Synthesis and Characterization of g-C3N4/Ag3PO4/TiO2/PVDF Membrane with Remarkable Self-Cleaning Properties for Rhodamine B Removal
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
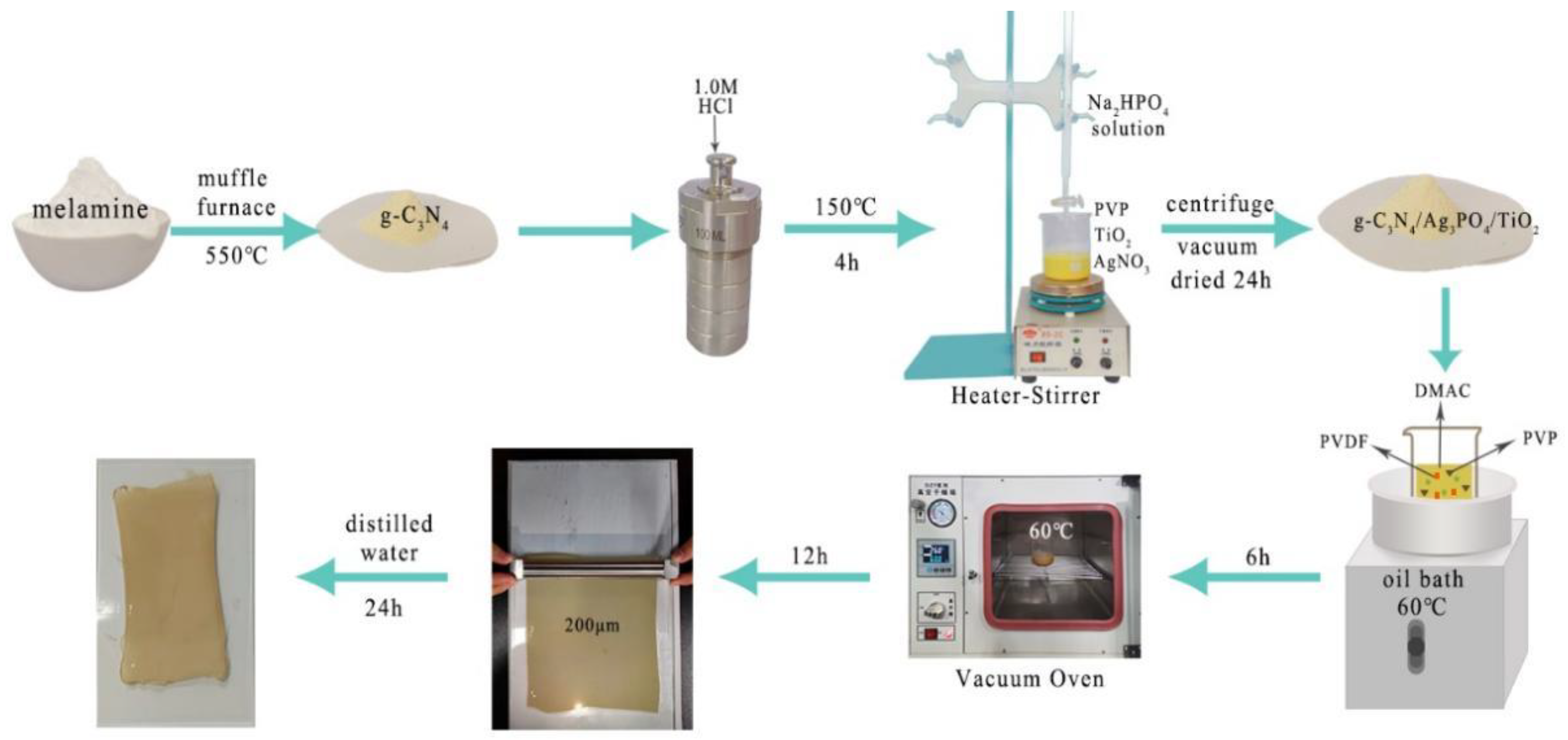
2.2. Synthesis of g-C3N4
2.3. Synthesis of g-C3N4/Ag3PO4/TiO2 Nanocomposites
2.4. Preparation of Photocatalytic Composite Membranes
2.5. Characterization of the g-C3N4/Ag3PO4/TiO2 Nanocomposites
2.6. Membrane Characterization
2.7. Basic Properties of Membranes
2.7.1. Permeability Measurements
2.7.2. Removal of RhB Using the Photocatalytic Membrane
3. Results and Discussion
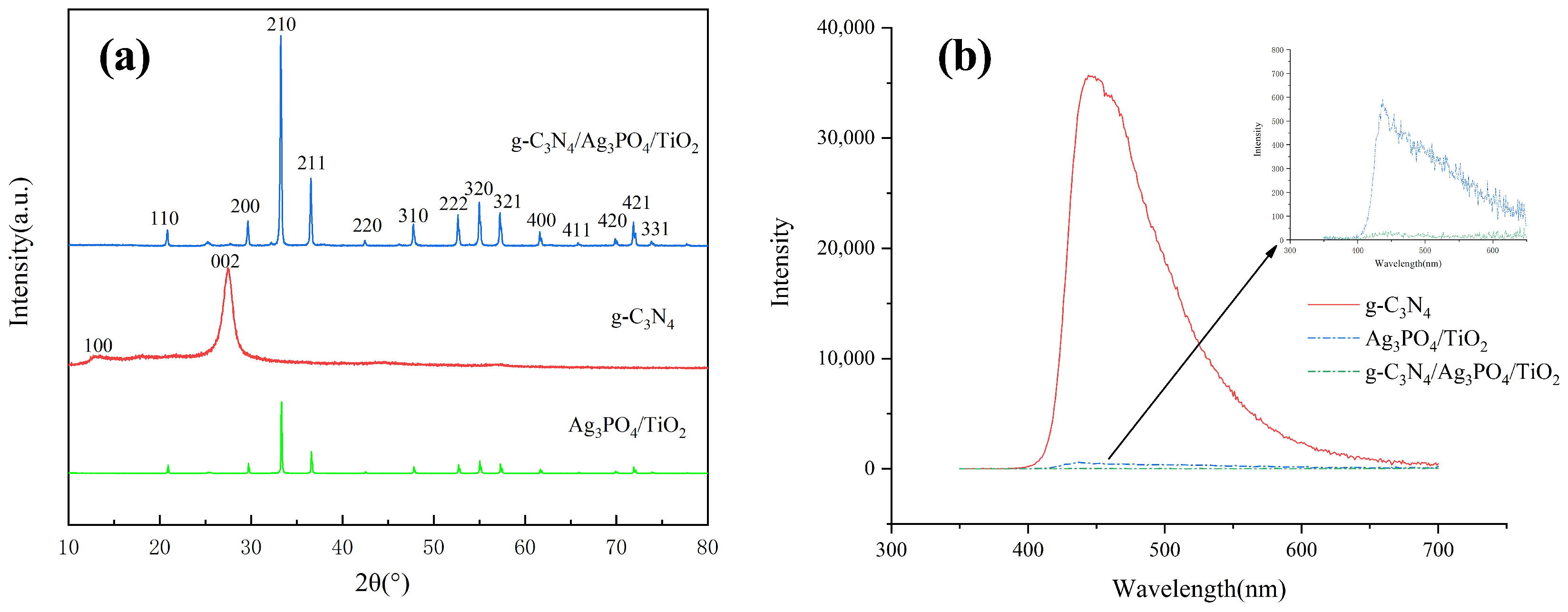
3.1. Characterization of the g-C3N4/Ag3PO4/TiO2 Nanocomposites
3.2. Membrane Characterizations
3.2.1. Membrane Morphology
3.2.2. Basic Properties of Membranes
3.2.3. Antifouling Performance of Membranes
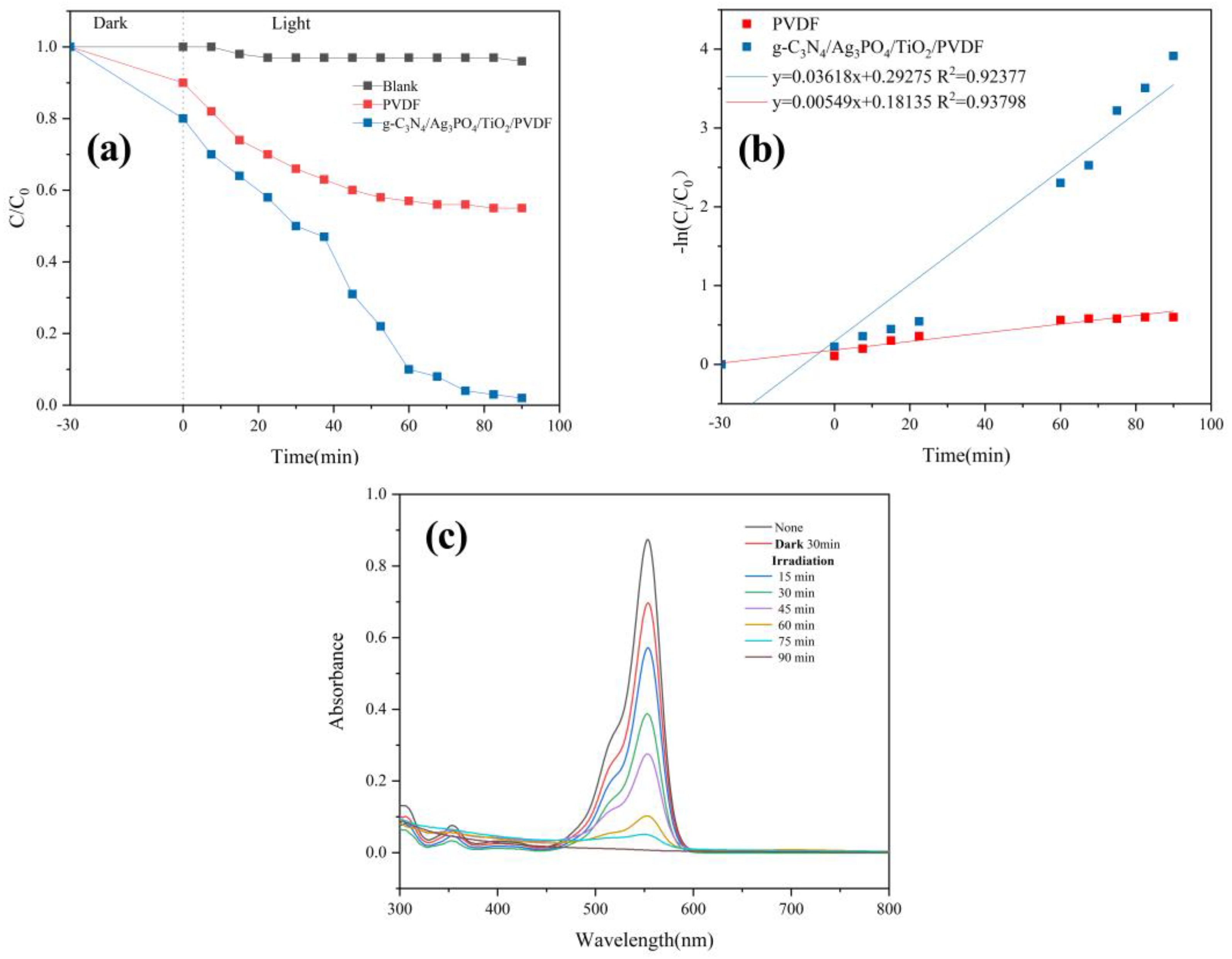
3.2.4. Photocatalytic Properties of Membranes
3.3. Mechanism of the g-C3N4/Ag3PO4/TiO2/PVDF Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, A.; Jia, R.; Wang, Y.L.; Sun, S.H.; Xin, X.D.; Wang, M.Q.; Zhao, Q.H.; Zhu, H.H. Abatement of sulfadiazine in water under a modified ultrafiltration membrane (PVDF-PVP-TiO2-dopamine) filtration-photocatalysis system. Sep. Purif. Technol. 2020, 234, 116099. [Google Scholar] [CrossRef]
- Li, X.; He, S.B.; Feng, C.L.; Zhu, Y.K.; Pang, Y.; Hou, J.; Luo, K.; Liao, X.S. Non-competitive and competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto SDS in process of micellar-enhanced ultrafiltration. Sustainability 2018, 10, 92. [Google Scholar] [CrossRef]
- Wan, J.; Huang, J.H.; Yu, H.B.; Liu, L.S.; Shi, Y.H.; Liu, C.H. Fabrication of self-assembled 0D-2D Bi2MoO6-g-C3N4 photocatalytic composite membrane based on PDA intermediate coating with visible light self-cleaning performance. J. Colloid Interface Sci. 2021, 601, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Huang, J.H.; Ouyang, Y.C.; Yi, K.X.; Yu, H.B.; Zhang, W.; Zhang, C.Y.; Li, S.Z. QQ-PAC core-shell structured quorum quenching beads for potential membrane antifouling properties. Enzym. Microb. Technol. 2021, 148, 109813. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wang, P.; Li, J.N.; Wang, Q.; Xu, P.; You, S.J.; Zheng, Q.Z.; Zhang, G.S. Photocatalytic PVDF ultrafiltration membrane blended with visible-light responsive Fe(III)-TiO2 catalyst: Degradation kinetics, catalytic performance and reusability. Chem. Eng. J. 2021, 417, 129340. [Google Scholar] [CrossRef]
- Naseem, S.; Wu, C.M.; Motora, K.G. Novel multifunctional RbxWO3@Fe3O4 immobilized Janus membranes for desalination and synergic-photocatalytic water purification. Desalination 2021, 517, 115256. [Google Scholar] [CrossRef]
- Huang, X.Z.; Liu, H.W.; Zhang, X.; Jiang, H.R. High Performance All-Solid-State Flexible Micro-Pseudocapacitor Based on Hierarchically Nanostructured Tungsten Trioxide Composite. ACS Appl. Mater. Interfaces 2015, 7, 27845–27852. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Huang, J.H.; Zeng, G.M.; Cheng, W.J.; Hu, J.L.; Shi, L.X.; Yi, K.X. Evaluation of self-cleaning performance of the modified g-C3N4 and GO based PVDF membrane toward oil-in-water separation under visible-light. Chemosphere 2019, 230, 40–50. [Google Scholar] [CrossRef]
- Kolesnyk, I.; Kujawa, J.; Bubela, H.; Konovalova, V.; Burban, A.; Cyganiuk, A.; Kujawski, W. Photocatalytic properties of PVDF membranes modified with g-C3N4 in the process of Rhodamines decomposition. Sep. Purif. Technol. 2020, 250, 117231. [Google Scholar] [CrossRef]
- Huang, J.H.; Hu, J.L.; Shi, Y.H.; Zeng, G.M.; Cheng, W.J.; Yu, H.B.; Gu, Y.L.; Shi, L.X.; Yi, K.X. Evaluation of self-cleaning and photocatalytic properties of modified g-C3N4 based PVDF membranes driven by visible light. J. Colloid Interface Sci. 2019, 541, 356–366. [Google Scholar] [CrossRef]
- Chen, X.J.; Huang, G.; Li, Y.P.; An, C.J.; Feng, R.F.; Wu, Y.H.; Shen, J. Functional PVDF ultrafiltration membrane for Tetrabromobisphenol-A (TBBPA) removal with high water recovery. Water Res. 2020, 181, 115952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Liu, P.L. Preparation of porous ZrO2 solid superacid shell/void/TiO2 core particles and effect of doping them on PVDF membranes properties. Chem. Eng. Sci. 2015, 135, 67–75. [Google Scholar] [CrossRef]
- Chen, X.J.; Huang, G.; An, C.J.; Feng, R.F.; Wu, Y.H.; Huang, C. Plasma-induced PAA-ZnO coated PVDF membrane for oily wastewater treatment: Preparation, optimization, and characterization through Taguchi OA design and synchrotron-based X-ray analysis. J. Membr. Sci. 2019, 582, 70–82. [Google Scholar] [CrossRef]
- Ayyaru, S.; Dinh, T.T.L.; Ahn, Y.H. Enhanced antifouling performance of PVDF ultrafiltration membrane by blending zinc oxide with support of graphene oxide nanoparticle. Chemosphere 2020, 241, 125068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Cai, Y.; Han, Y.; Zhang, T.Q.; Liu, Y.; Zeng, K.L.; Zhao, C. Photocatalytic Poly(vinylidene fluoride) membrane of Ag3PO4/GO/APTES for water treatment. Colloids Surf. A 2020, 597, 124779. [Google Scholar] [CrossRef]
- Fan, G.D.; Chen, C.G.; Chen, X.L.; Li, Z.S.; Bao, S.L.; Luo, J.; Tang, D.S.; Yan, Z.S. Enhancing the antifouling and rejection properties of PVDF membrane by Ag3PO4-GO modification. Sci. Total Environ. 2021, 801, 149611. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Effect of reduced graphene oxide/TiO2 nanocomposite with different molar ratios on the performance of PVDF ultrafiltration membranes. Sep. Purif. Technol. 2015, 140, 32–42. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumran, S.; Jegatheesan, V. Factors affecting the degradation of remazol turquoise blue (RTB) dye by titanium dioxide (TiO2) entrapped photocatalytic membrane. J. Environ. Manag. 2020, 272, 111090. [Google Scholar] [CrossRef]
- Che, H.N.; Liu, L.H.; Che, G.B.; Dong, H.J.; Liu, C.B.; Li, C.M. Control of energy band, layer structure and vacancy defect of graphitic carbon nitride by intercalated hydrogen bond effect of NO−3 toward improving photocatalytic performance. Biochem. Eng. J. 2019, 357, 209–219. [Google Scholar] [CrossRef]
- Yu, H.B.; Huang, J.H.; Jiang, L.B.; Yuan, X.Z.; Yi, K.X.; Zhang, W.; Zhang, J.; Chen, H.Y. Steering photo-excitons towards active sites: Intensified substrates affinity and spatial charge separation for photocatalytic molecular oxygen activation and pollutant removal. Chem. Eng. J. 2021, 408, 127334. [Google Scholar] [CrossRef]
- Cai, G.H.; Wang, J.P.; Wu, X.Q.; Zhan, Y.Y.; Liang, S.J. Scalable one-pot synthesis of porous 0D/2D C3N4 nanocomposites for efficient visible-light driven photocatalytic hydrogen evolution. Appl. Surf. Sci. 2018, 459, 224–232. [Google Scholar] [CrossRef]
- Cui, Y.H.; Yang, L.L.; Meng, M.J.; Zhang, Q.; Li, B.R.; Wu, Y.L.; Zhang, Y.L.; Lang, J.H.; Li, C.X. Facile preparation of antifouling g-C3N4/Ag3PO4 nanocomposite photocatalytic polyvinylidene fluoride membranes for effective removal of rhodamine B. Korean J. Chem. Eng. 2019, 36, 236–247. [Google Scholar] [CrossRef]
- Liu, L.S.; Huang, J.H.; Yu, H.B.; Wan, J.; Liu, L.Y.; Yi, K.X.; Zhang, W.; Zhang, C.Y. Construction of MoO3 nanopaticles /g-C3N4 nanosheets 0D/2D heterojuntion photocatalysts for enhanced photocatalytic degradation of antibiotic pollutant. Chemosphere 2021, 282, 131049. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Fu, Z.; Li, L.; Ma, M.; Liu, Y. Step-scheme heterojunction g-C3N4/TiO2 for efficient photocatalytic degradation of tetracycline hydrochloride under, UV light. Colloids Surf. A 2022, 649, 129475. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, Y.; Zhou, M.; Cheng, H.; Chen, H.; Dorus, B.; Lu, M.; Le, T. A 2D/3D g-C3N4/ZnO heterojunction enhanced visible-light driven photocatalytic activity for sulfonamides degradation. Ceram. Int. 2022, 48, 7283–7290. [Google Scholar] [CrossRef]
- Van, K.N.; Huu, H.T.; Nguyen Thi, V.N.; Le Thi, T.L.; Truong, D.H.; Truong, T.T.; Dao, N.N.; Vo, V.; Tran, D.L.; Vasseghian, Y. Facile construction of S-scheme SnO2/g-C3N4 photocatalyst for improved photoactivity. Chemosphere 2022, 289, 133120. [Google Scholar] [CrossRef] [PubMed]
- Khezami, L.; Ben Aissa, M.A.; Modwi, A.; Ismail, M.; Guesmi, A.; Algethami, F.K.; Ben Ticha, M.; Assadi, A.A.; Nguyen-Tri, P. Harmonizing the photocatalytic activity of g-C3N4 nanosheets by ZrO2 stuffing: From fabrication to experimental study for the wastewater treatment. Biochem. Eng. J. 2022, 182, 108411. [Google Scholar] [CrossRef]
- Yin, C.; Liu, Y.L.; Lv, X.Y.; Lv, S.Y.; Cheng, H.; Kang, X.R.; Li, X. Carbon dots as heterojunction transport mediators effectively enhance BiOI/g-C3N4 synergistic persulfate degradation of antibiotics. Appl. Surf. Sci. 2022, 601, 154249. [Google Scholar] [CrossRef]
- Raeisi-Kheirabadi, N.; Nezamzadeh-Ejhieh, A. A Z-scheme g-C3N4/Ag3PO4 nanocomposite: Its photocatalytic activity and capability for water splitting. Int. J. Hydrog. Energy 2020, 45, 33381–33395. [Google Scholar] [CrossRef]
- Abbasi-Asl, H.; Sabzehmeidani, M.M.; Ghaedi, M. Efficient degradation of metronidazole antibiotic by TiO2/Ag3PO4/g–C3N4 ternary composite photocatalyst in a continuous flow-loop photoreactor. J. Environ. 2021, 9, 105963. [Google Scholar] [CrossRef]
- Liu, L.; Qi, Y.H.; Lu, J.R.; Lin, S.L.; An, W.J.; Liang, Y.H.; Cui, W.Q. A stable Ag3PO4@g-C3N4 hybrid core@shell composite with enhanced visible light photocatalytic degradation. Appl. Catal. B Environ. 2016, 183, 133–141. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tahir, M. Facile fabrication of well-designed 2D/2D porous g-C3N4–GO nanocomposite for photocatalytic methane reforming (DRM) with CO2 towards enhanced syngas production under visible light. Fuel 2021, 305, 121558. [Google Scholar] [CrossRef]
- He, P.Z.; Song, L.M.; Zhang, S.J.; Wu, X.Q.; Wei, Q.W. Synthesis of g-C3N4/Ag3PO4 heterojunction with enhanced photocatalytic performance. Mater. Res. Bull. 2014, 51, 432–437. [Google Scholar] [CrossRef]
- Rosman, N.; Norharyati Wan Salleh, W.; Aqilah Mohd Razali, N.; Nurain Ahmad, S.Z.; Hafiza Ismail, N.; Aziz, F.; Harun, Z.; Fauzi Ismail, A.; Yusof, N. Ibuprofen removal through photocatalytic filtration using antifouling PVDF-ZnO/Ag2CO3/Ag2O nanocomposite membrane. Mater. Today Proc. 2021, 42, 69–74. [Google Scholar] [CrossRef]
- Pan, T.D.; Liu, Y.; Li, Z.J.; Fan, J.; Wang, L.; Liu, J.; Shou, W. A Sm-doped Egeria-densa-like ZnO nanowires@PVDF nanofiber membrane for high-efficiency water clean. Sci. Total Environ. 2020, 737, 139818. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.A.; Zhang, G.G.; Wang, X.C. A facile synthesis of Br-modified g-C3N4 semiconductors for photoredox water splitting. Appl. Catal. B Environ. 2016, 192, 116–125. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, M.; Qu, Z.H.; Cui, X.; Liu, Z.Y.; Piao, C.C.; Li, S.G.; Wang, J.; Song, Y.T. Fabrication of highly active Z-scheme Ag/g-C3N4-Ag-Ag3PO4 (1 1 0) photocatalyst photocatalyst for visible light photocatalytic degradation of levofloxacin with simultaneous hydrogen production. Biochem. Eng. J. 2020, 382, 122394. [Google Scholar] [CrossRef]
- Arumugham, T.; Amimodu, R.G.; Kaleekkal, N.J.; Rana, D. Nano CuO/g-C3N4 sheets-based ultrafiltration membrane with enhanced interfacial affinity, antifouling and protein separation performances for water treatment application. J. Environ. Sci. 2019, 82, 57–69. [Google Scholar] [CrossRef]
- Shao, L.Q.; Jiang, D.L.; Xiao, P.; Zhu, L.M.; Meng, S.C.; Chen, M. Enhancement of g-C3N4 nanosheets photocatalysis by synergistic interaction of ZnS microsphere and RGO inducing multistep charge transfer. Appl. Catal. B Environ. 2016, 198, 200–210. [Google Scholar] [CrossRef]
- Cacho-Bailo, F.; Téllez, C.; Coronas, J. Interactive Thermal Effects on Metal–Organic Framework Polymer Composite Membranes. Chem. Eur. J. 2016, 22, 9533–9536. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Liu, Z.Y.; Gao, Y.; Shu, L. Ag modified g-C3N4 composite entrapped PES UF membrane with visible-light-driven photocatalytic antifouling performance. RSC Adv. 2017, 7, 42919–42928. [Google Scholar] [CrossRef]
- Seyyed Shahabi, S.; Azizi, N.; Vatanpour, V. Synthesis and characterization of novel g-C3N4 modified thin film nanocomposite reverse osmosis membranes to enhance desalination performance and fouling resistance. Sep. Purif. Technol. 2019, 215, 430–440. [Google Scholar] [CrossRef]
- Chen, J.X.; Li, Z.Y.; Wang, C.B.; Wu, H.; Liu, G. Synthesis and characterization of g-C3N4 nanosheet modified polyamide nanofiltration membranes with good permeation and antifouling properties. RSC Adv. 2016, 6, 112148–112157. [Google Scholar] [CrossRef]
- Ravichandran, K.; Sindhuja, E. Fabrication of cost effective g-C3N4+Ag activated ZnO photocatalyst in thin film form for enhanced visible light responsive dye degradation. Mater. Chem. Phys. 2019, 221, 203–215. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites:A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Du, Y.E.; Li, W.X.; Bai, Y.; Huangfu, Z.W.; Wang, W.J.; Chai, R.D.; Chen, C.D.; Yang, X.J.; Feng, Q. Facile synthesis of TiO2/Ag3PO4 composites with co-exposed high-energy facets for efficient photodegradation of rhodamine B solution under visible light irradiation. RSC Adv. 2020, 10, 24555–24569. [Google Scholar] [CrossRef]
- Lv, J.L.; Dai, K.; Zhang, J.F.; Lu, L.H.; Liang, C.H.; Geng, L.; Wang, Z.L.; Yuan, G.Y.; Zhu, G.P. In situ controllable synthesis of novel surface plasmon resonance-enhanced Ag2WO4/Ag/Bi2MoO6 composite for enhanced and stable visible light photocatalyst. Appl. Surf. Sci. 2017, 391, 507–515. [Google Scholar] [CrossRef]
- Liu, W.; Shen, J.; Yang, X.F.; Liu, Q.Q.; Tang, H. Dual Z-scheme g-C3N4/Ag3PO4/Ag2MoO4 ternary composite photocatalyst for solar oxygen evolution from water splitting. Appl. Surf. Sci. 2018, 456, 369–378. [Google Scholar] [CrossRef]
- Dong, Z.F.; Wu, Y.; Thirugnanam, N.; Li, G.L. Double Z-scheme ZnO/ZnS/g-C3N4 ternary structure for efficient photocatalytic H2 production. Appl. Surf. Sci. 2018, 430, 293–300. [Google Scholar] [CrossRef]
- Wen, J.Q.; Xie, J.; Chen, X.B.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Yu, H.B.; Huang, J.H.; Jiang, L.B.; Leng, L.J.; Yi, K.X.; Zhang, W.; Zhang, C.Y.; Yuan, X.Z. In situ construction of Sn-doped structurally compatible heterojunction with enhanced interfacial electric field for photocatalytic pollutants removal and CO2 reduction. Appl. Catal. B Environ. 2021, 298, 120618. [Google Scholar] [CrossRef]
- Méricq, J.P.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]








| Membrane | Thickness (μm) | Porosity (%) | Tensile Strength (MPa) |
|---|---|---|---|
| raw membrane | 176 ± 19 | 70 ± 7 | 9.3 ± 1.0 |
| composite membrane | 186 ± 17 | 85 ± 3 | 7.5 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Li, X.; Huang, J.; Pang, H.; Wan, Q.; Luo, K.; Pang, Y.; Wang, L. Synthesis and Characterization of g-C3N4/Ag3PO4/TiO2/PVDF Membrane with Remarkable Self-Cleaning Properties for Rhodamine B Removal. Int. J. Environ. Res. Public Health 2022, 19, 15551. https://doi.org/10.3390/ijerph192315551
Liu R, Li X, Huang J, Pang H, Wan Q, Luo K, Pang Y, Wang L. Synthesis and Characterization of g-C3N4/Ag3PO4/TiO2/PVDF Membrane with Remarkable Self-Cleaning Properties for Rhodamine B Removal. International Journal of Environmental Research and Public Health. 2022; 19(23):15551. https://doi.org/10.3390/ijerph192315551
Chicago/Turabian StyleLiu, Renguo, Xue Li, Jinhui Huang, Haoliang Pang, Qiongfang Wan, Kun Luo, Ya Pang, and Lingyu Wang. 2022. "Synthesis and Characterization of g-C3N4/Ag3PO4/TiO2/PVDF Membrane with Remarkable Self-Cleaning Properties for Rhodamine B Removal" International Journal of Environmental Research and Public Health 19, no. 23: 15551. https://doi.org/10.3390/ijerph192315551
APA StyleLiu, R., Li, X., Huang, J., Pang, H., Wan, Q., Luo, K., Pang, Y., & Wang, L. (2022). Synthesis and Characterization of g-C3N4/Ag3PO4/TiO2/PVDF Membrane with Remarkable Self-Cleaning Properties for Rhodamine B Removal. International Journal of Environmental Research and Public Health, 19(23), 15551. https://doi.org/10.3390/ijerph192315551





