Association between Global Monkeypox Cases and Meteorological Factors
Abstract
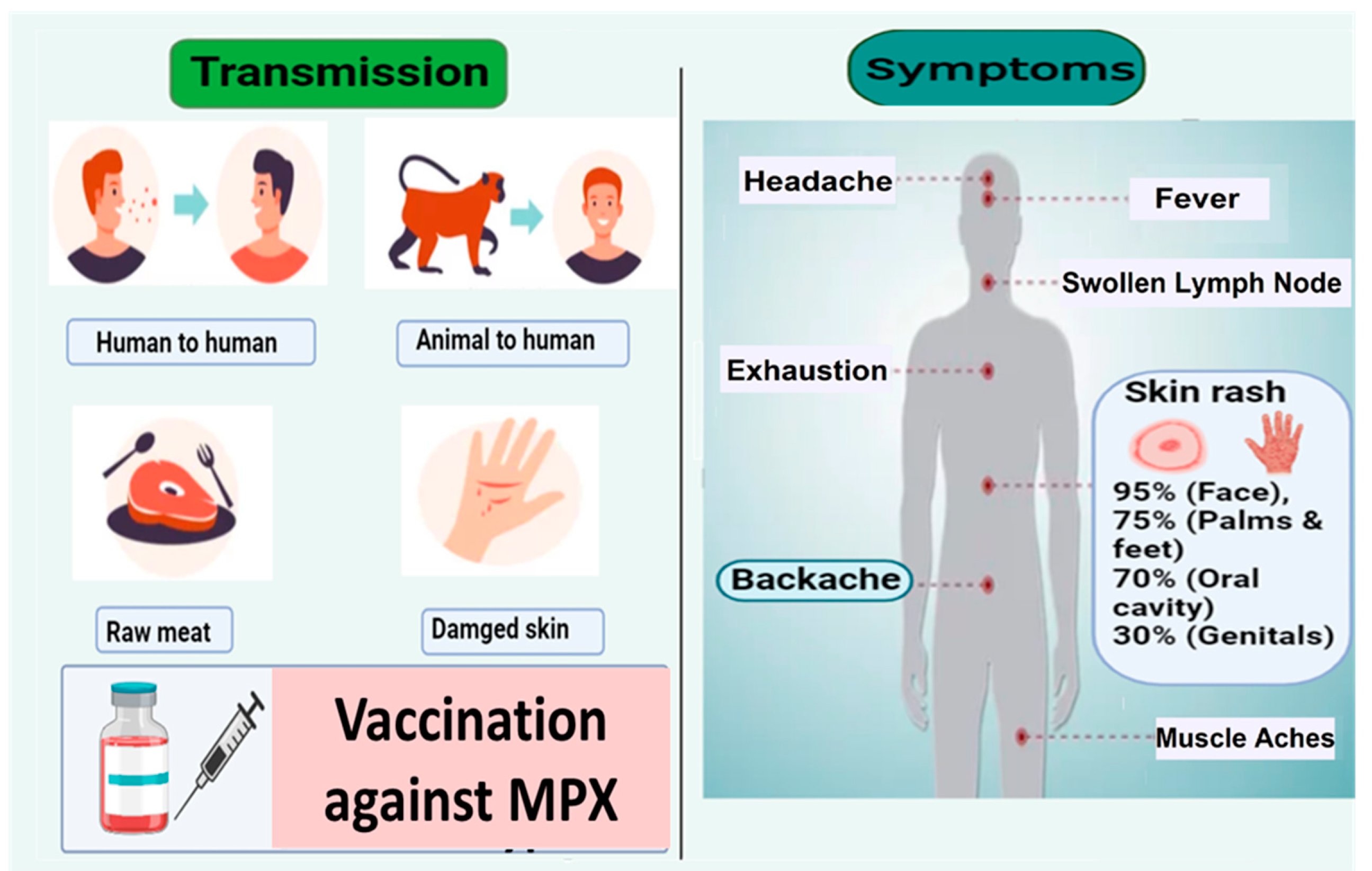
:1. Introduction
2. Materials and Methods
2.1. Daily Confirmed Monkeypox Cases
2.2. Meteorological Factors
2.3. Time Series Models
2.3.1. SES Model (Simple Exponential Smoothing)
2.3.2. ARIMA Model (Auto-Regressive Integrated Moving Average)
2.3.3. Prophet Model (Automatic Forecasting Time-Series Model)
2.3.4. Autoregressive Integrated Moving Average with Explanatory Variables (ARIMAX)
2.3.5. Empirical Evaluation
2.3.6. Statistical Analysis
3. Results
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, M.A.; Haque, M.A.; Rahman, M.A.; Hossen, F.; Reza, M.; Barua, A.; Marzan, A.A.; Das, T.; Baral, S.K.; He, C.; et al. A Review on Measures to Rejuvenate Immune System: Natural Mode of Protection Against Coronavirus Infection. Front. Immunol. 2022, 13, 837290. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Marzan, A.A.; Islam, M.S.; Sultana, S.; Parvej, M.I.; Hossain, M.S.; Amin, M.T.; Hossain, F.E.; Barek, M.A.; Hossen, M.S.; et al. Sex-specific epidemiological and clinical characteristics of COVID-19 patients in the southeast region of Bangladesh. medRxiv 2021. [Google Scholar] [CrossRef]
- Ahmed, F.; Islam, M.A.; Kumar, M.; Hossain, M.; Bhattacharya, P.; Islam, M.T.; Hossen, F.; Hossain, M.S.; Islam, M.S.; Uddin, M.M.; et al. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation centre through wastewater surveillance in Bangladesh. medRxiv 2020. [Google Scholar] [CrossRef]
- Ahmed, F.; Islam, M.A.; Kumar, M.; Hossain, M.; Bhattacharya, P.; Islam, M.T.; Hossen, F.; Hossain, M.S.; Islam, M.S.; Uddin, M.M.; et al. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation Centre in Bangladesh: Variation along the sewer network. Sci. Total Environ. 2021, 776, 145724. [Google Scholar] [CrossRef]
- WHO. 2022. Available online: https://www.who.int/emergencies/situations/monkeypox-oubreak-2022 (accessed on 10 November 2022).
- Zumla, A.; Valdoleiros, S.R.; Haider, N.; Asogun, D.; Ntoumi, F.; Petersen, E.; Kock, R. Monkeypox outbreaks outside endemic regions: Scientific and social priorities. Lancet 2022, 22, 929–931. [Google Scholar] [CrossRef]
- Giorgi, F.M.; Pozzobon, D.; Meglio, A.D.; Mercatelli, D.; Giorgi, F.M.; Pozzobon, D. Genomic characterization of the recent monkeypox outbreak. bioRxiv 2022. [Google Scholar] [CrossRef]
- Jain, N.; Lansiaux, E.; Simanis, R. The new face of monkeypox virus: An emerging global emergency. New Microbes New Infect. 2022, 47, 100989. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2052297522000415 (accessed on 10 November 2022). [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.J.; Duncan, C.J.A.; et al. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1473309922002286 (accessed on 10 November 2022). [CrossRef]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. 2005, 41, 1742–1751. Available online: https://academic.oup.com/cid/article/41/12/1742/344953 (accessed on 10 November 2022). [CrossRef]
- McCollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. Available online: https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/cit703 (accessed on 10 November 2022). [CrossRef]
- Kantele, A.; Chickering, K.; Vapalahti, O.; Rimoin, A.W. Emerging diseases—The monkeypox epidemic in the Democratic Republic of the Congo. Clin. Microbiol. Infect. 2016, 22, 658–659. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1198743X16302282 (accessed on 10 November 2022). [CrossRef] [PubMed] [Green Version]
- Wallau, G.L.; Maciel-de-Freitas, R.; Schmidt-Chanasit, J. An unfolding monkeypox outbreak in Europe and beyond. Mil. Med. Res. 2022, 9, 31. Available online: https://mmrjournal.biomedcentral.com/articles/10.1186/s40779-022-00394-z (accessed on 10 November 2022). [CrossRef] [PubMed]
- Aromolo, I.F.; Maronese, C.A.; Avallone, G.; Beretta, A.; Boggio, F.L.; Murgia, G.; Marletta, D.A.; Barei, F.; Carrera, C.G.; Ramoni, S.; et al. Clinical spectrum of human monkeypox: An Italian single-centre case series. J. Eur. Acad. Dermatol. Venereol. JEADV 2022. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jdv.18612 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Nishiura, H.; Kashiwagi, T. Smallpox and Season: Reanalysis of Historical Data. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 1–10. Available online: http://www.hindawi.com/journals/ipid/2009/591935/ (accessed on 10 November 2022). [CrossRef] [Green Version]
- Dhama, K.; Chandran, D.; Chakraborty, S.; Yatoo, M.I.; Islam, M.A.; Bhattacharya, M.; Chakraborty, C.; Harapan, H.; Chaicumpa, W. Zoonotic concerns of Marburg virus: Current knowledge and counteracting strategies including One Health approach to limit animal-human interface: An update. Int. J. Surg. 2022, 106, 106941. Available online: https://linkinghub.elsevier.com/retrieve/pii/S174391912200718X (accessed on 10 November 2022). [CrossRef]
- Chakraborty, S.; Chandran, D.; Mohapatra, R.K.; Islam, M.A.; Alagawany, M.; Bhattacharya, M.; Chakraborty, C.; Dhama, K. Langya virus, a newly identified Henipavirus in China—Zoonotic pathogen causing febrile illness in humans, and its health concerns: Current knowledge and counteracting strategies—Correspondence. Int. J. Surg. 2022, 105, 106882. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1743919122006598 (accessed on 10 November 2022). [CrossRef]
- Chakraborty, S.; Mohapatra, R.K.; Chandran, D.; Alagawany, M.; Sv, P.; Islam, M.A.; Chakraborty, C.; Dhama, K. Monkeypox vaccines and vaccination strategies: Current knowledge and advances. An update—Correspondence. Int. J. Surg. 2022, 105, 106869. Available online: https://linkinghub.elsevier.com/retrieve/pii/S174391912200646X (accessed on 10 November 2022). [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R.; Roy, S.S.; Islam, M.A.; Chakraborty, S.; Nandi, S.S.; Dhama, K. Deep learning research should be encouraged for diagnosis and treatment of antibiotic resistance of microbial infections in treatment associated emergencies in hospitals. Int. J. Surg. 2022, 105, 106857. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1743919122006343 (accessed on 10 November 2022). [CrossRef]
- Chakraborty, S.; Chandran, D.; Mohapatra, R.K.; Yatoo, M.I.; Islam, A.; Sharma, A.K.; Alagawany, M.; Dhama, K. Marburg Virus Disease—A Mini-Review. J. Exp. Biol. Agric. Sci. 2022, 10, 689–696. [Google Scholar] [CrossRef]
- Chandran, D.; Dhama, K.; Chakraborty, S.; Mohapatra, R.K.; Yatoo, M.I. Monkeypox: An Update on Current Knowledge and Research Advances. J. Exp. Biol. Agric. Sci. 2022, 10, 679–688. [Google Scholar] [CrossRef]
- NASA. POWER. Available online: https://power.larc.nasa.gov/ (accessed on 10 November 2022).
- de Livera, A.M.; Hyndman, R.J.; Snyder, R.D. Forecasting time series with complex seasonal patterns using exponential smoothing. J. Am. Stat. Assoc. 2011, 106, 1513–1527. [Google Scholar] [CrossRef] [Green Version]
- Tseng, Y.J.; Shih, Y.L. Developing epidemic forecasting models to assist disease surveillance for influenza with electronic health records. Int. J. Comput. Appl. 2020, 42, 616–621. [Google Scholar] [CrossRef]
- Chaurasia, V.; Pal, S. Application of machine learning time series analysis for prediction COVID-19 pandemic. Res. Biomed. Eng. 2020, 38, 35–47. [Google Scholar] [CrossRef]
- Adhikari, R.; Agrawal, R.K. An Introductory Study on Time Series Modeling and Forecasting. arXiv 2013, arXiv:1302.6613. [Google Scholar]
- Tariq, H.; Hanif, M.K.; Sarwar, M.U.; Bari, S.; Sarfraz, M.S.; Oskouei, R.J. Employing Deep Learning and Time Series Analysis to Tackle the Accuracy and Robustness of the Forecasting Problem. Secur. Commun Netw. 2021, 2021, 1–10. Available online: https://www.hindawi.com/journals/scn/2021/5587511/ (accessed on 10 November 2022). [CrossRef]
- Hasan, M.N.; Haider, N.; Stigler, F.L.; Khan, R.A.; McCoy, D.; Zumla, A.; Kock, R.A.; Uddin, M.J. The Global Case-Fatality Rate of COVID-19 Has Been Declining Since May 2020. Am. J. Trop. Med. Hyg. 2021, 104, 2176. [Google Scholar] [CrossRef] [PubMed]
- Kourentzes, N.; Petropoulos, F. Forecasting with multivariate temporal aggregation: The case of promotional modelling. Int. J. Prod. Econ. 2016, 181, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Haider, N.; Hasan, M.N.; Khan, R.A.; McCoy, D.; Ntoumi, F.; Dar, O.; Ansumana, R.; Uddin, M.J.; Zumla, A.; Kock, R.A. The Global case-fatality rate of COVID-19 has been declining disproportionately between top vaccinated countries and the rest of the world. medRxiv 2022. [Google Scholar]
- Roy, S.; Bhowmik, D.R.; Begum, R.; Amin, M.T.; Islam, M.A.; Ahmed, F.; Hossain, M.S. Aspirin attenuates the expression of adhesion molecules, risk of obesity, and adipose tissue inflammation in high-fat diet-induced obese mice. Prostaglandins Other Lipid Mediat. 2022, 162, 106664. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1098882322000545 (accessed on 10 November 2022). [CrossRef]
- Roy, S.; Ripon, M.A.R.; Begum, R.; Bhowmik, D.R.; Amin, M.T.; Islam, M.A.; Ahmed, F.; Hossain, M.S. Arachidonic acid supplementation attenuates adipocyte inflammation but not adiposity in high fat diet induced obese mice. Biochem. Biophys Res. Commun. 2022, 60, 90–95. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0006291X22004223 (accessed on 10 November 2022). [CrossRef]
- Haque, M.A.; Wang, F.; Chen, Y.; Hossen, F.; Islam, M.A.; Hossain, M.A.; Siddique, N.; He, C.; Ahmed, F. Bacillus spp. Contamination: A Novel Risk Originated From Animal Feed to Human Food Chains in South-Eastern Bangladesh. Front. Microbiol. 2022, 12, 3852. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2021.783103/full (accessed on 10 November 2022). [CrossRef]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Cansal, S. Climate change increases cross-species viral transmission risk. Nature 2022, 607, 555–562. Available online: https://www.nature.com/articles/s41586-022-04788-w (accessed on 10 November 2022). [CrossRef]
- Hossain, F.E.; Islam, S.; Islam, M.A.; Islam, S.; Ahmed, F. Detection of virulence genes of APEC (avian pathogenic Escherichia coli) isolated from poultry in Noakhali, Bangladesh. Biores. Commun. 2021, 7, 967–972. Available online: https://www.banglajol.info/index.php/BRC/article/view/54253 (accessed on 10 November 2022). [CrossRef]
- Bi, P.; Wang, J.; Hiller, J.E. Weather: Driving force behind the transmission of severe acute respiratory syndrome in China? Intern. Med. J. 2007, 37, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Son, W.; Ryu, Y.; Choi, S.B.; Kwon, O.; Ahn, I. Effects of temperature, humidity, and diurnal temperature range on influenza incidence in a temperate region. Influenza Other Respi. Viruses 2020, 14, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkodie, S.A.; Owusu, P.A. Global effect of city-to-city air pollution, health conditions, climatic & socio-economic factors on COVID-19 pandemic. Sci. Total Environ. 2021, 778, 146394. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0048969721014625 (accessed on 10 November 2022). [CrossRef]
- Jakariya, M.; Ahmed, F.; Islam, M.A.; Ahmed, T.; Marzan, A.A.; Hossain, M.; Reza, H.M.; Bhattacharya, P.; Hossain, A.; Nahla, T.; et al. Wastewater based surveillance system to detect SARS-CoV-2 genetic material for countries with on-site sanitation facilities: An experience from Bangladesh. medRxiv 2021. [Google Scholar]
- Altamimi, A.; Ahmed, A.E. Climate factors and incidence of Middle East respiratory syndrome coronavirus. J. Infect. Public. Health. 2020, 13, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Rakib, S.H. Design of a cost-effective Ultraviolet Disinfection unit to minimize the cross-contamination of COVID-19 in transport. In Proceedings of the 2022 International Conference on Advancement in Electrical and Electronic Engineering (ICAEEE), Gazipur, Bangladesh, 24–26 February 2022; pp. 2–7. [Google Scholar]
- Rakib, S.H.; Masum, S.; Patwari, M.R.I.; Fahima, R.A.; Farhana, A.; Islam, M.A. Design and Development of a low cost Ultraviolet Disinfection system to reduce the cross infection of SARS-CoV-2 in ambulances. In Proceedings of the 2021 International Conference on Electronics, Communications and Information Technology (ICECIT), Khulna, Bangladesh, 14–16 September 2021; pp. 1–4. Available online: https://ieeexplore.ieee.org/document/9641131/ (accessed on 10 November 2022).
- Nasirpour, M.H.; Sharifi, A.; Ahmadi, M.; Ghoushchi, J.S. Revealing the relationship between solar activity and COVID-19 and forecasting of possible future viruses using multi-step autoregression (MSAR). Env. Sci. Pollut. Res. 2021, 28, 38074–38084. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Sharifi, A.; Dorosti, S.; Ghoushchi, J.S.; Ghanbari, N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Sci. Total Environ. 2020, 729, 138705. [Google Scholar] [CrossRef] [PubMed]
- Menebo, M.M. Temperature and precipitation associate with Covid-19 new daily cases: A correlation study between weather and Covid-19 pandemic in Oslo, Norway. Sci. Total Environ. 2020, 737, 139659. Available online: https://linkinghub.elsevier.com/retrieve/pii/S004896972033179X (accessed on 10 November 2022). [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. Available online: http://www.nejm.org/doi/10.1056/NEJMoa2207323 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Tiwari, A.; Adhikari, S.; Kaya, D.; Islam, M.A.; Malla, B.; Sherchan, S.P.; Al-Mustapha, A.I.; Kumar, M.; Aggarwal, S.; Bhattacharya, P.; et al. Monkeypox outbreak: Wastewater and environmental surveillance perspective. Sci. Total Environ. 2023, 856, 159166. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0048969722062659 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Jakariya, M.; Ahmed, F.; Islam, M.A.; Al Marzan, A.; Hasan, M.N.; Hossain, M.; Ahmed, T.; Hossain, A.; Reza, H.M.; Hossen, M.F.; et al. Wastewater-based epidemiological surveillance to monitor the prevalence of SARS-CoV-2 in developing countries with onsite sanitation facilities. Environ. Pollut. 2022, 311, 119679. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.U.; Zhang, L.; Parreira, V.R.; Brown, R.S.; Chettleburgh, C.; Dannah, N.; Delatolla, R.; Gilbride, K.A.; Graber, T.E.; Islam, G.; et al. Metagenomics of Wastewater Influent from Wastewater Treatment Facilities across Ontario in the Era of Emerging SARS-CoV-2 Variants of Concern. Microbiol. Resour. Announc. 2022, 11, e00362-22. [Google Scholar] [CrossRef]
- McAndrew, T.; Majumder, M.S.; Lover, A.A.; Venkatramanan, S.; Bocchini, P.; Besiroglu, T.; Codi, A.; Braun, D.; Dempsey, G.; Abbott, S.; et al. Early human judgment forecasts of human monkeypox, May 2022. Lancet Digit. Health 2022, 4, e569–e571. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2589750022001273 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Islam, M.A.; Hasan, M.N.; Bhattacharya, P.; Jakariya, M.; Dhama, K.; Ahmed, F. Association of household fuel with ARI (acute respiratory illness) under five years children in Bangladesh: Increasing mortality in developing countries. Front. Public Health Sec, 2022; in press. [Google Scholar] [CrossRef]
- Lowen, A.C.; Steel, J. Roles of Humidity and Temperature in Shaping Influenza Seasonality. J. Virol. 2014, 88, 7692–7695. Available online: https://journals.asm.org/doi/10.1128/JVI.03544-13 (accessed on 10 November 2022). [CrossRef] [PubMed] [Green Version]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza Virus Transmission Is Dependent on Relative Humidity and Temperature. PLoS Pathog. 2007, 3, e151. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, C. The Effects of Weather and Climate on the Seasonality of Influenza: What We Know and What We Need to Know. Geogr. Compass. 2010, 4, 718–730. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1749-8198.2010.00343.x (accessed on 10 November 2022). [CrossRef]
- Greenhalgh, T.; Peng, Z.; Jimenez, J.L.; Bahnfleth, W.; Dancer, S.J.; Bourouiba, L. Quantifying transmission risk of SARS-CoV-2 in different situations. BMJ 2022, 376, o106. Available online: https://www.bmj.com/lookup/doi/10.1136/bmj.o106 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Islam, A.; Rahman, A.; Jakariya; Bahadur, N.M.; Hossen, F.; Mukharjee, S.K.; Hossain, M.S.; Tasneem, A.; Haque, M.A.; Sera, F.; et al. A 30-day follow-up study on the prevalence of SARS-COV-2 genetic markers in wastewater from the residence of COVID-19 patient and comparison with clinical positivity. Sci. Total Environ. 2022, 159350. [Google Scholar] [CrossRef] [PubMed]
- Sakib, M.M.H.; Nishat, A.A.; Islam, M.T.; Raihan Uddin, M.A.; Iqbal, M.S.; Bin Hossen, F.F.; Ahmed, M.I.; Bashir, M.S.; Hossain, T.; Tohura, U.S.; et al. Computational screening of 645 antiviral peptides against the receptor-binding domain of the spike protein in SARS-CoV-2. Comput. Biol. Med. 2021, 136, 104759. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Hossen, F.; Rahman, A.; Sultana, K.F.; Hasan, M.N.; Haque, A.; Sosa-Hernández, J.E.; Oyervides-Muñoz, M.A.; Parra-Saldívar, R.; Ahmed, T.; et al. An opinion on Wastewater-Based Epidemiological Monitoring (WBEM) with Clinical Diagnostic Test (CDT) for detecting high-prevalence areas of community COVID-19 Infections. Curr. Opin. Environ. Sci. Health 2022, 100396. [Google Scholar] [CrossRef] [PubMed]



| Variables | Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| Temperature (°C) | 21.21 ± 1.38 | 17.45 | 23.24 |
| Dew/frost point temperature (°C) | 14.49 ± 1.17 | 11.34 | 15.93 |
| Relative humidity (%) | 70.25 ± 1.48 | 65.55 | 74.08 |
| Precipitation (mm/day) | 4.53 ± 1.44 | 1.87 | 9.54 |
| Surface pressure (kPa) | 96.32 ± 0.11 | 96.04 | 96.61 |
| Wind speed (m/s) | 2.11 ± 0.14 | 1.8 | 2.55 |
| Daily confirmed cases (number of people) | 419.57 ± 459.29 | 0 | 1977 |
| Method and Period | R2 | RMSE | MAE | |
|---|---|---|---|---|
| Simple Exponential Smoothing | ||||
| Overall | 33.20% | 374.37 | 260.44 | |
| Auto-Regressive Integrated Moving Average | ||||
| Overall ARIMA | 63.04% | 276.04 | 182.39 | |
| Auto-Regressive Integrated Moving Average with explanatory variables | ||||
| Overall ARIMAX | 64.88% | 271.45 | 185.04 | |
| Automatic Forecasting time-series model (Prophet Model) | ||||
| Overall | 56.47% | 378.68 | 298.32 | |
| Mann-Kendell trend analysis | ||||
| Tau | p-value | |||
| 0.238 | <0.001 | |||
| Sen’s slop test | ||||
| Sen’s Slope | 95% CI | |||
| 1.62 | 0.84 to 2.76 | |||
| Variables | ARIMAX Model | Negative Binomial Model | ||||
|---|---|---|---|---|---|---|
| Coef. | 95%CI | p-Value | Coef. | 95%CI | p-Value | |
| Temperature | 51.56 | −274.55 to 377.68 | 0.76 | 0.86 | −0.42 to 2.13 | 0.13 |
| Dew/frost point | −69.59 | −366.61 to 227.43 | 0.65 | −0.61 | −1.93 to 0.71 | 0.19 |
| Relative humidity | 17.32 | −83.71 to 118.35 | 0.74 | −0.14 | −0.53 to 0.26 | 0.36 |
| Precipitation | −19.41 | −57.25 to 18.42 | 0.31 | −0.05 | −0.19 to 0.08 | 0.51 |
| Surface pressure | 23.42 | −9.9 to 56.75 | 0.17 | −1.45 | −3.29 to 0.4 | 0.45 |
| Wind | −10.87 | −45.67 to 23.92 | 0.54 | −0.36 | −1.82 to 1.09 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.A.; Sangkham, S.; Tiwari, A.; Vadiati, M.; Hasan, M.N.; Noor, S.T.A.; Mumin, J.; Bhattacharya, P.; Sherchan, S.P. Association between Global Monkeypox Cases and Meteorological Factors. Int. J. Environ. Res. Public Health 2022, 19, 15638. https://doi.org/10.3390/ijerph192315638
Islam MA, Sangkham S, Tiwari A, Vadiati M, Hasan MN, Noor STA, Mumin J, Bhattacharya P, Sherchan SP. Association between Global Monkeypox Cases and Meteorological Factors. International Journal of Environmental Research and Public Health. 2022; 19(23):15638. https://doi.org/10.3390/ijerph192315638
Chicago/Turabian StyleIslam, Md. Aminul, Sarawut Sangkham, Ananda Tiwari, Meysam Vadiati, Mohammad Nayeem Hasan, Syed Toukir Ahmed Noor, Jubayer Mumin, Prosun Bhattacharya, and Samendra P. Sherchan. 2022. "Association between Global Monkeypox Cases and Meteorological Factors" International Journal of Environmental Research and Public Health 19, no. 23: 15638. https://doi.org/10.3390/ijerph192315638













