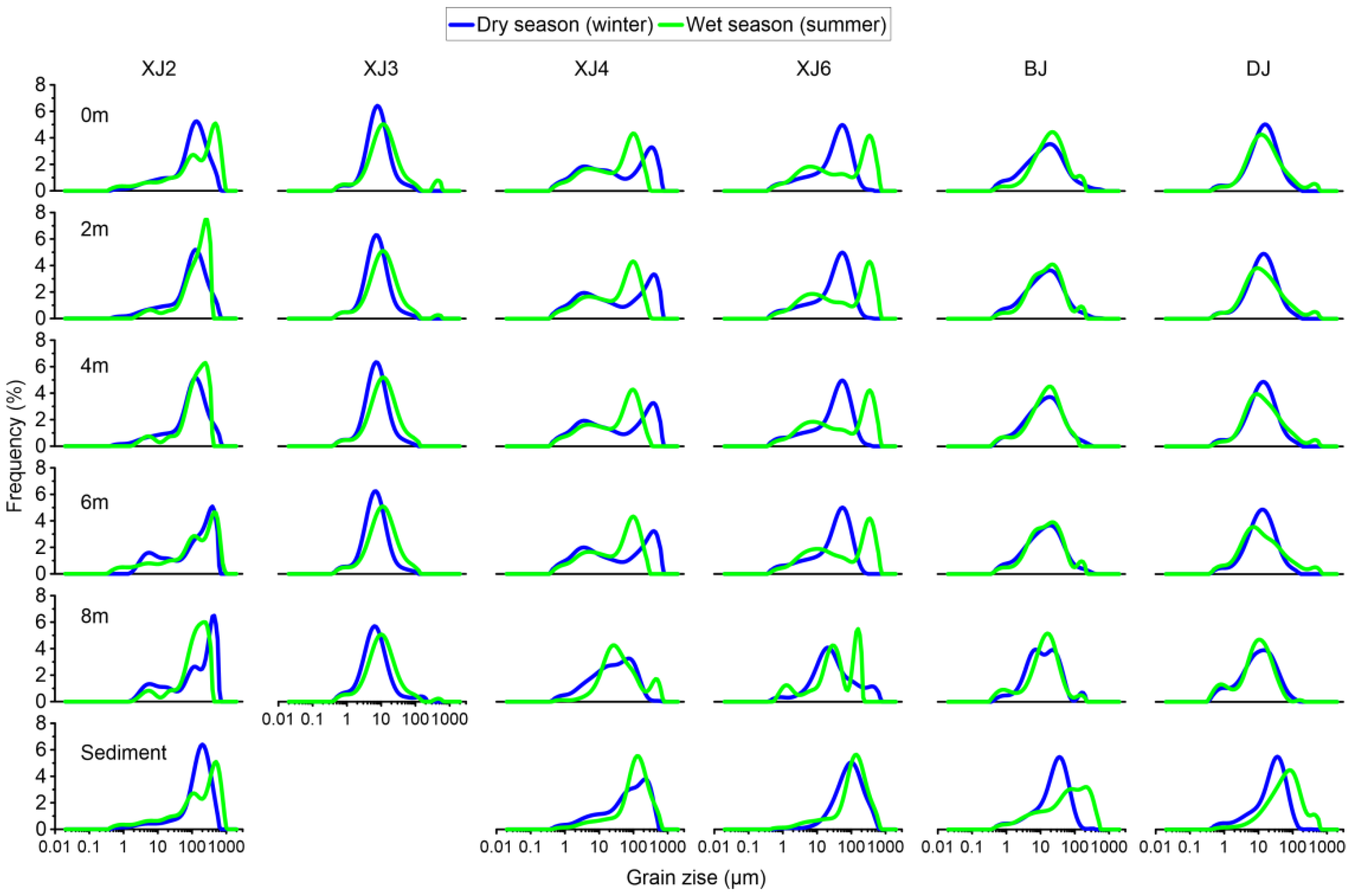
3.2. Spatial Patterns of Biomarkers in the Water Column and Surface Sediments as a Consequence of BCP Effects
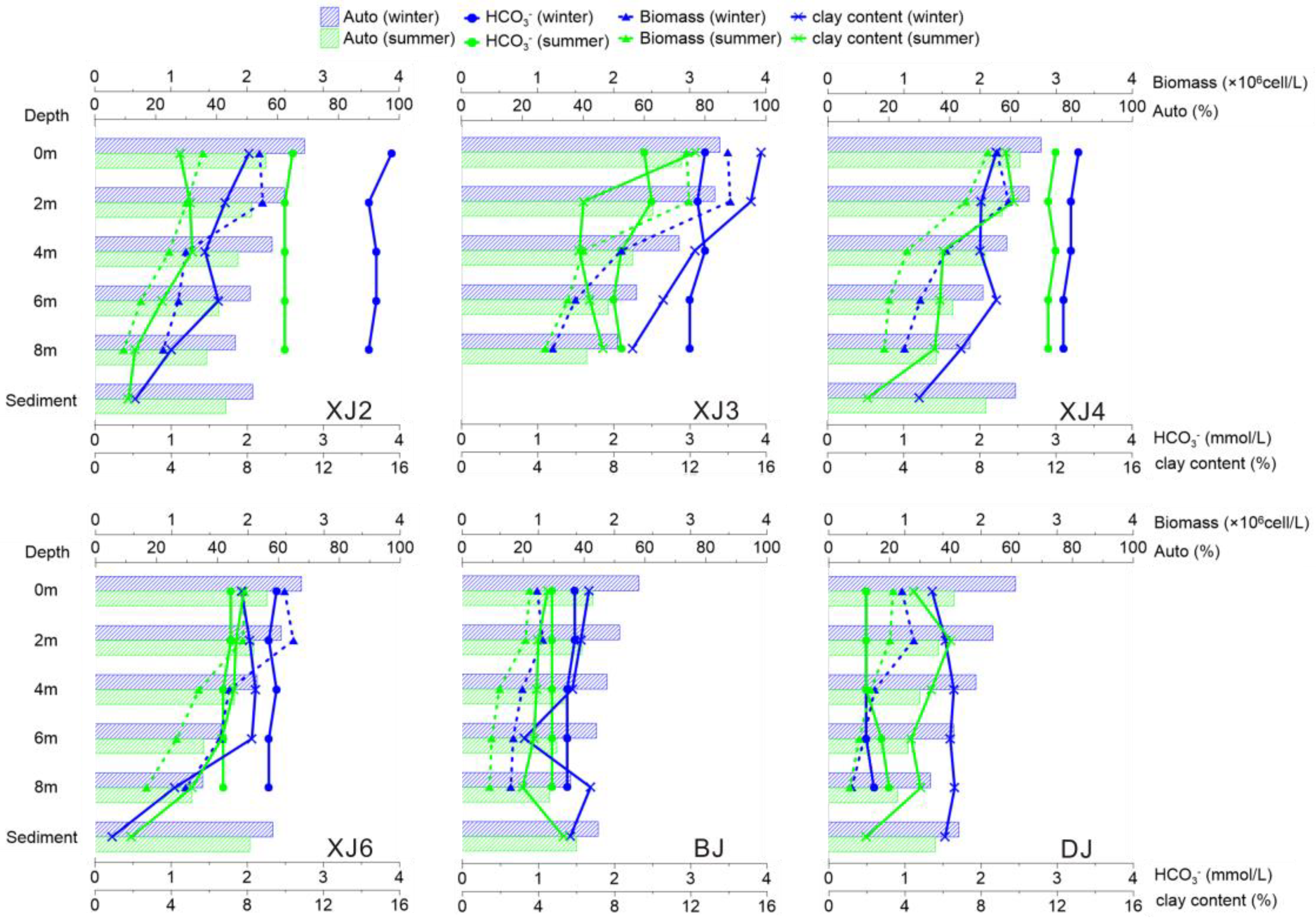
3.2.1. Distribution of Lipid Biomarkers in the Water Column and Surface Sediments
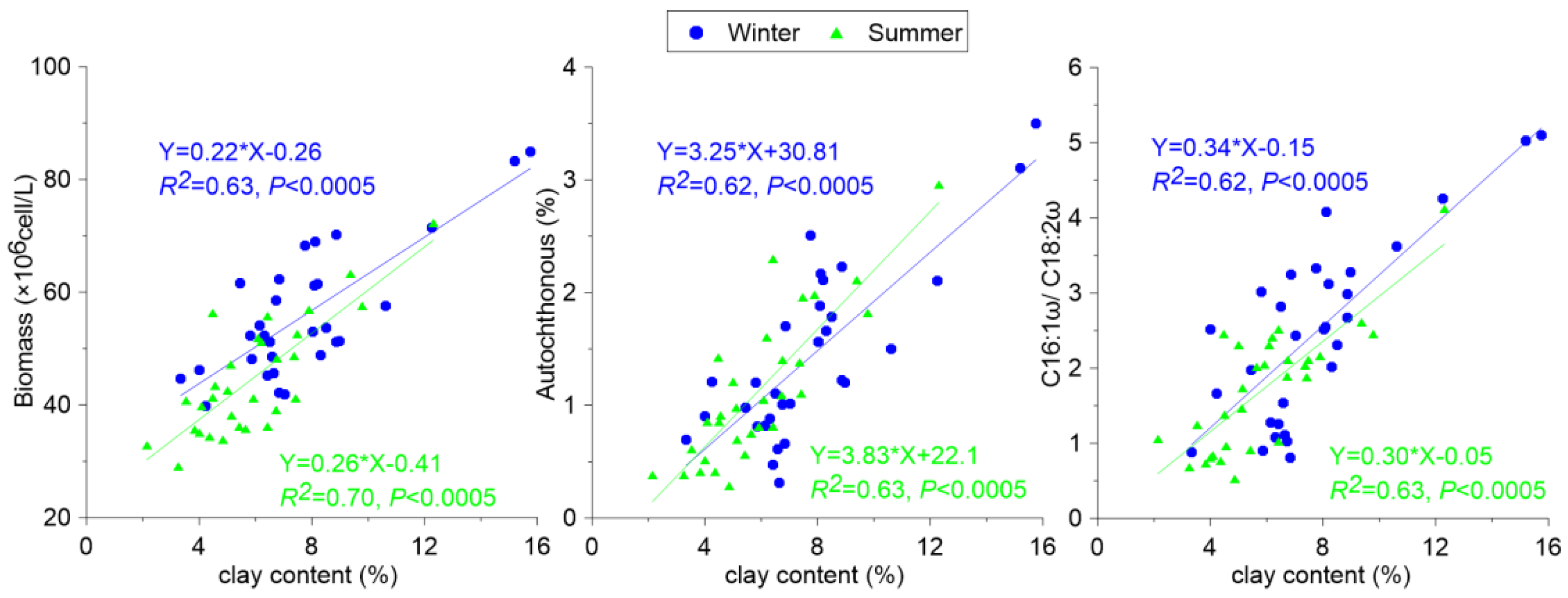
3.2.2. Grain Size Distribution and Its Influence on the BCP Effects
3.3. Carbon Sink Estimation Based on Photosynthesis Coupled with Carbonate Weathering
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rixen, T.; Goyet, C.; Ittekkot, V. Diatoms and their influence on the biologically mediated uptake of atmospheric CO2 in the Arabian Sea upwelling system. Biogeosciences 2006, 3, 1–13. [Google Scholar] [CrossRef]
- Goodwin, P.; Lauderdale, J.M. Carbonate ion concentrations, ocean carbon storage, and atmospheric CO2. Glob. Biogeochem. Cycles 2013, 27, 882–893. [Google Scholar] [CrossRef][Green Version]
- Sun, H.G.; Han, J.T.; Zhang, S.R.; Lu, X.X. Transformation of dissolved inorganic carbon (DIC) into particulate organic carbon (POC) in the lower Xijiang River, SE China, an isotopic approach. Biogeosci. Discuss. 2011, 8, 9471–9501. [Google Scholar]
- Liu, Z. Review on the role of terrestrial aquatic photosynthesis in the global carbon cycle. Proc. Earth Planet. Sci. 2013, 7, 513–516. [Google Scholar] [CrossRef]
- Romera-Castillo, C.; Álvarez-Salgado, X.A.; Galí, M.; Gasol, J.M.; Marrase, C. Combined effect of light exposure and microbial activity on distinct dissolved organic matter pools. A seasonal field study in an oligotrophic coastal system (Blanes Bay, NW Mediterranean). Mar. Chem. 2013, 148, 44–51. [Google Scholar] [CrossRef]
- Yang, R.; Chen, B.; Liu, H.; Liu, Z.; Yan, H. Carbon sequestration and decreased CO2, emission caused by terrestrial aquatic photosynthesis: Insights from diel hydrochemical variations in an epikarst spring and two spring-fed ponds in different seasons. Appl. Geochem. 2015, 63, 248–260. [Google Scholar] [CrossRef]
- Medeiros, P.M.; Simoneit, B.R.T. Multi-biomarker characterization of sedimentary organic carbon in small rivers draining the Northwestern United States. Org. Geochem. 2008, 39, 52–74. [Google Scholar] [CrossRef]
- Matsunaga, K. An estimation of allochthonous and autochthonous organic matter of the fresh sediments on the basis of Ti content. Jpn. J. Limnol. 2009, 43, 113–120. [Google Scholar] [CrossRef]
- Waterson, E.J.; Canuel, E.A. Sources of sedimentary organic matter in the Mississippi River and adjacent Gulf of Mexico as revealed by lipid biomarker and δ13CTOC analyses. Org. Geochem. 2008, 39, 422–439. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Z.; Sun, H.; Yang, R.; Chen, B. Organic carbon source tracing and DIC fertilization effect in the Pearl River: Insights from lipid biomarker and geochemical analysis. Appl. Geochem. 2016, 73, 132–141. [Google Scholar] [CrossRef]
- Bianchi, T.S. Biogeochemistry of Estuaries; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Liu, Z.; Dreybrodt, W. Significance of the carbon sink produced by H2O-carbonate-CO2-aquatic phototroph interaction on land. Sci. Bull. 2015, 60, 182–191. [Google Scholar] [CrossRef]
- Liu, Z.; Macpherson, G.L.; Groves, C.; Martin, J.B.; Yuan, D.X.; Zeng, S. Large and active CO2, uptake by coupled carbonate weathering. Earth-Sci. Rev. 2018, 182, 42–49. [Google Scholar] [CrossRef]
- Goni, M.A.; Monacci, N.; Gisewhite, R.; Ogston, A.; Crockett, J.; Nittrouer, C. Distribution and sources of particulate organic matter in the water column and sediments of the Fly River Delta, Gulf of Papua (Papua New Guinea). Estuar. Coast. Shelf Sci. 2006, 69, 225–245. [Google Scholar] [CrossRef]
- Battin, T.J.; Kaplan, L.A.; Findlay, S.; Hopkinson, C.S.; Marti, E.; Packman, A.I.; Newbold, J.D.; Sabater, F. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 2009, 1, 95–100. [Google Scholar] [CrossRef]
- Aufdenkampe, A.K.; Mayorga, E.; Raymond, P.A.; Melack, J.M.; Doney, S.C.; Alin, S.R.; Aalto, R.E.; Yoo, K. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front. Ecol. Environ. 2011, 9, 53–60. [Google Scholar] [CrossRef]
- Tao, S.; Eglinton, T.I.; Montluçon, D.B.; Mcintyre, C.; Zhao, M. Pre-aged soil organic carbon as a major component of the Yellow River suspended load: Regional significance and global relevance. Earth Planet. Sci. Lett. 2015, 414, 77–86. [Google Scholar] [CrossRef]
- Mayer, L.M. Extent of coverage of mineral surfaces by organic matter in marine sediments. Geochim. Cosmochim. Acta 1999, 63, 207–215. [Google Scholar] [CrossRef]
- Keil, R.G.; Mayer, L.M.; Quay, P.D.; Richey, J.E.; Hedges, J.I. Loss of organic matter from riverine particles in deltas. Geochim. Cosmochim. Acta 1997, 61, 1507–1511. [Google Scholar] [CrossRef]
- Ransom, B.; Kim, D.; Kastner, M.; Wainwright, S. Organic matter preservation on continental slopes: Importance of mineralogy and surface area. Geochim. Cosmochim. Acta 1998, 62, 1329–1345. [Google Scholar] [CrossRef]
- Bock, M.J.; Mayer, L.M. Mesodensity organo-clay associations in a near-shore sediment. Mar. Geol. 2000, 163, 65–75. [Google Scholar] [CrossRef]
- Curry, K.J.; Bennett, R.H.; Mayer, L.M.; Curry, A.; Abrli, M.; Biesiot, P.M.; Hulbert, M.H. Direct visualization of clay microfabric signatures driving organic matter preservation in fine-grained sediment. Geochim. Cosmochim. Acta 2007, 71, 1709–1720. [Google Scholar] [CrossRef]
- Galy, V.; France-Lanord, C.; Lartiges, B. Loading and fate of particulate organic carbon from the Himalaya to the Ganga–Brahmaputra delta. Geochim. Cosmochim. Acta 2008, 72, 1767–1787. [Google Scholar] [CrossRef]
- West, A.J.; Lin, C.W.; Lin, T.C.; Hilton, R.G.; Liu, S.H.; Chang, C.T.; Lin, K.C.; Galy, A.; Sparkes, R.B.; Hovius, N. Mobilization and transport of coarse woody debris to the oceans triggered by an extreme tropical storm. Limnol. Oceanogr. 2011, 56, 77–85. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Algal Lipids and Their Metabolism. In Algae for Biofuels and Energy; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Mohanty, B.P.; Bhattacharjee, S.; Paria, P.; Mahanty, A.; Sharma, A.P. Lipid biomarkers of lens aging. Appl. Biochem. Biotech. 2013, 169, 192–200. [Google Scholar] [CrossRef]
- Eglinton, G.; Hamilton, R.J. Leaf epicuticular waxes. Science 1967, 156, 1322–1335. [Google Scholar] [CrossRef]
- Robinson, N.; Cranwell, P.A.; Eglinton, G. Sources of the lipids in the bottom sediments of an English oligo-mesotrophic lake. Freshw. Biol. 1987, 17, 15–33. [Google Scholar] [CrossRef]
- Gao, L.; Hou, J.; Toney, J.; Macdonald, D.; Huang, Y. Mathematical modeling of the aquatic macrophyte inputs of mid-chain n-alkyl lipids to lake sediments: Implications for interpreting compound specific hydrogen isotopic records. Geochim. Cosmochim. Acta 2011, 75, 3781–3791. [Google Scholar] [CrossRef]
- Dunstan, G.A.; Volkman, J.K.; Barrett, S.M.; Leroi, J.M.; Jeffrey, S.W. Essential polyunsaturated fatty acids from 14 species of diatom (Bacillariophycea). Phytochemistry 1993, 35, 155–161. [Google Scholar] [CrossRef]
- Harwood, J.L.; Russell, N.J. Lipids in Plants and Microbes; George Allen and Unwin: London, UK, 1984. [Google Scholar]
- Volkman, J.K. A review of sterol markers for marine and terrigenous organic matter. Org. Geochem. 1986, 9, 83–99. [Google Scholar] [CrossRef]
- Yunker, M.B.; MacDonald, R.W.; Veltkamp, D.J.; Cretney, W.J. Terrestrial and marine biomarkers in a seasonally ice-covered Arctic estuary-integration of multivariate and biomarker approaches. Mar. Chem. 1995, 49, 1–50. [Google Scholar] [CrossRef]
- Tue, N.T.; Quy, T.D.; Hamaoka, H.; Mai, T.N.; Omori, K. Sources and exchange of particulate organic matter in an estuarine mangrove ecosystem of Xuan Thuy National Park, Vietnam. Estuar. Coasts 2012, 35, 1060–1068. [Google Scholar] [CrossRef]
- Zhang, S.R.; Lu, X.X.; Higgitt, D.L.; Chen, C.T.A.; Han, J.T.; Sun, H.G. Recent changes of water discharge and sediment load in the Zhujiang (Pearl River) basin, China. Glob. Planet. Chang. 2008, 60, 365–380. [Google Scholar] [CrossRef]
- Sun, H.G.; Han, J.; Li, D.; Zhang, S.R.; Lu, X.X. Chemical weathering inferred from riverine water chemistry in the lower Xijiang basin, South China. Sci. Total Environ. 2010, 408, 4749–4760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, C.; Gemmer, M.; Chen, Y.; Liu, C. Changing properties of precipitation concentration in the Pearl River basin, China. Stochast. Environ. Res. Risk Assess. 2009, 23, 377–385. [Google Scholar] [CrossRef]
- Ministry of Water Resource, P.R.C. China River Sediment Bulletin; Ministry of Water Resource, P.R.C. 1954–2010; China Water Power Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- He, L.; Yang, Q.; Ou, S. Temporal variation of runoff and sediment on the main channels of Xijiang River and Beijiang River. Guangdong Water Res. Hydropower 2003, 1, 57–62. (In Chinese) [Google Scholar]
- Cheng, Y.Q. Geological Atlas of China (1:5,000,000); China Cartographic Pressing House: Beijing, China, 1990. (In Chinese) [Google Scholar]
- Liu, Z.; Li, Q.; Sun, H.; Wang, J. Seasonal, diurnal and storm-scale hydrochemical variations of typical epikarst springs in subtropical karst areas of SW China, Soil CO2 and dilution effects. J. Hydrol. 2007, 337, 207–223. [Google Scholar] [CrossRef]
- Wigley, T. WATSPEC, a computer program for determining the equilibrium speciation of aqueous solutions. In British Geomorphological Research Group. Technical Bulletin; Geo Abstracts ltd.: Norwich, UK, 1977; Volume 20, pp. 1–48. [Google Scholar]
- MEP, Ministry of Environmental Protection of the People’s Republic of China. Monitoring and Analysis Method of Water and Waste Water, 4th ed.; Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Mortillaro, J.M.; Abril, G.; Moreira-Turcq, P.; Sobrinho, R.L.; Perez, M.; Meziane, T. Fatty acid and stable isotope (δ13C, δ15N) signatures of particulate organic matter in the lower Amazon River: Seasonal contrasts and connectivity between floodplain lakes and the mainstem. Org. Geochem. 2011, 42, 1159–1168. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, J.; Liu, L.; Lin, K.; Zhu, Y. The reference condition for Eutrophication Indictor in the Yangtze River Estuary and adjacent—Waters response variables. Acta Ecol. Sin. 2013, 33, 2780–2789. [Google Scholar] [CrossRef][Green Version]
- Dunstan, G.A.; Volkman, J.K.; Barrett, S.M.; Garland, C.D. Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. J. Appl. Phycol. 1993, 5, 71–83. [Google Scholar] [CrossRef]
- Viso, A.C.; Marty, J.C. Fatty acids from 28 marine microalgae. Phytochemistry 1993, 34, 1521–1533. [Google Scholar] [CrossRef]
- Li, W.; Dagaut, J.; Saliot, A. The application of sterol biomarkers to the study of the sources of particulate organic matter in the Solo River system and Serayu River, Java, Indonesia. Biogeochemistry 1995, 31, 139–154. [Google Scholar] [CrossRef]
- Meyers, P.A. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paieoclimatic processes. Org. Geochem. 1997, 27, 213–250. [Google Scholar] [CrossRef]
- Walling, D.E.; Moorehead, P.W. Spatial and temporal variation of the particle-size characteristics of fluvial suspended sediment. Geograf. Ann. 1987, 69, 47–59. [Google Scholar] [CrossRef]
- Walling, D.E.; Moorehead, P.W. The particle size characteristics of fluvial suspended sediment, an overview. Hydrobiologia 1989, 176, 125–149. [Google Scholar] [CrossRef]
- Wei, X.; Shen, C.; Li, D.; Wu, Z.F.; He, J.H.; Zhu, L.A. The inventory and fluxes of carbon in soil of Pearl River basin. Ecol. Environ. 2004, 13, 670–673. (In Chinese) [Google Scholar]

| Site | Location | Tributary Description | Sample Types | TSS * (mg/L) | T * (°C) | Precipitation * (mm/a) | ||
|---|---|---|---|---|---|---|---|---|
| Surface Water | Column Water | Sediment | ||||||
| XJ1 | 25°36′18″ N; 103°49′33″ E | Upstream of Nanpanjiang | √ | 1290 | 18.6 | 1000.0 | ||
| XJ2 | 24°01′13″ N; 103°36′20″ E | Midstream of Nanpanjiang | √ | √ | √ | 680 | 20.2 | 963.7 |
| BPJ1 | 26°29′59″ N; 103°44′07″ E | Upstream of Beipanjiang | √ | 2610 | 18.7 | 1127.6 | ||
| XJ3 | 24°57′47″ N; 106°08′55″ E | Junction of Nanpanjiang and Beipanjiang | √ | √ | √ | 170 | 19.1 | 921.1 |
| XJ4 | 23°44′04″ N; 109°13′43″ E | Downstream of Hongshuihe | √ | √ | √ | 628 | 20.8 | 1499.8 |
| YJ1 | 22°48′38″ N; 108°18′46″ E | Junction of Zuojiang and Youjiang | √ | 241 | 21.6 | 1304.2 | ||
| LQJ1 | 24°24′09″ N; 109°36′25″ E | Downstream of Luoqingjiang | √ | 132 | 20.0 | 1257.7 | ||
| LJ1 | 25°13′07″ N; 109°23′47″ E | Upstream of Liujiang | √ | 143 | 19.0 | 1493.0 | ||
| LJ2 | 24°14′36″ N; 109°43′13″ E | Junction of Liujiang and Luoqingjiang | √ | 132 | 21.6 | 1304.2 | ||
| XJ5 | 23°27′50″ N; 110°09′39″ E | Junction of Qianjiang and Yujiang | √ | 350 | 21.4 | 1726.7 | ||
| GJ1 | 25°31′17″ N; 110°11′41″ E | Upstream of Guijiang | √ | 67 | 18.0 | 2052.0 | ||
| GJ2 | 25°06′19″ N; 110°24′55″ E | Upstream of Guijiang | √ | 92 | 18.7 | 1926.0 | ||
| XJ6 | 23°28′40″ N; 111°17′11″ E | Downstream of Xijiang | √ | √ | √ | 311 | 21.1 | 1503.6 |
| BJ | 23°41′21″ N; 113°03′26″ E | Downstream of Beijiang | √ | √ | √ | 129 | 20.7 | 1900.1 |
| DJ | 23°09′28″ N; 114°16′12″ E | Downstream of Dongjiang | √ | √ | √ | 107 | 22.1 | 1821.2 |
| Fatty Acids | Sterols | n-Alkanes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site | SSFA (%) | BSFA (%) | MUFA (%) | PUFA (%) | 28Δ5,22 (μg/L) | 29Δ5 (μg/L) | 27Δ5/29Δ5,22 +29Δ5 | TARHC | CPIHC | C17 (μg/L) |
| XJ1 | 72.0/63.2 | 2.2/9.6 | 19.7/10.6 | 6.1/16.6 | 1.2/0.7 | 0.2/0.4 | 4.79/3.56 | 0.38/0.46 | 1.56/2.01 | 296.3/208.6 |
| XJ2 | 66.5/49.6 | 8.3/18.2 | 17.5/12.6 | 7.7/19.6 | 1.5/0.8 | 0.4/1.1 | 4.32/3.12 | 0.61/0.72 | 2.09/3.06 | 255.3/158.8 |
| BPJ1 | 60.3/52.2 | 8.4/11.5 | 16.5/13.9 | 14.7/22.4 | 0.9/0.6 | 0.2/0.5 | 3.53/3.11 | 0.55/0.61 | 2.12/2.49 | 266.3/188.5 |
| XJ3 | 61.2/58.3 | 4.1/5.1 | 23.8/21.6 | 10.8/15.0 | 1.7/1.5 | 0.1/0.2 | 7.29/6.89 | 0.26/0.29 | 1.18/1.61 | 311.2/278.5 |
| XJ4 | 65.4/55.6 | 3.8/14.7 | 19.2/15.2 | 11.6/14.5 | 0.9/0.6 | 0.2/0.6 | 3.90/3.01 | 0.47/0.51 | 2.92/4.03 | 216.3/156.6 |
| YJ1 | 53.5/49.0 | 10.8/22.1 | 13.7/10.8 | 22.1/24.7 | 1.5/1.1 | 0.2/0.7 | 1.89/1.68 | 0.59/0.61 | 3.43/3.98 | 243.2/146.3 |
| LQJ1 | 62.9/64.8 | 12.7/16.8 | 6.6/5.1 | 17.8/13.3 | 1.1/0.6 | 0.3/0.4 | 2.79/2.81 | 0.61/0.73 | 3.79/4.28 | 163.2/112.6 |
| LJ1 | 55.0/58.9 | 21.7/24.8 | 10.0/5.5 | 13.4/10.8 | 0.4/0.4 | 0.2/0.8 | 1.45/1.22 | 0.48/0.41 | 2.28/3.56 | 35.6/31.1 |
| LJ2 | 77.1/73.9 | 3.7/9.7 | 14.8/15.3 | 4.4/1.1 | 0.4/0.5 | 0.2/0.4 | 0.81/0.89 | 0.52/0.58 | 4.99/5.01 | 35.6/28.2 |
| XJ5 | 62.2/64.9 | 4.8/8.9 | 12.8/14.2 | 20.1/12.0 | 0.6/0.6 | 0.3/0.3 | 2.02/2.88 | 0.52/0.66 | 2.88/2.58 | 189.0/136.1 |
| GJ1 | 62.7/58.2 | 17.2/19.5 | 8.7/13.2 | 11.4/9.1 | 0.4/0.4 | 0.2/0.4 | 0.67/0.56 | 0.49/0.51 | 3.32/2.96 | 47.7/61.9 |
| GJ2 | 63.3/70.1 | 16.5/15.8 | 7.2/9.8 | 13.0/4.3 | 0.7/0.9 | 0.2/0.4 | 1.59/1.41 | 0.46/0.55 | 3.52/4.12 | 69.9/72.8 |
| XJ6 | 58.6/53.3 | 12.2/15.2 | 15.6/8.5 | 13.6/23.0 | 1.3/0.6 | 0.3/0.5 | 1.99/1.58 | 0.51/0.62 | 2.33/2.80 | 68.0/50.1 |
| BJ | 72.5/68.2 | 6.4/10.2 | 11.7/11.3 | 9.5/10.3 | 0.9/0.5 | 0.4/0.5 | 0.89/0.93 | 0.68/0.75 | 4.71/5.06 | 88.8/68.3 |
| DJ | 63.9/53.6 | 10.1/16.0 | 9.9/10.1 | 16.0/20.5 | 0.3/0.4 | 0.4/0.6 | 1.11/1.21 | 0.72/0.81 | 3.16/4.88 | 45.2/39.8 |
| mean | 63.8 ± 6.1/59.6 ± 7.39 | 9.5 ± 5.5/14.1 ± 5.2 | 13.9 ± 4.8/11.8 ± 3.9 | 12.8 ± 4.8/14.5 ± 6.6 | 0.9 ± 0.4/0.7 ± 0.3 | 0.3 ± 0.1/0.5 ± 0.2 | 2.60 ± 1.7/2.32 ± 1.5 | 0.52 ± 0.11/0.58 ± 0.13 | 2.99 ± 1.03/3.49 ± 1.06 | 149.3 ± 100.2115.8 ± 71.7 |
| Site | Depth (m) | Fatty Acids | Sterols | n-Alkanes | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSFA (%) | BSFA (%) | MUFA (%) | PUFA (%) | 28Δ5,22 (μg/L) | 29Δ5 (μg/L) | 27Δ5/29Δ5,22 +29Δ5 | TARHC | CPIHC | C17 (μg/L) | ||||||||||||
| W | S | W | S | W | S | W | S | W | S | W | S | W | S | W | S | W | S | W | S | ||
| XJ2 | 0 | 66.5 | 49.6 | 8.3 | 18.2 | 17.5 | 12.6 | 7.7 | 19.6 | 1.5 | 0.8 | 0.4 | 1.1 | 4.32 | 3.12 | 0.61 | 0.72 | 2.09 | 3.06 | 255.3 | 158.8 |
| 2 | 64.8 | 47.1 | 9.9 | 19.3 | 16.7 | 13.2 | 8.6 | 20.4 | 1.6 | 0.7 | 0.5 | 1 | 4.21 | 3.01 | 0.63 | 0.73 | 2.11 | 3.05 | 231.5 | 129.6 | |
| 4 | 62.6 | 45.8 | 8.1 | 20.1 | 18.8 | 12.1 | 10.5 | 22.0 | 1.3 | 0.6 | 0.4 | 0.9 | 4.24 | 2.86 | 0.69 | 0.74 | 2.32 | 3.09 | 201.6 | 115.7 | |
| 6 | 60.2 | 42.2 | 9.3 | 23.2 | 17.6 | 11.8 | 12.9 | 22.8 | 1.1 | 0.7 | 0.6 | 0.8 | 4.01 | 2.41 | 0.71 | 0.78 | 2.27 | 3.11 | 168.2 | 114.2 | |
| 8 | 48.8 | 41.9 | 16.1 | 22.8 | 19.8 | 12.8 | 15.3 | 22.5 | 1.1 | 0.4 | 0.4 | 0.8 | 3.89 | 2.62 | 0.73 | 0.76 | 2.14 | 3.16 | 155.7 | 109.8 | |
| S | 53.6 | 38.2 | 15.8 | 23.6 | 13.8 | 16.1 | 16.8 | 22.1 | 32.5 | 21.3 | 15.4 | 24.1 | 4.18 | 2.97 | 0.62 | 0.68 | 2.13 | 2.25 | 171.2 | 121.8 | |
| XJ3 | 0 | 61.2 | 61.2 | 4.1 | 5.1 | 23.8 | 21.6 | 10.8 | 15.0 | 1.7 | 1.5 | 0.1 | 0.2 | 7.29 | 6.89 | 0.26 | 0.29 | 1.18 | 1.61 | 311.2 | 278.5 |
| 2 | 61.6 | 58.9 | 5.3 | 5.4 | 22.6 | 20.8 | 10.5 | 14.9 | 1.7 | 1.5 | 0.1 | 0.2 | 7.31 | 6.88 | 0.26 | 0.27 | 1.13 | 1.65 | 289.6 | 267.3 | |
| 4 | 60.8 | 58.3 | 6.8 | 6.4 | 23.1 | 19.5 | 9.3 | 15.8 | 1.6 | 1.3 | 0.1 | 0.3 | 7.11 | 6.51 | 0.23 | 0.22 | 1.23 | 1.71 | 257.1 | 254.9 | |
| 6 | 60.1 | 57.2 | 6.5 | 7.6 | 23.8 | 17.6 | 9.6 | 17.6 | 1.5 | 1.5 | 0.2 | 0.2 | 7.01 | 6.37 | 0.21 | 0.31 | 1.18 | 1.66 | 261 | 239.1 | |
| 8 | 58.2 | 56.9 | 7.9 | 8.9 | 24.1 | 22.1 | 9.8 | 12.1 | 1.4 | 1.1 | 0.1 | 0.2 | 6.99 | 6.68 | 0.28 | 0.23 | 1.14 | 1.58 | 245.8 | 211.3 | |
| S * | |||||||||||||||||||||
| XJ4 | 0 | 65.4 | 55.6 | 3.8 | 14.7 | 19.2 | 15.2 | 11.6 | 14.5 | 0.9 | 0.6 | 0.2 | 0.6 | 3.9 | 3.01 | 0.47 | 0.51 | 2.92 | 4.03 | 216.3 | 156.6 |
| 2 | 61.3 | 57.3 | 4.3 | 12.6 | 22.2 | 14.9 | 12.2 | 15.2 | 0.9 | 0.6 | 0.2 | 0.6 | 3.81 | 2.89 | 0.45 | 0.55 | 2.95 | 3.77 | 215.8 | 138.9 | |
| 4 | 58.2 | 51.9 | 7.7 | 16.2 | 15.3 | 16.6 | 18.8 | 15.3 | 0.8 | 0.5 | 0.3 | 0.6 | 3.63 | 2.71 | 0.51 | 0.52 | 2.91 | 3.62 | 208.1 | 166.8 | |
| 6 | 51.6 | 45.8 | 9.3 | 21.1 | 19.8 | 18.2 | 19.3 | 14.9 | 0.8 | 0.6 | 0.5 | 0.5 | 4.01 | 2.77 | 0.46 | 0.46 | 2.87 | 3.78 | 201.6 | 127.5 | |
| 8 | 42.1 | 38.9 | 8.8 | 24.6 | 24.5 | 20.2 | 24.6 | 16.3 | 0.7 | 0.4 | 0.3 | 0.4 | 3.87 | 2.58 | 0.45 | 0.48 | 2.61 | 4.11 | 186.2 | 132.9 | |
| S | 45.3 | 40.6 | 11.9 | 21.9 | 21.0 | 19.3 | 21.8 | 18.2 | 142.7 | 116.2 | 24.5 | 22.1 | 6.43 | 6.12 | 0.46 | 0.55 | 2.77 | 3.87 | 267.9 | 251.5 | |
| XJ6 | 0 | 58.6 | 53.3 | 12.2 | 15.2 | 15.6 | 8.5 | 13.6 | 23.0 | 1.3 | 0.6 | 0.3 | 0.5 | 1.99 | 1.58 | 0.51 | 0.62 | 2.33 | 2.8 | 68 | 50.1 |
| 2 | 56.2 | 48.6 | 12.9 | 17.8 | 15.1 | 9.9 | 15.8 | 23.7 | 1.3 | 0.5 | 0.4 | 0.5 | 2.01 | 1.49 | 0.55 | 0.66 | 2.35 | 2.76 | 63.2 | 46.3 | |
| 4 | 48.7 | 46.9 | 13.6 | 18.4 | 20.5 | 8.6 | 17.2 | 26.1 | 1.2 | 0.4 | 0.2 | 0.3 | 1.96 | 1.38 | 0.57 | 0.73 | 2.41 | 2.84 | 56.9 | 45.1 | |
| 6 | 42.1 | 40.2 | 14.1 | 17.3 | 24.5 | 14.4 | 19.3 | 28.1 | 1.1 | 0.3 | 0.3 | 0.4 | 1.86 | 1.39 | 0.62 | 0.81 | 2.26 | 2.66 | 60.1 | 41.6 | |
| 8 | 39.8 | 36.5 | 14.3 | 19.8 | 24.4 | 18.2 | 21.5 | 25.5 | 0.9 | 0.5 | 0.3 | 0.4 | 1.73 | 1.42 | 0.63 | 0.73 | 2.18 | 2.51 | 45.6 | 39.5 | |
| S | 46.9 | 45.2 | 15.5 | 19.0 | 19.8 | 14.7 | 17.8 | 21.1 | 86.3 | 72.1 | 31.3 | 46.8 | 2.05 | 1.65 | 0.54 | 0.66 | 2.17 | 2.89 | 167.3 | 105.2 | |
| BJ | 0 | 72.5 | 68.2 | 6.4 | 10.2 | 11.7 | 11.3 | 9.5 | 10.3 | 0.9 | 0.5 | 0.4 | 0.5 | 0.89 | 0.93 | 0.68 | 0.75 | 4.71 | 5.06 | 88.8 | 68.3 |
| 2 | 65.6 | 67.2 | 8.7 | 11.5 | 14.5 | 9.8 | 11.2 | 11.5 | 0.9 | 0.5 | 0.4 | 0.4 | 0.87 | 0.92 | 0.69 | 0.73 | 4.71 | 5.01 | 86.4 | 66.2 | |
| 4 | 66.7 | 66.1 | 8.4 | 12.6 | 11.4 | 9.0 | 13.5 | 12.3 | 0.8 | 0.6 | 0.7 | 0.4 | 0.91 | 0.96 | 0.68 | 0.78 | 4.74 | 4.71 | 82.1 | 61.8 | |
| 6 | 68.2 | 64.2 | 6.2 | 13.1 | 13.8 | 10.8 | 11.8 | 11.9 | 0.5 | 0.4 | 0.3 | 0.6 | 0.84 | 0.91 | 0.66 | 0.81 | 4.81 | 4.75 | 78.9 | 55.6 | |
| 8 | 63.5 | 63.8 | 11.3 | 14.1 | 12.9 | 9.3 | 12.3 | 12.8 | 0.7 | 0.7 | 0.5 | 0.4 | 0.91 | 0.89 | 0.71 | 0.63 | 4.55 | 4.68 | 68.1 | 46.3 | |
| S | 62.8 | 61.3 | 8.8 | 15.1 | 15.2 | 12.0 | 13.2 | 11.6 | 25.1 | 19.8 | 13.8 | 22.9 | 0.93 | 0.81 | 0.65 | 0.68 | 4.36 | 4.71 | 31.6 | 26.7 | |
| DJ | 0 | 63.9 | 53.6 | 10.1 | 16.0 | 9.9 | 10.1 | 16.0 | 20.5 | 0.3 | 0.4 | 0.4 | 0.6 | 1.11 | 1.21 | 0.72 | 0.81 | 3.16 | 4.88 | 45.2 | 39.8 |
| 2 | 62.5 | 52.5 | 8.9 | 14.1 | 11.3 | 11.7 | 17.3 | 21.7 | 0.3 | 0.2 | 0.4 | 0.5 | 1.12 | 1.14 | 0.72 | 0.75 | 3.17 | 4.89 | 46.7 | 38.1 | |
| 4 | 62.1 | 51.3 | 9.2 | 11.9 | 10.5 | 13.2 | 18.2 | 23.6 | 0.2 | 0.3 | 0.3 | 0.4 | 1.05 | 1.12 | 0.73 | 0.79 | 3.22 | 4.85 | 44.2 | 36.3 | |
| 6 | 60.1 | 52.9 | 9.9 | 13.5 | 12.8 | 11.5 | 17.2 | 22.1 | 0.4 | 0.4 | 0.3 | 0.6 | 1.03 | 1.15 | 0.77 | 0.74 | 3.19 | 4.66 | 42.8 | 37.8 | |
| 8 | 58.9 | 52.7 | 11.0 | 9.9 | 13.2 | 12.8 | 16.9 | 24.6 | 0.4 | 0.3 | 0.3 | 0.5 | 1.02 | 1.04 | 0.72 | 0.73 | 3.12 | 4.81 | 43.6 | 39.6 | |
| S | 63.7 | 55.8 | 8.3 | 7.6 | 12.2 | 14.1 | 15.8 | 22.5 | 21.4 | 16.2 | 16.9 | 24.8 | 0.85 | 0.99 | 0.74 | 0.89 | 3.64 | 4.53 | 41.2 | 36.1 | |
| Site | Depth (m) | d(0.5) (μm) | DO (mg/L) | pH | EC (μs/cm) | T (℃) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | ||
| XJ2 | 0 | 108.58 | 165.21 | 9.77 | 7.60 | 8.34 | 8.08 | 381 | 428 | 18.1 | 23.4 |
| 2 | 102.58 | 150.26 | 9.14 | 8.41 | 7.99 | 8.06 | 313 | 435 | 17.8 | 23.6 | |
| 4 | 101.16 | 132.66 | 9.05 | 8.22 | 7.96 | 8.01 | 311 | 431 | 17.8 | 21.3 | |
| 6 | 131.36 | 129.63 | 8.92 | 7.69 | 7.89 | 7.90 | 309 | 425 | 17.8 | 23.9 | |
| 8 | 157.07 | 128.33 | 8.76 | 7.41 | 7.80 | 7.82 | 306 | 418 | 17.6 | 23.8 | |
| XJ3 | 0 | 7.61 | 11.35 | 7.89 | 10.27 | 7.57 | 8.56 | 381 | 320 | 18.4 | 28.9 |
| 2 | 6.93 | 10.78 | 7.61 | 11.98 | 7.93 | 8.58 | 402 | 329 | 18.6 | 28.3 | |
| 4 | 6.86 | 10.77 | 7.69 | 9.54 | 7.88 | 8.57 | 382 | 322 | 18.1 | 27.9 | |
| 6 | 6.35 | 10.27 | 7.87 | 8.16 | 7.87 | 8.16 | 383 | 326 | 18.0 | 26.6 | |
| 8 | 6.07 | 9.46 | 7.86 | 8.03 | 7.88 | 8.02 | 381 | 329 | 18.0 | 26.7 | |
| XJ4 | 0 | 29.62 | 42.22 | 8.83 | 7.92 | 7.97 | 7.79 | 350 | 336 | 18.6 | 25.7 |
| 2 | 29.38 | 41.52 | 8.98 | 7.61 | 7.95 | 7.86 | 353 | 335 | 18.6 | 26.3 | |
| 4 | 29.20 | 41.42 | 8.62 | 7.67 | 7.95 | 7.78 | 349 | 337 | 18.7 | 25.8 | |
| 6 | 28.48 | 40.98 | 8.55 | 7.33 | 7.94 | 7.83 | 348 | 336 | 18.6 | 26.0 | |
| 8 | 24.26 | 33.38 | 8.61 | 7.30 | 7.58 | 7.89 | 349 | 336 | 18.7 | 26.5 | |
| XJ6 | 0 | 36.08 | 49.02 | 8.70 | 6.77 | 7.76 | 7.63 | 310 | 227 | 17.9 | 28.0 |
| 2 | 35.56 | 48.22 | 8.75 | 6.46 | 7.80 | 7.88 | 312 | 172.4 | 17.8 | 28.6 | |
| 4 | 35.42 | 47.05 | 8.73 | 6.80 | 7.79 | 7.85 | 312 | 174.7 | 17.8 | 28.7 | |
| 6 | 35.20 | 44.15 | 8.71 | 6.99 | 7.79 | 7.86 | 312 | 167.4 | 17.6 | 28.9 | |
| 8 | 23.91 | 31.07 | 8.70 | 6.93 | 7.78 | 7.81 | 312 | 167.9 | 17.6 | 29.1 | |
| BJ | 0 | 13.37 | 18.59 | 9.54 | 5.72 | 7.63 | 7.07 | 237 | 96.2 | 15.6 | 29.2 |
| 2 | 12.82 | 14.25 | 10.37 | 5.65 | 7.89 | 7.54 | 241 | 96.6 | 15.0 | 29.8 | |
| 4 | 12.70 | 13.85 | 10.27 | 5.54 | 7.87 | 7.69 | 239 | 93.6 | 14.8 | 31.5 | |
| 6 | 12.43 | 13.69 | 10.33 | 5.19 | 7.85 | 7.33 | 240 | 94.0 | 14.8 | 30.0 | |
| 8 | 10.83 | 12.69 | 10.33 | 4.54 | 7.92 | 7.47 | 240 | 104.6 | 14.9 | 28.6 | |
| DJ | 0 | 13.78 | 13.69 | 9.00 | 6.01 | 7.12 | 7.52 | 135 | 172.6 | 17.5 | 28.3 |
| 2 | 12.39 | 12.10 | 9.36 | 6.26 | 7.15 | 7.63 | 138 | 171.8 | 17.5 | 29.8 | |
| 4 | 11.99 | 12.08 | 9.28 | 5.79 | 7.34 | 7.58 | 136 | 168.1 | 17.1 | 29.6 | |
| 6 | 11.48 | 10.27 | 9.23 | 5.75 | 7.48 | 7.52 | 136 | 174.4 | 17.1 | 28.7 | |
| 8 | 9.73 | 8.737 | 9.15 | 5.70 | 7.43 | 7.48 | 135 | 170.6 | 17.3 | 29.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Liu, Z.; Sun, H.; Zhao, M.; He, H. Lipid Biomarker Investigation of the Delivery and Preservation of Autochthonous Organic Carbon in the Pearl River and Its Contribution to the Carbon Sink: Evidence from the Water and Surface Sediment. Int. J. Environ. Res. Public Health 2022, 19, 15392. https://doi.org/10.3390/ijerph192215392
Yang M, Liu Z, Sun H, Zhao M, He H. Lipid Biomarker Investigation of the Delivery and Preservation of Autochthonous Organic Carbon in the Pearl River and Its Contribution to the Carbon Sink: Evidence from the Water and Surface Sediment. International Journal of Environmental Research and Public Health. 2022; 19(22):15392. https://doi.org/10.3390/ijerph192215392
Chicago/Turabian StyleYang, Mingxing, Zaihua Liu, Hailong Sun, Min Zhao, and Haibo He. 2022. "Lipid Biomarker Investigation of the Delivery and Preservation of Autochthonous Organic Carbon in the Pearl River and Its Contribution to the Carbon Sink: Evidence from the Water and Surface Sediment" International Journal of Environmental Research and Public Health 19, no. 22: 15392. https://doi.org/10.3390/ijerph192215392
APA StyleYang, M., Liu, Z., Sun, H., Zhao, M., & He, H. (2022). Lipid Biomarker Investigation of the Delivery and Preservation of Autochthonous Organic Carbon in the Pearl River and Its Contribution to the Carbon Sink: Evidence from the Water and Surface Sediment. International Journal of Environmental Research and Public Health, 19(22), 15392. https://doi.org/10.3390/ijerph192215392






