Sensitivity of Pathogenic Bacteria Strains to Treated Mine Water
Abstract
1. Introduction
2. Materials and Methods
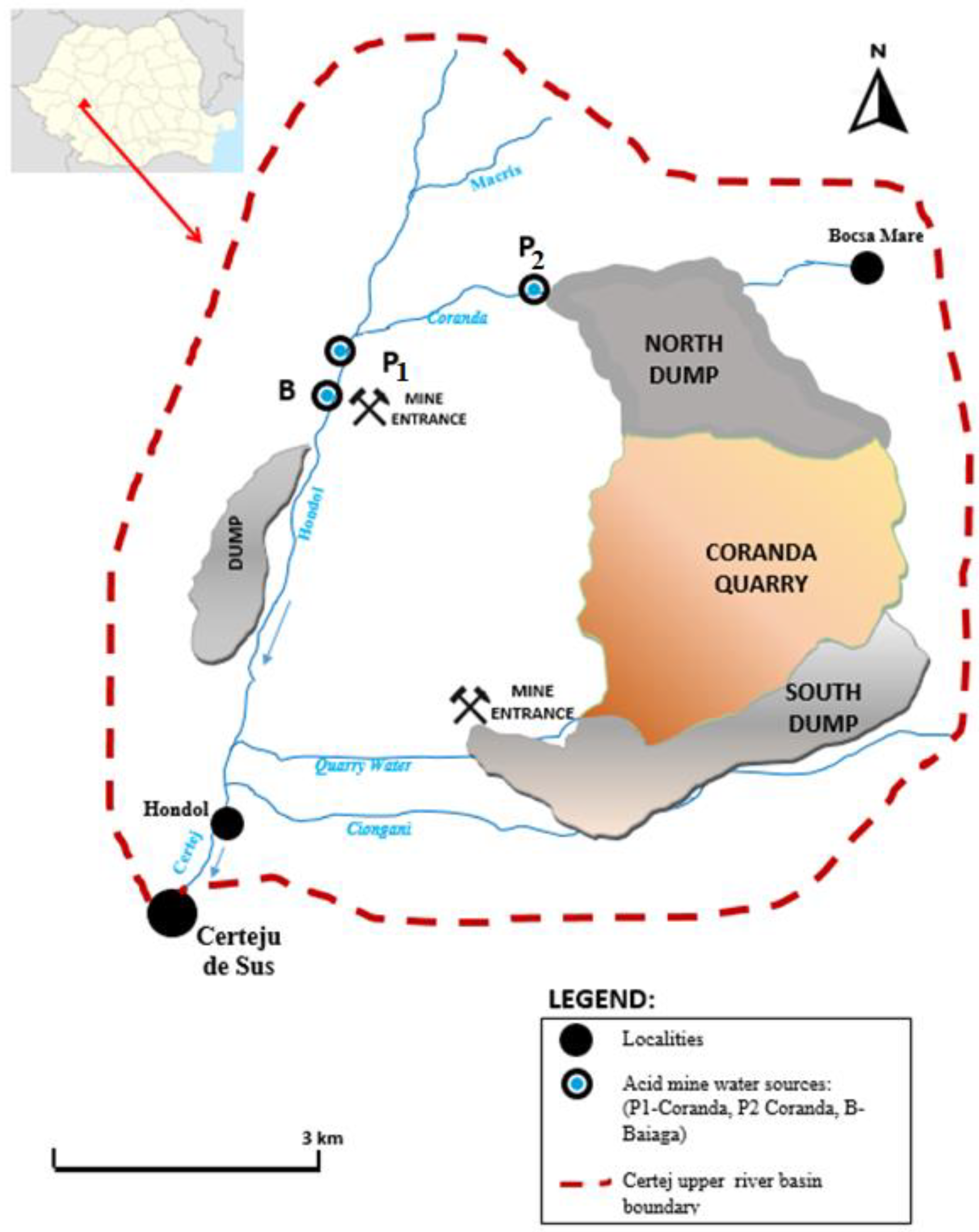
2.1. Study Area
2.2. Experiment Conditions
2.3. Bacterial Growth Inhibition
2.4. Genotoxicity Test
2.5. Statistical Analysis
3. Results
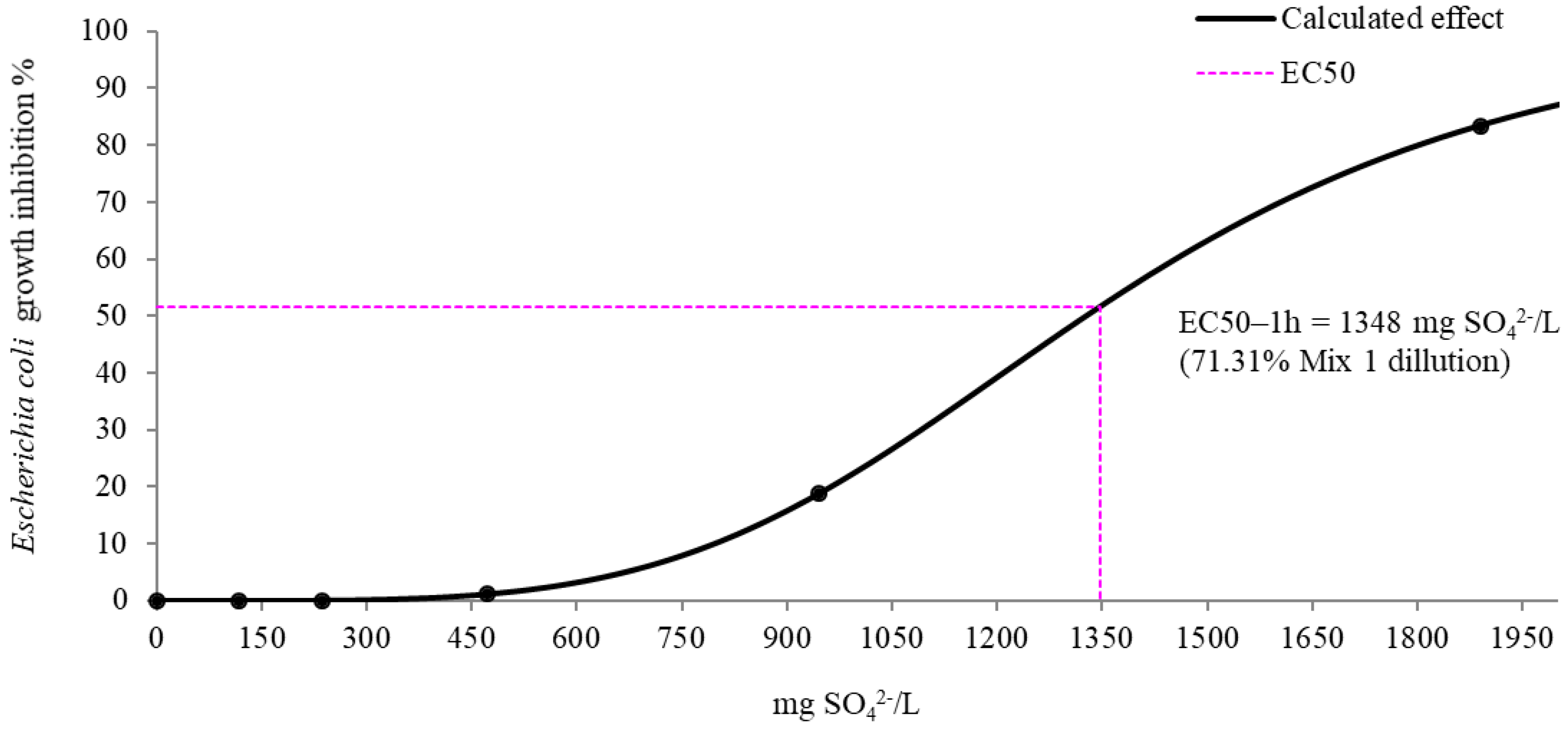
3.1. Bacterial Growth Inhibition
3.2. Genotoxicity Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valavanidis, A. Current environmental issues and emerging global challenges in the 21st century for environmental protection and sustainable development. Sci. Rev. 2019, 1, 1–53. [Google Scholar]
- Blasco, J.; Chapman, P.; Compana, O.; Hampel, M. Marine Ecotoxicology, Current Knowledge and Future Issues; Academic Press: London, UK; New York, NY, USA, 2016; pp. 1–334. [Google Scholar]
- Walker, C. Ecotoxicology. Effects of Pollutants in the Natural Environment; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–233. [Google Scholar]
- Carvalho, F.P. Mining industry and sustainable development: Time for change. Food. Energy Secur. 2017, 6, 61–67. [Google Scholar] [CrossRef]
- Jain, R.; Cui, Z.C.; Domen, J.K. Environmental Impact of Mining and Mineral Processing; Elsevier: Amsterdam, The Netherlands; Butterworth-Heinemann Publ.: Oxford, UK, 2016; p. 322. [Google Scholar]
- Nordstrom, D.K. Mine waters: Acidic to circumneutral. Elements 2011, 7, 393–398. [Google Scholar] [CrossRef]
- Jarosławiecka, A.K.; Piotrowska-Seget, Z. The effect of heavy metals on microbial communities in industrial soil in the area of Piekary Slaskie and Bukowno (Poland). Microbiol. Res. 2022, 13, 626–642. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- van Dam, R.A.; Harford, A.J.; Lunn, S.A.; Gagnon, M.M. Identifying the Cause of Toxicity of a Saline Mine Water. PLoS ONE 2014, 9, e106857. [Google Scholar] [CrossRef]
- Fernando, W.A.M.; Ilankoon, I.M.S.K.; Syed, T.H.; Yellishetty, M. Challenges and opportunities in the removal of sulphate ions in contaminated mine water: A review. Miner. Eng. 2018, 117, 74–90. [Google Scholar] [CrossRef]
- Cunha, M.P.; Ferraz, R.M.; Sancinetti, G.P.; Rodriguez, R.P. Long-term performance of a UASB reactor treating acid mine drainage: Effects of sulfate loading rate, hydraulic retention time, and COD/SO42− ratio. Biodegradation 2019, 30, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Dinu, L.; Stefanescu, M.; Balaiu, I.; Cosma, C.; Cristea, I.; Badescu, V. Acid mine water treatment using high density sludge technology. J. Environ. Prot. Ecol. 2014, 15, 1700–1717. [Google Scholar]
- Nariyan, E.; Wolkersdorfer, C.; Sillanpää, M. Sulfate removal from acid mine water from the deepest active European mine by precipitation and various electrocoagulation configurations. J. Environ. Manag. 2018, 227, 162–171. [Google Scholar] [CrossRef]
- Pyankov, S.V.; Maximovich, N.G.; Khayrulina, E.A.; Berezina, O.A.; Shikhov, A.N.; Abdullin, R.K. Monitoring acid mine drainage’s effects on surface water in the Kizel Coal Basin with Sentinel-2 Satellite Images. Mine Water Environ. 2021, 40, 606–621. [Google Scholar] [CrossRef]
- Olías, M.; Nieto, J.M.; Sarmiento, A.M.; Cerón, J.C.; Cánovas, C.R. Seasonal water quality variations in a river affected by acid mine drainage: The Odiel River (South West Spain). Sci. Total Environ. 2004, 333, 267–281. [Google Scholar] [CrossRef]
- Osvaldo, A.; Montesinos, M.; Loza, N. Efficient Methodologies in the Treatment of Acid Water from Mines with Recovery of Byproducts. In Mine Water Management for Future Generations; Stanley, P., Wolkersdorfer, C., Wolkersdorfer, K., Eds.; Natural Resources Wales, The Coal Authority, Welsh Government, Cardiff University: Cardiff, Wales, UK, 2021; pp. 1–7. [Google Scholar]
- Grande, J.A.; Luís, A.T.; Córdoba, F.; Leiva, M.; Dávila, J.M.; Fortes, J.C.; Santisteban, M.; Ferreira da Silva, E.; Sarmiento, A.M. Odiel River (SW Spain), a singular scenario affected by acid mine drainage (AMD): Graphical and statistical models to assess diatoms and water hydrogeochemistry interactions. Int. J. Environ. Res. Public Health 2021, 18, 8454. [Google Scholar] [CrossRef]
- Gheorghe, S.; Lucaciu, I.; Stanescu, E.; Stoica, C.J. Romanian aquatic toxicity testing strategy under REACH. Environ. Prot. Ecol. 2013, 14, 601–611. [Google Scholar]
- Gheorghe, S.; Vasile, G.G.; Stoica, C.; Nita-Lazar, M.; Lucaciu, I.; Banciu, A. Phytotoxicity tests applied on sewage sludge resulted from urban wastewater treatment plants. Rev. Chim. 2016, 67, 1469–1473. [Google Scholar]
- Gheorghe, S.; Stoica, C.; Paun, I.; Lucaciu, I.; Nita-Lazar, M.; Cristofor, S. Ecotoxicological tests used as warning system for Danube Delta quality assessment. J. Environ. Prot. Ecol. 2016, 17, 171–181. [Google Scholar]
- Stoica, C.; Gheorghe, S.; Lucaciu, I.; Stanescu, E.; Paun, I.; Niculescu, D. The impact of chemical compounds on benthic invertebrates from the Danube and Danube Delta Systems. Soil Sedim. Contamin. 2014, 23, 763. [Google Scholar] [CrossRef]
- Stoica, C.; Dinu, L.; Lucaciu, I.; Nita-Lazar, M.; Oncu, V. The Toxic Effect of Conventional Treated Mine Water on Aquatic Organisms. Rev. Chim. 2020, 71, 67–72. [Google Scholar] [CrossRef]
- Griffith, M.; James, L.; Herman, H. Uptake of sulfate from ambient water by freshwater animals. Water 2020, 12, 1496. [Google Scholar] [CrossRef]
- Dusinska, M.; Runden-Pran, E.; Correia Carreira, S.; Saunders, M. Adverse Effects of Engineered Nanomaterials (Chapter 14: Critical Evaluation of Toxicity Tests); Elsevier Inc.: New York, NY, USA, 2012; pp. 63–83. [Google Scholar]
- Davies, T.D. Sulphate Toxicity to Freshwater and Molybdenum Toxicity to Rainbow Trout (Oncorhynchus mykiss). Master’s Thesis, The University of British Columbia, Vancouver, BC, Canada, 2002; p. 127. [Google Scholar]
- Tarique Zeyad, M.; Kumar, M.; Malik, A. Mutagenicity, genotoxicity and oxidative stress induced by pesticide industry wastewater using bacterial and plant bioassays. Biotechnol. Rep. 2019, 24, e00389. [Google Scholar] [CrossRef]
- Davies, T.D.; Hall, K.J. Importance of calcium in modifing the acute toxicity of sodium sulphate to Hyalella azteca and Daphnia magna. Environ. Toxicol. Chem. 2007, 26, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- EBPI Inc. SOS-Chromotest Procedure S9 Activation and Express Strains, Version 6.5; EBPI Inc.: Mississauga, ON, Canada, 2016. [Google Scholar]
- Hrynkiewicz, K.; Baum, C. Application of microorganisms in bioremediation of environment from heavy metals. In Environmental Deterioration and Human Health; Springer: Berlin/Heidelberg, Germany, 2014; pp. 215–227. [Google Scholar]
- Nita-Lazar, M.; Galaon, T.; Banciu, A.; Paun, I.; Stoica, C.; Lucaciu, I.J. Screening of various harmful compounds in a new bacterial biological model. Environ. Prot. Ecol. 2016, 17, 237–247. [Google Scholar]
- Sleator, R.D.; Hill, C. Bacterial osmoadaptation: The role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 2002, 26, 49–71. [Google Scholar] [CrossRef]
- Brahmacharimayum, B.; Kumar Ghosh, P. Effects of different environmental and operating conditions on sulfate bioreduction in shake flasks by mixed bacterial culture predominantly Pseudomonas aeruginosa. Desalin. Water Treat. 2015, 57, 1–11. [Google Scholar] [CrossRef]
- Szatmári, D.; Sárkány, P.; Kocsis, B.; Nagy, T.; Miseta, A.; Barkó, S.; Longauer, B.; Robinson, R.C.; Nyitrai, M. Intracellular ion concentrations and cation-dependent remodelling of bacterial MreB assemblies. Sci. Rep. 2020, 10, 12002. [Google Scholar] [CrossRef]
- Kocak, C.; Yetilmezsoy, K.; Gonullu, M.T.; Petek, M. A statistical evaluation of the potential genotoxic activity in the surface waters of the Golden Horn Estuary. Mar. Pollut. Bull. 2010, 60, 1708–1717. [Google Scholar] [CrossRef]
- Zak, D.; Hupfer, M.; Cabezas, A.; Jurasinski, G.; Audet, J.; Kleeberg, A.; McInnes, R.; Kristiansen, S.M.; Jes Petersen, R.; Liu, H.; et al. Sulphate in freshwater ecosystems: A review of sources, biogeochemical cycles, ecotoxicological effects and bioremediation. Earth Sci. Rev. 2021, 212, 103446. [Google Scholar] [CrossRef]
- Hodor, C.; Danci, O.; Manci, C.; Ionescu, D.T. Studiu de Evaluare Adecvata: Exploatarea Minereurilor Auro-Argentifere Din Perimetrul Certej. 2013, p. 103. (In Romanian). Available online: https://www.certejudesus.ro/Docs/Anunturi/EA%20Deva%20Gold%20-%20Floroaia.pdf (accessed on 24 October 2022).
- Alexander, R.; Juras, S.; Miller, R.; Kalanchey, R. Technical Report for the Certej Project, Romania; Eldorado Gold: Vancouver, BC, Canada, 2014; p. 190. [Google Scholar]
- Abraham, B.; Senila, M.; Levei, E.-A.; Roman, C.; Incze, A.M.; Cordos, E. Surface Water Pollution with Heavy Metals in Certej Mining Basin. Available online: https://tucson.ars.ag.gov/isco/isco15/pdf/Abraham%20B_Surface%20water%20pollution.pdf (accessed on 14 October 2022).
- SR 13315; SR 13315/C91; Water Quality. Determination of Iron Content. Atomic Absorption Spectrometric Method. Institute for Standardization of Serbia: Belgrade, Serbia, 2008.
- SR 8662-2-R 8662-2; Water Quality. Determination of Manganese Content. Atomic Absorption Spectrometric Method. Institute for Standardization of Serbia: Belgrade, Serbia, 1996.
- SR EN ISO 12020; Water Quality. Determination of Aluminum Content. The Method by Atomic Absorption Spectrometry. Institute for Standardization of Serbia: Belgrade, Serbia, 2004.
- SR EN ISO 17294-2; Water Quality. Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Part 2: Determination of Selected Elements Including Uranium Isotopes. Institute for Standardization of Serbia: Belgrade, Serbia, 2017.
- SR EN ISO 11885; Water Quality. Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). Institute for Standardization of Serbia: Belgrade, Serbia, 2009.
- SR EN ISO 10304-1; Water Quality. Determination of Dissolved Anions by Liquid Phase Ion Chromatography. Part 1: Determination of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate Ions. Institute for Standardization of Serbia: Belgrade, Serbia, 2009.
- SR EN ISO 14911; Water Quality. Determination by Ion Chromatography of the Dissolved Ions of Li+, Na+, NH4+, K+, Mn2+, Ca2+, Mg2+, Sr2+ and Ba2+. Method for Water and Wastewater. Institute for Standardization of Serbia: Belgrade, Serbia, 2003.
- Nguyen, K.; Kumar, P. Morphological phenotypes, cell division, and gene expression of Escherichia coli under high concentration of sodium sulfate. Microorganisms 2022, 10, 274. [Google Scholar] [CrossRef]
- Percival, S.L.; Williams, D.W. Escherichia coli (Chapter Six). In Microbiology of Waterborne Disease, Microbiological Aspects and Risks, 2nd ed.; Percival, S.L., Williams, D.W., Gray, N.F., Yates, M.V., Chalmers, R.M., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 89–117. [Google Scholar]
- Teitzel, G.M.; Parsek, M.R. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2003, 69, 2313–2320. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 1–29. [Google Scholar] [CrossRef]
- Langendonk, R.F.; Neill, D.R.; Fothergill, J.L. The building blocks of antimicrobial resistance in Pseudomonas aeruginosa: Implications for current resistance-breaking therapies. Front. Cell Infect. Microbiol. 2021, 11, 665759. [Google Scholar] [CrossRef] [PubMed]
- Gugala, N.; Vu, D.; Parkins, M.D.; Turner, R.J. Specificity in the susceptibilities of Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus clinical isolates to six metal antimicrobials. Antibiotics 2019, 8, 51. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Olanrewaju, O.S.; Babalola, O.O. Sulfate-Reducing Bacteria as an Effective Tool for Sustainable Acid Mine Bioremediation. Front Microbiol. 2018, 22, 1986. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Shi, H.; Liu, Y.; Chen, S. Soil bacteria, genes, and metabolites stimulated during sulfur cycling and cadmium mobilization under sodium sulfate stress. Environ. Res. 2021, 201, 111599. [Google Scholar] [CrossRef]
- Lasier, P.J.; Hardin, I.R. Observed and predicted reproduction of Ceriodaphnia dubia exposed to chloride, sulfate, and bicarbonate. Environ. Toxicol. Chem. 2010, 29, 347–358. [Google Scholar] [CrossRef]
- Meays, C.; Nordin, R. Tehnical Appendix; Ministry of Environment Province of British Columbia: Victoria, BC, Canada, 2013; pp. 1–56.
- Kushkevych, I.; Dordević, D.; Vitĕzova, M. Analysis of pH dose-dependent growth of sulfate-reducing bacteria. Open Med. 2019, 14, 66–74. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Jing, J.; Zhao, P.; Luo, Z.; Cao, M.; Ma, Z.; Jia, T.; Chai, B. Ecological patterns and ada, ptability of bacterial communities in alkaline copper mine drainage. Water Res. 2018, 133, 99–109. [Google Scholar] [CrossRef]
- Miaochun, F.; Yanbing, L.; Haibo, H.; Yang, L.; Liang, Z.; Entao, W.; Weimin, C.; Gehong, W. Microbial communities in riparian soils of a settling pond for mine drainage treatment. Water Res. 2016, 96, 198–207. [Google Scholar] [CrossRef]
- Liu, H.; Luo, L.; Jiang, G.; Li, G.; Zhu, C.; Meng, W.; Zhang, J.; Jiao, Q.; Du, P.; Li, X.; et al. Sulfur enhances cadmium bioaccumulation in Cichorium intybus by altering soil properties, heavy metal availability and microbial community in contaminated alkaline soil. Sci. Total Environ. 2022, 837, 155879. [Google Scholar] [CrossRef]
- Li, X.; Meng, D.; Li, J.; Yin, H.; Liu, H.; Liu, X.; Cheng, C.; Xiao, Y.; Liu, Z.; Yan, M. Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ. Pollut. 2017, 231, 908–917. [Google Scholar] [CrossRef]
- Oluwafikemi, T.I.; Emmanuel, M.N.; Balungile, M.; Jan, G.M.; Lyndy, J.M. Evaluation of the genotoxic potential of water impacted by acid mine drainage from a coal mine in Mpumalanga, South Africa, using the Ames test and Comet assay. Water SA 2021, 47, 456–465. [Google Scholar] [CrossRef]
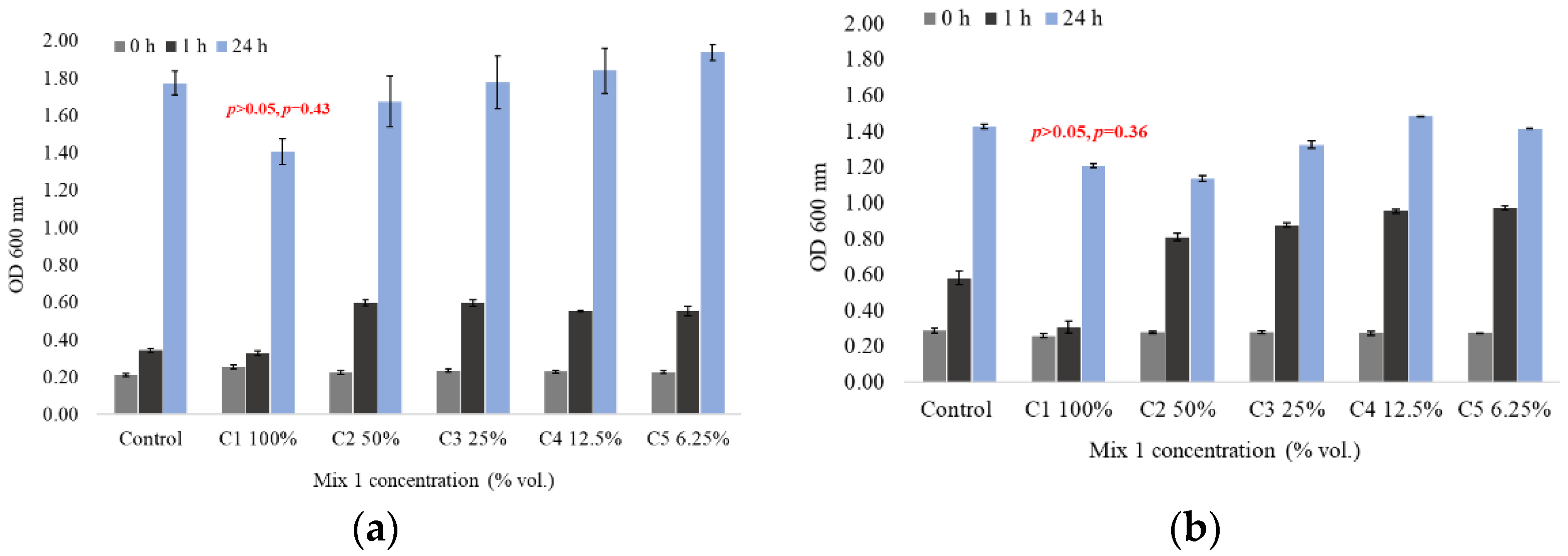
| Pseudomonas Aeruginosa | Dilution (% Vol.) of Mix 1 | Inhibition Effect% |
| LID-1 h | <6.25 | - |
| LID-24 h | 6.25 | 0 |
| LOEC-1 h | 6.25 | 3.37 ± 1.23 |
| LOEC-24 h | 12.25 | 0.35 ± 0.02 |
| Escherichia coli | Dilution (% Vol.) of Mix 1 | Inhibition Effect% |
| LID-1 h | 12.25 | 0 |
| LID-24 h | 12.25 | 0 |
| LOEC-1 h | 25 | 1.21 ± 0.04 |
| LOEC-24 h | 25 | 8.21 ± 1.02 |
| Effect Endpoint | Dilution (% Vol.) of Mix 1 | |
| Pseudomonas aeruginosa | Escherichia coli | |
| EC20-1 h | 52.57 ± 4.35% (994 mg/L) | 66.83 ± 6.32% (1263 mg/L) |
| EC20-2 h | 64.96 ± 5.25% (1228 mg/L) | 68.74 ± 8.36% (1299 mg/L) |
| EC20-4 h | 65.00 ± 7.30% (1229 mg/L) | 75.78 ± 10.51% (1432 mg/L) |
| EC20-24 h | 94.74 ± 10.43% (1790 mg/L) | - |
| Pseudomonas aeruginosa | Escherichia coli | |
| EC50-1 h | >100% (>1890 mg/L) | 71.31 ± 9.33% (1348 mg/L) |
| EC50-2 h | >100% (>1890 mg/L) | 101.47 ± 12.56% (1917 mg/L) |
| EC50-4h | >100% (>1890 mg/L) | 117.86 ± 11.43% (2227 mg/L) |
| EC50-24 h | >100% (>1890 mg/L) | >100% (1890 mg/L) |
| Mix 1 % Vol. | G | β-Gal | IF | Reference IF * | SR% | Reference SR * |
|---|---|---|---|---|---|---|
| 100% | 0.99 | 1.09 | 1.09 | 1.5–2.0 | 99.6 | 80% |
| 50% | 1.07 | 1.05 | 0.98 | 100 | ||
| 25% | 0.94 | 0.93 | 0.99 | 93.7 | ||
| 12.5% | 0.98 | 0.95 | 0.97 | 97.9 | ||
| 6.25% | 0.99 | 0.97 | 0.97 | 99.7 | ||
| 3.125% | 0.94 | 0.89 | 0.95 | 94.2 | ||
| 1.56% | 0.99 | 0.94 | 0.94 | 99.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoica, C.; Dinu, L.R.; Lucaciu, I.E.; Oncu, V.; Gheorghe, S.; Nita-Lazar, M. Sensitivity of Pathogenic Bacteria Strains to Treated Mine Water. Int. J. Environ. Res. Public Health 2022, 19, 15535. https://doi.org/10.3390/ijerph192315535
Stoica C, Dinu LR, Lucaciu IE, Oncu V, Gheorghe S, Nita-Lazar M. Sensitivity of Pathogenic Bacteria Strains to Treated Mine Water. International Journal of Environmental Research and Public Health. 2022; 19(23):15535. https://doi.org/10.3390/ijerph192315535
Chicago/Turabian StyleStoica, Catalina, Laurentiu Razvan Dinu, Irina Eugenia Lucaciu, Voicu Oncu, Stefania Gheorghe, and Mihai Nita-Lazar. 2022. "Sensitivity of Pathogenic Bacteria Strains to Treated Mine Water" International Journal of Environmental Research and Public Health 19, no. 23: 15535. https://doi.org/10.3390/ijerph192315535
APA StyleStoica, C., Dinu, L. R., Lucaciu, I. E., Oncu, V., Gheorghe, S., & Nita-Lazar, M. (2022). Sensitivity of Pathogenic Bacteria Strains to Treated Mine Water. International Journal of Environmental Research and Public Health, 19(23), 15535. https://doi.org/10.3390/ijerph192315535







