Left Atrial Wall Motion Velocity Assessed during Atrial Fibrillation Predicts Sinus Rhythm Maintenance after Electrical Cardioversion in Patients with Persistent Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data
2.3. Sinus Rhythm Restoration
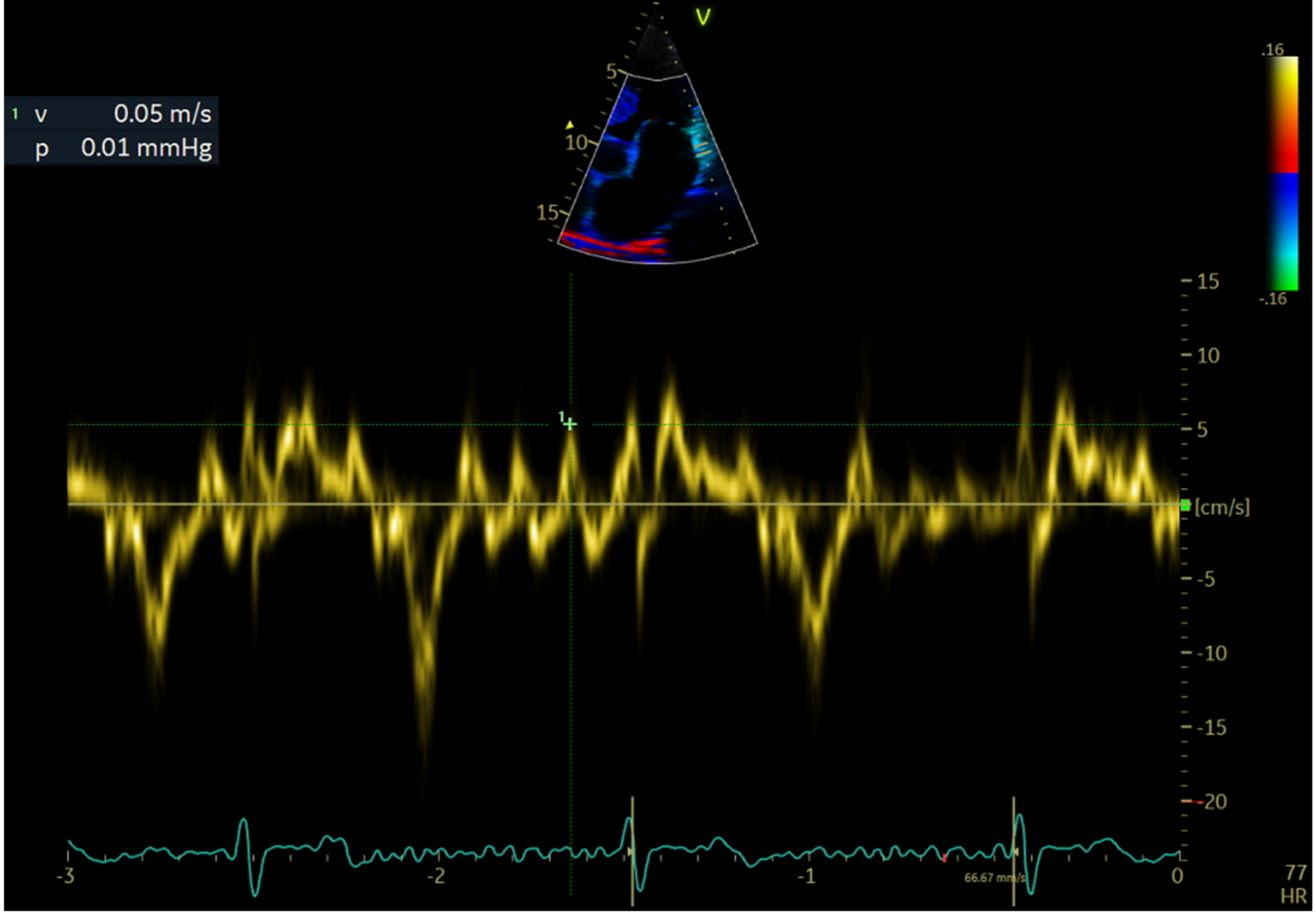
2.4. Echocardiographic Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Nieuwlaat, R.; Prins, M.H.; Le Heuzey, J.Y.; Vardas, P.E.; Aliot, E.; Santini, M.; Cobbe, S.M.; Widdershoven, J.W.; Baur, L.H.; Lévy, S.; et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: Follow-up of the Euro Heart Survey on atrial fibrillation. Eur. Heart J. 2008, 29, 1181–1189. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Jones, D.M.; Wang, T.J.; Leip, E.P.; Larson, M.G.; Levy, D.; Vasan, R.S.; D’Agostino, R.B.; Massaro, J.M.; Beiser, A.; Wolf, P.A.; et al. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation 2004, 110, 1042–1046. [Google Scholar] [CrossRef]
- Bartkowiak, R.; Wozakowska-Kapłon, B.; Janiszewska, G. Plasma NT-proANP in patients with persistent atrial fibrillation who underwent successful cardioversion. Kardiol. Pol. 2010, 68, 48–54. [Google Scholar]
- Gumprecht, J.; Szulik, M.; Domek, M.; Mazurek, M.; Shantsila, A.; Oxborough, D.; Lip, G.Y.H. Novel Echocardiographic Biomarkers in the Management of Atrial Fibrillation. Curr. Cardiovasc. Imaging Rep. 2019, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Vizzardi, E.; Curnis, A.; Latini, M.G.; Salghetti, F.; Rocco, E.; Lupi, L.; Rovetta, R.; Quinzani, F.; Bonadei, I.; Bontempi, L.; et al. Risk factors for atrial fibrillation recurrence: A literature review. J. Cardiovasc. Med. (Hagerstown) 2014, 15, 235–253. [Google Scholar] [CrossRef]
- Wałek, P.; Gorczyca, I.; Grabowska, U.; Spałek, M.; Wożakowska-Kapłon, B. The prognostic value of soluble suppression of tumourigenicity 2 and galectin-3 for sinus rhythm maintenance after cardioversion due to persistent atrial fibrillation in patients with normal left ventricular systolic function. Europace 2020, 22, 1470–1479. [Google Scholar] [CrossRef]
- Wożakowska-Kapłon, B.; Bartkowiak, R. Biomarkers for prognosis in atrial fibrillation: Unfulfilled hopes. Pol. Arch. Med. Wewn. 2015, 125, 400–401. [Google Scholar] [CrossRef] [Green Version]
- Wijffels, M.C.; Kirchhof, C.J.; Dorland, R.; Allessie, M.A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef]
- Chung, H.; Lee, B.K.; Min, P.K.; Choi, E.Y.; Yoon, Y.W.; Hong, B.K.; Rim, S.J.; Kwon, H.M.; Kim, J.Y. Left Ventricular Filling Pressure as Assessed by the E/e′ Ratio Is a Determinant of Atrial Fibrillation Recurrence after Cardioversion. Yonsei Med. J. 2016, 57, 64–71. [Google Scholar] [CrossRef]
- De Vos, C.B.; Limantoro, I.; Pisters, R.; Delhaas, T.; Schotten, U.; Cheriex, E.C.; Tieleman, R.G.; Crijns, H.J. The mechanical fibrillation pattern of the atrial myocardium is associated with acute and long-term success of electrical cardioversion in patients with persistent atrial fibrillation. Heart Rhythm 2014, 11, 1514–1521. [Google Scholar] [CrossRef]
- Marchese, P.; Bursi, F.; Delle Donne, G.; Malavasi, V.; Casali, E.; Barbieri, A.; Melandri, F.; Modena, M.G. Indexed left atrial volume predicts the recurrence of non-valvular atrial fibrillation after successful cardioversion. Eur. J. Echocardiogr. 2011, 12, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Marchese, P.; Malavasi, V.; Rossi, L.; Nikolskaya, N.; Donne, G.D.; Becirovic, M.; Colantoni, A.; Luciani, A.; Modena, M.G. Indexed left atrial volume is superior to left atrial diameter in predicting nonvalvular atrial fibrillation recurrence after successful cardioversion: A prospective study. Echocardiography 2012, 29, 276–284. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Castelli, A.; Andria, A.; Mattioli, G. Clinical and echocardiographic features influencing recovery of atrial function after cardioversion of atrial fibrillation. Am. J. Cardiol. 1998, 82, 1368–1371. [Google Scholar] [CrossRef]
- Okçün, B.; Yigit, Z.; Küçükoglu, M.S.; Mutlu, H.; Sansoy, V.; Güzelsoy, D.; Uner, S. Predictors for maintenance of sinus rhythm after cardioversion in patients with nonvalvular atrial fibrillation. Echocardiography 2002, 19, 351–357. [Google Scholar] [CrossRef]
- Antonielli, E.; Pizzuti, A.; Pálinkás, A.; Tanga, M.; Gruber, N.; Michelassi, C.; Varga, A.; Bonzano, A.; Gandolfo, N.; Halmai, L.; et al. Clinical value of left atrial appendage flow for prediction of long-term sinus rhythm maintenance in patients with nonvalvular atrial fibrillation. J. Am. Coll. Cardiol. 2002, 39, 1443–1449. [Google Scholar] [CrossRef] [Green Version]
- Luong, C.L.; Thompson, D.J.; Gin, K.G.; Jue, J.; Nair, P.; Lee, P.K.; Tsang, M.Y.; Barnes, M.E.; Colley, P.; Tsang, T.S. Usefulness of the Atrial Emptying Fraction to Predict Maintenance of Sinus Rhythm After Direct Current Cardioversion for Atrial Fibrillation. Am. J. Cardiol. 2016, 118, 1345–1349. [Google Scholar] [CrossRef]
- Melduni, R.M.; Lee, H.C.; Bailey, K.R.; Miller, F.A., Jr.; Hodge, D.O.; Seward, J.B.; Gersh, B.J.; Ammash, N.M. Real-time physiologic biomarker for prediction of atrial fibrillation recurrence, stroke, and mortality after electrical cardioversion: A prospective observational study. Am. Heart J. 2015, 170, 914–922. [Google Scholar] [CrossRef]
- Wałek, P.; Sielski, J.; Gorczyca, I.; Roskal-Wałek, J.; Starzyk, K.; Jaskulska-Niedziela, E.; Bartkowiak, R.; Wożakowska-Kapłon, B. Left atrial mechanical remodelling assessed as the velocity of left atrium appendage wall motion during atrial fibrillation is associated with maintenance of sinus rhythm after electrical cardioversion in patients with persistent atrial fibrillation. PLoS ONE 2020, 15, e0228239. [Google Scholar] [CrossRef]
- Wałek, P.; Sielski, J.; Starzyk, K.; Gorczyca, I.; Roskal-Wałek, J.; Wożakowska-Kapłon, B. Echocardiographic assessment of left atrial morphology and function to predict maintenance of sinus rhythm after electrical cardioversion in patients with non-valvular persistent atrial fibrillation and normal function or mild dysfunction of left ventricle. Cardiol. J. 2020, 27, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Dell’Era, G.; Rondano, E.; Franchi, E.; Marino, P.N. Atrial asynchrony and function before and after electrical cardioversion for persistent atrial fibrillation. Eur. J. Echocardiogr. 2010, 11, 577–583. [Google Scholar] [CrossRef]
- Di Salvo, G.; Caso, P.; Lo Piccolo, R.; Fusco, A.; Martiniello, A.R.; Russo, M.G.; D’Onofrio, A.; Severino, S.; Calabró, P.; Pacileo, G.; et al. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: A color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation 2005, 112, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Doruchowska, A.; Wita, K.; Bochenek, T.; Szydło, K.; Filipecki, A.; Staroń, A.; Wróbel, W.; Krzych, L.; Trusz-Gluza, M. Role of left atrial speckle tracking echocardiography in predicting persistent atrial fibrillation electrical cardioversion success and sinus rhythm maintenance at 6 months. Adv. Med.Sci. 2014, 59, 120–125. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.P.; Yoon, H.J.; Park, H.S.; Cho, Y.K.; Nam, C.W.; Hur, S.H.; Kim, Y.N.; Kim, K.B. Association between Doppler flow of atrial fibrillatory contraction and recurrence of atrial fibrillation after electrical cardioversion. J. Am. Soc. Echocardiogr. 2014, 27, 1107–1112. [Google Scholar] [CrossRef]
- Moreno-Ruiz, L.A.; Madrid-Miller, A.; Martínez-Flores, J.E.; González-Hermosillo, J.A.; Arenas-Fonseca, J.; Zamorano-Velázquez, N.; Mendoza-Pérez, B. Left atrial longitudinal strain by speckle tracking as independent predictor of recurrence after electrical cardioversion in persistent and long standing persistent non-valvular atrial fibrillation. Int. J. Cardiovasc. Imaging 2019, 35, 1587–1596. [Google Scholar] [CrossRef] [Green Version]
- Rondano, E.; Dell’Era, G.; De Luca, G.; Piccinino, C.; Bellomo, G.; Marino, P.N. Left atrial asynchrony is a major predictor of 1-year recurrence of atrial fibrillation after electrical cardioversion. J. Cardiovasc. Med. (Hagerstown) 2010, 11, 499–506. [Google Scholar] [CrossRef]
- Shaikh, A.Y.; Maan, A.; Khan, U.A.; Aurigemma, G.P.; Hill, J.C.; Kane, J.L.; Tighe, D.A.; Mick, E.; McManus, D.D. Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: A prospective study. Cardiovasc. Ultrasound 2012, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, M.; Fung, J.W.; Yip, G.W.; Zhang, Y.; Ho, P.P.; Tse, D.M.; Yu, C.M.; Sanderson, J.E. Atrial strain rate echocardiography can predict success or failure of cardioversion for atrial fibrillation: A combined transthoracic tissue Doppler and transoesophageal imaging study. Int. J. Cardiol. 2007, 114, 202–209. [Google Scholar] [CrossRef]
- Müller, P.; Schiedat, F.; Bialek, A.; Bösche, L.; Ewers, A.; Kara, K.; Dietrich, J.W.; Mügge, A.; Deneke, T. Total atrial conduction time assessed by tissue doppler imaging (PA-TDI Interval) to predict early recurrence of persistent atrial fibrillation after successful electrical cardioversion. J. Cardiovasc. Electrophysiol. 2014, 25, 161–167. [Google Scholar] [CrossRef]
- Wałek, P.; Ciesla, E.; Gorczyca, I.; Wożakowska-Kapłon, B. Left atrial wall dyskinesia assessed during contractile phase as a predictor of atrial fibrillation recurrence after electrical cardioversion performed due to persistent atrial fibrillation. Medicine (Baltimore) 2020, 99, e23333. [Google Scholar] [CrossRef]
- Wałek, P.; Gorczyca, I.; Sielski, J.; Wożakowska-Kapłon, B. Left atrial emptying fraction determined during atrial fibrillation predicts maintenance of sinus rhythm after direct current cardioversion in patients with persistent atrial fibrillation. PLoS ONE 2020, 15, e0238002. [Google Scholar] [CrossRef]
- Wałek, P.; Grabowska, U.; Cieśla, E.; Gorczyca, I.; Wożakowska-Kapłon, B. Left atrial longitudinal strain in the contractile phase as a predictor of sinus rhythm maintenance after electrical cardioversion performed due to persistent atrial fibrillation. Kardiol. Pol. 2021, 79, 458–460. [Google Scholar] [CrossRef]
- Wałek, P.; Grabowska, U.; Cieśla, E.; Sielski, J.; Roskal-Wałek, J.; Wożakowska-Kapłon, B. Analysis of the Correlation of Galectin-3 Concentration with the Measurements of Echocardiographic Parameters Assessing Left Atrial Remodeling and Function in Patients with Persistent Atrial Fibrillation. Biomolecules 2021, 11, 1108. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Pastore, M.C.; De Carli, G.; Mandoli, G.E.; D’Ascenzi, F.; Focardi, M.; Contorni, F.; Mondillo, S.; Cameli, M. The prognostic role of speckle tracking echocardiography in clinical practice: Evidence and reference values from the literature. Heart Fail. Rev. 2021, 26, 1371–1381. [Google Scholar] [CrossRef]
- Uretsky, S.; Shah, A.; Bangalore, S.; Rosenberg, L.; Sarji, R.; Cantales, D.R.; Macmillan-Marotti, D.; Chaudhry, F.A.; Sherrid, M.V. Assessment of left atrial appendage function with transthoracic tissue Doppler echocardiography. Eur. J. Echocardiogr. 2009, 10, 363–371. [Google Scholar] [CrossRef]



| Variables | Study Population n = 126 (100%) | Sinus Rhythm Maintenance n = 55 (43.7%) | Cardioversion Failure or AF Recurrence n = 71 (56.7%) | p-Value |
|---|---|---|---|---|
| Age (years), mean (SD) | 65.10 (10.17) | 63.38 (11.64) | 66.44 (8.74) | 0.273 |
| Age < 65 years, n (%) | 49 (38.89) | 24 (43.64) | 25 (35.21) | 0.486 |
| Age 65–74 years, n (%) | 58 (46.03) | 22 (40.00) | 36 (50.70) | |
| Age ≥ 75 years, n (%) | 19 (15.08) | 9 (15.08) | 10 (14.08) | |
| Male sex, n (%) | 75 (59.52) | 40 (72.73) | 35 (49.30) | 0.008 |
| BMI (kg/m2), mean (SD) | 30.48 (4.64) | 30.80 (3.99) | 30.24 (5.11) | 0.291 |
| Hypertension, n (%) | 107 (84.92) | 45 (81.82) | 62 (87.32) | 0.392 |
| Diabetes mellitus, n (%) | 28 (22.22) | 13 (23.64) | 15 (21.13) | 0.737 |
| Coronary artery disease, n (%) | 20 (15.87) | 9 (16.36) | 11 (15.49) | 0.895 |
| Stroke/TIA, n (%) | 12 (9.52) | 4 (7.27) | 8 (11.27) | 0.449 |
| CHA2DS2-VAScscore, mean (SD) | 2.83 (1.54) | 2.65 (1.49) | 2.97 (1.57) | 0.243 |
| CHA2DS2-VASc = 0, n (%) | 6 (4.76) | 3 (5.45) | 3 (4.23) | 0.863 |
| CHA2DS2-VASc = 1, n (%) | 21 (16.67) | 10 (18.18) | 11 (15.49) | |
| CHA2DS2-VASc ≥ 2, n (%) | 99 (78.57) | 42 (76.36) | 57 (80.28) | |
| Smokers, n (%) | 10 (7.94) | 3 (5.45) | 7 (9.86) | 0.364 |
| Heart failure, n (%) | 40 (31.75) | 20 (36.36) | 20 (28.17) | 0.327 |
| MDRD (mL/m2) mean (SD) | 64.79 (16.67) | 69.28 (16.59) | 61.31 (15.99) | 0.005 |
| Creatinine (mg/dL), mean (SD) | 1.13 (0.23) | 1.10 (0.19) | 1.14 (0.25) | 0.454 |
| TnT (ng/L), n = 126, mean (SD) | 11.23 (8.80) | 11.86 (8.70) | 10.75 (8.91) | 0.685 |
| HbA1c (%), n = 106, mean (SD) | 6.11 (0.88) | 6.02 (0.91) | 6.17 (0.85) | 0.156 |
| Medication use before DCCV | ||||
| Beta-blockers, n (%) | 114 (90.48) | 53 (96.36) | 61 (85.92) | 0.048 |
| Amiodarone, n (%) | 13 (10.32) | 4 (7.27) | 9 (12.68) | 0.322 |
| ACE inhibitors/ARB, n (%) | 106 (84.13) | 46 (83.64) | 60 (84.51) | 0.894 |
| Statins, n (%) | 81 (64.29) | 38 (69.09) | 43 (60.56) | 0.322 |
| Diuretics, n (%) | 59 (46.83) | 19 (34.55) | 40 (56.34) | 0.015 |
| Spironolactone/eplerenone, n (%) | 23 (18.25) | 13 (23.64) | 10 (14.08) | 0.169 |
| Medication use post DCCV | ||||
| Beta-blockers, n (%) | 104 (82.54) | 48 (87.27) | 56 (78.87) | 0.218 |
| Amiodarone, n (%) | 40 (31.75) | 17 (30.91) | 23 (32.39) | 0.859 |
| Propafenone, n (%) | 33 (26.19) | 17 (30.91) | 16 (22.54) | 0.289 |
| ACE inhibitors/ARB, n (%) | 104)82.54) | 46 (83.64) | 58 (81.69) | 0.775 |
| Statins, n (%) | 81 (64.29) | 35 (63.64) | 46 (64.79) | 0.893 |
| Diuretics, n (%) | 61 (48.41) | 19 (34.55) | 42 (59.15) | 0.006 |
| Spironolactone/eplerenonen (%) | 28 (22.22) | 15 (27.27) | 13 (18.31) | 0.230 |
| Variable | Study Population n = 126 (100%) | Sinus Rhythm Maintenance n = 55 (43.7%) | Cardioversion Failure or AF Recurrence n = 71 (56.7%) | p-Value |
|---|---|---|---|---|
| RVOT (mm) | 31.17 (3.90) | 32.04 (4.11) | 30.51 (3.62) | 0.029 |
| IVS (mm) | 10.73 (1.69) | 10.69 (1.67) | 10.76 (1.71) | 0.926 |
| LVEDD (mm) | 51.13 (6.50) | 51.62 (6.31) | 50.75 (6.66) | 0.457 |
| LVESD (mm) | 35.92 (7.60) | 36.44 (7.73) | 35.52 (7.52) | 0.504 |
| LVEDV (mL) | 117.21 (34.60) | 124.04 (32.56) | 111.93 (35.42) | 0.051 |
| LVESV (mL) | 52.49 (20.63) | 54.59 (18.41) | 50.87 (22.19) | 0182 |
| LVSV (mL) | 65.00 (21.18) | 69.47 (19.43) | 61.55 (21.96) | 0.013 |
| LVEF (%) | 57.13 (9.80) | 56.98 (7.82) | 57.24 (11.15) | 0.884 |
| LAAP (mm) | 43.98 (4.40) | 43.47 (4.14) | 44.38 (4.57) | 0.252 |
| LAVI (mL/m2) | 47.09 (12.28) | 42.84 (9.43) | 50.39 (13.24) | <0.001 |
| LAEDVI (mL/m2) | 34.22 (12.05) | 29.15 (8.63) | 38.14 (12.89) | <0.001 |
| LAEF (%) | 26.84 (10.09) | 31.13 (7.99) | 23.52 (10.35) | <0.001 |
| RAA s (cm2) | 22.29 (5.18) | 21.30 (5.53) | 23.02 (4.82) | 0.071 |
| RAA d (cm2) | 16.66 (4.41) | 16.07 (4.79) | 17.09 (4.09) | 0.061 |
| s′ lat (cm/s) | 6.57 (1.90) | 7.13 (1.83) | 6.13 (1.85) | 0.002 |
| e′ lat (cm/s) | 11.57 (2.96) | 12.37 (2.69) | 10.95 (3.02) | 0.007 |
| s′ mid (cm/s) | 5.46 (1.62) | 6.18 (1.73) | 4.91 (1.29) | <0.001 |
| e′ mid (cm/s) | 8.25 (2.33) | 9.10 (2.63) | 7.59 (1.82) | 0.001 |
| s′ mean (cm/s) | 6.01 (1.66) | 6.65 (1.68) | 5.51 (1.46) | <0.001 |
| e′ mean (cm/s) | 9.91 (2.36) | 10.74 (2.29) | 9.27 (2.22) | <0.001 |
| E (m/s) | 0.88 (0.19) | 0.83 (0.15) | 0.93 (0.21) | <0.006 |
| LAWMV (cm/s) | 3.22 (1.07) | 3.69 (0.84) | 2.86 (1.09) | <0.001 |
| Variable | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| LAVI (ml/m2) | 0.94 | 0.91–0.98 | 0.001 | 0.96 | 0.92–1.00 | 0.050 |
| e′ mean (cm/s) | 1.36 | 1.13–1.63 | 0.001 | 1.18 | 0.96–1.47 | 0.116 |
| LAWMV (cm/s) | 2.29 | 1.54–3.39 | <0.001 | 1.81 | 1.19–2.77 | 0.006 |
| Male sex | 2.74 | 1.29–5.83 | 0.009 | 1.86 | 0.79–4.39 | 0.156 |
| Diuretics post | 0.36 | 0.18–0.76 | 0.007 | 0.60 | 0.26–1.41 | 0.242 |
| Variable | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| LAVI (ml/m2) | 0.94 | 0.91–0.98 | 0.001 | 0.97 | 0.93–1.00 | 0.081 |
| LAEF (%) | 1.09 | 1.05–1.15 | <0.001 | 1.04 | 0.99–1.10 | 0.082 |
| e′ mean (cm/s) | 1.36 | 1.13–1.63 | 0.001 | 1.19 | 0.95–1.49 | 0.132 |
| E (m/s) | 0.06 | 0.01–0.43 | 0.006 | 0.32 | 0.03–3.83 | 0.369 |
| LAWMV (cm/s) | 2.29 | 1.54–3.39 | <0.001 | 1.72 | 1.10–2.69 | 0.017 |
| Variable | AUC (95% CI) | p-Value | Cut-Off Value (cm/s) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| LAWMV | 0.911 (0.862–0.961) | <0.001 | 3.0 | 100 | 81.3 | 100 | 18.7 |
| Variable | AUC (95% CI) | p-Value | Cut-Off Value (cm/s) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| LAWMV | 0.738 (0.651–0.825) | <0.001 | 3.0 | 0.927 | 0.493 | 0.586 | 0.897 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wałek, P.; Roskal-Wałek, J.; Dłubis, P.; Tracz, J.; Wożakowska-Kapłon, B. Left Atrial Wall Motion Velocity Assessed during Atrial Fibrillation Predicts Sinus Rhythm Maintenance after Electrical Cardioversion in Patients with Persistent Atrial Fibrillation. Int. J. Environ. Res. Public Health 2022, 19, 15508. https://doi.org/10.3390/ijerph192315508
Wałek P, Roskal-Wałek J, Dłubis P, Tracz J, Wożakowska-Kapłon B. Left Atrial Wall Motion Velocity Assessed during Atrial Fibrillation Predicts Sinus Rhythm Maintenance after Electrical Cardioversion in Patients with Persistent Atrial Fibrillation. International Journal of Environmental Research and Public Health. 2022; 19(23):15508. https://doi.org/10.3390/ijerph192315508
Chicago/Turabian StyleWałek, Paweł, Joanna Roskal-Wałek, Patryk Dłubis, Justyna Tracz, and Beata Wożakowska-Kapłon. 2022. "Left Atrial Wall Motion Velocity Assessed during Atrial Fibrillation Predicts Sinus Rhythm Maintenance after Electrical Cardioversion in Patients with Persistent Atrial Fibrillation" International Journal of Environmental Research and Public Health 19, no. 23: 15508. https://doi.org/10.3390/ijerph192315508






