Effect of Persulfate Activation by Electrogenerated H2O2 and Anodic Oxidation on the Color Removal of Dye Solutions at Pt and BDD Anodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Oxidation Experiments
2.3. Analytic Methods
3. Results and Discussion
3.1. Effect of PS Concentration on Color Removal
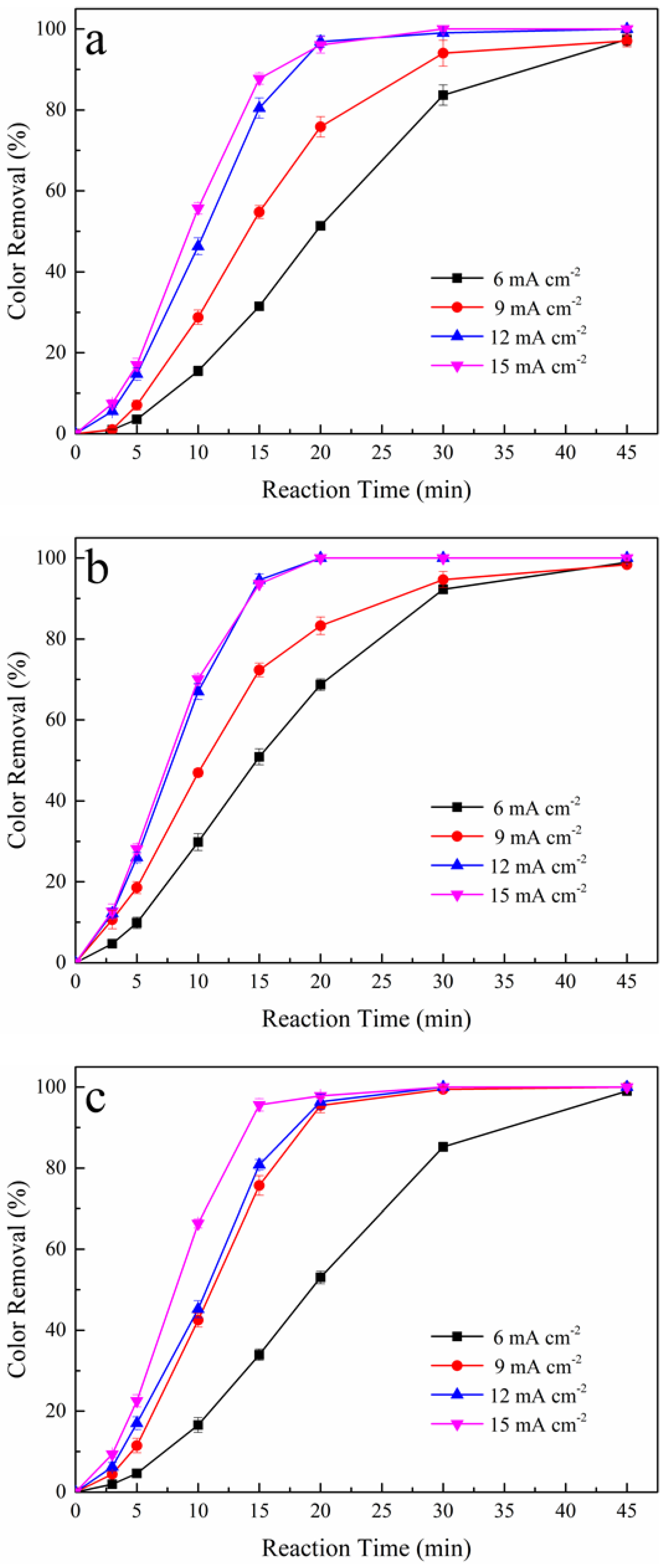
3.2. Effect of Applied Current Density on Color Removal
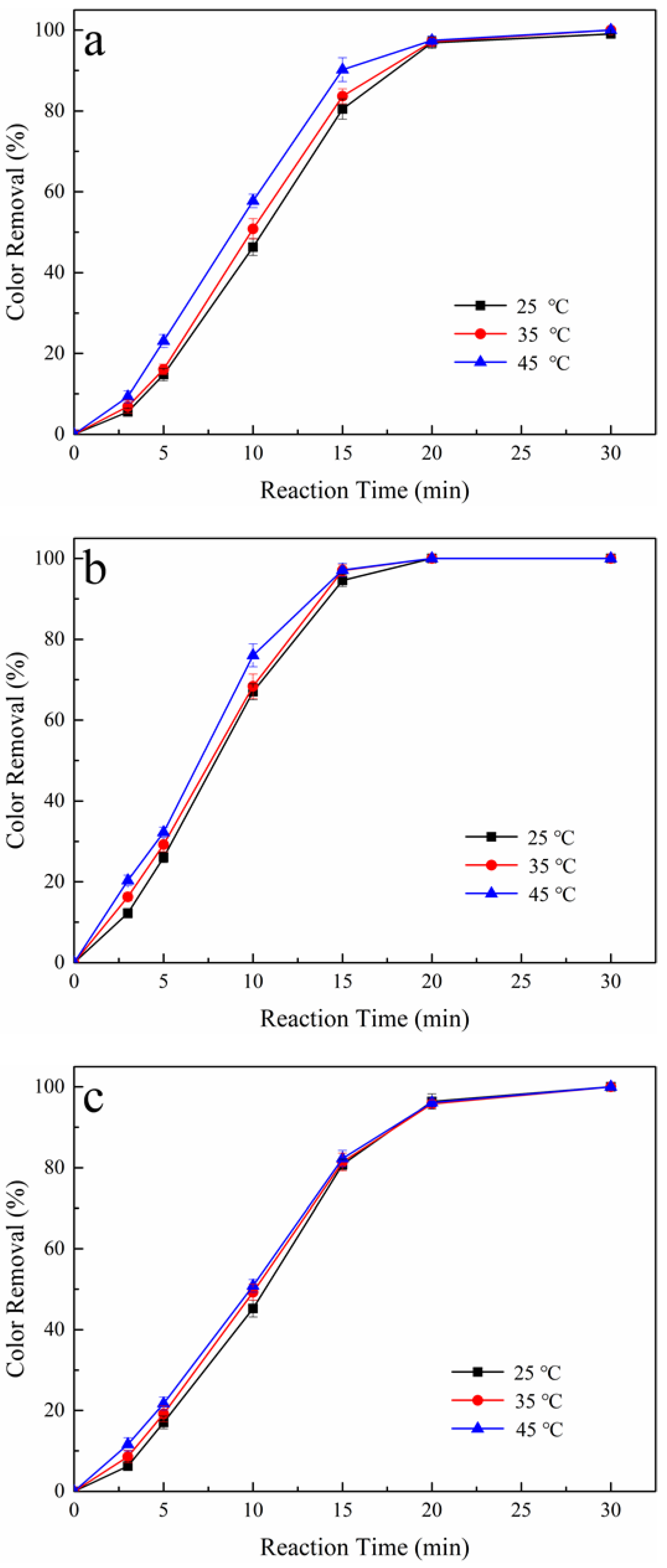
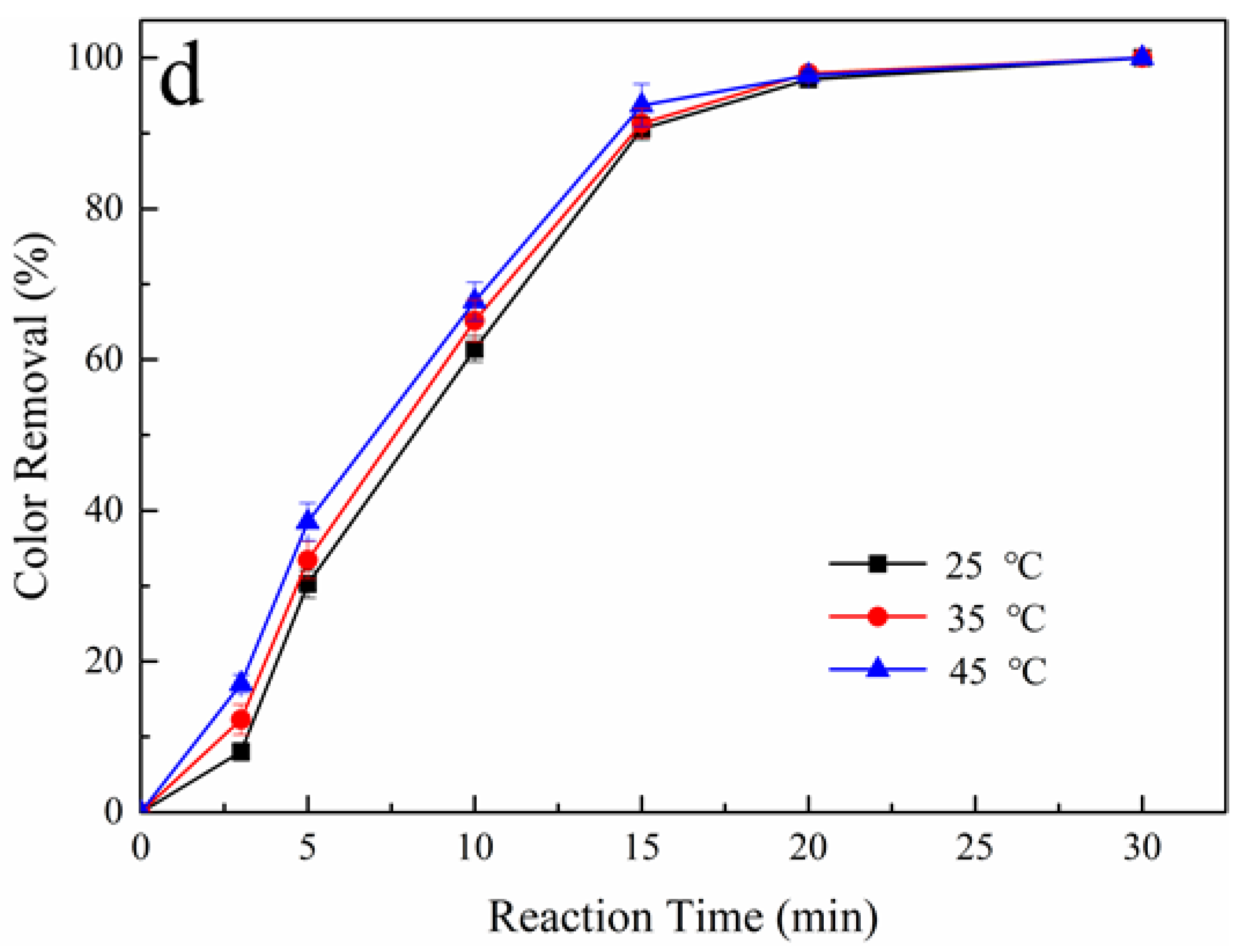
3.3. Effect of Reaction Temperature on Color Removal
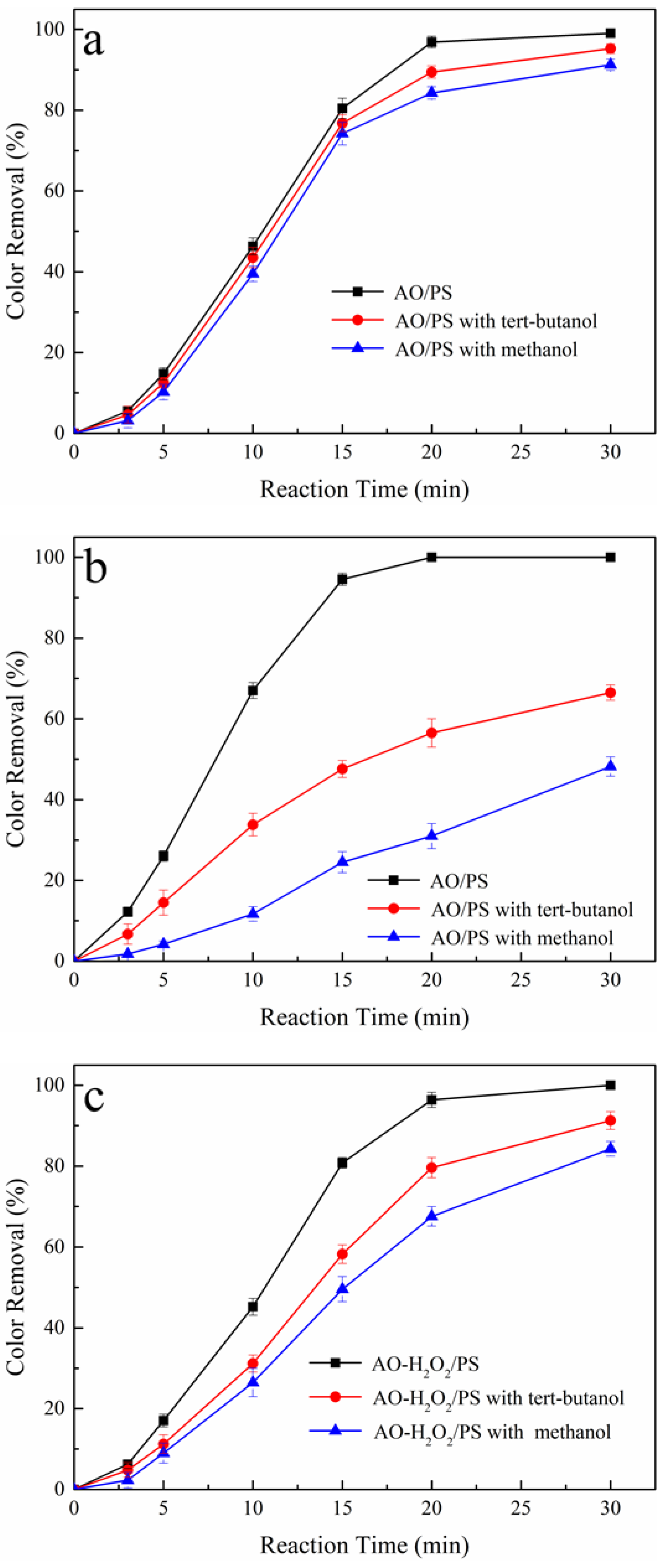
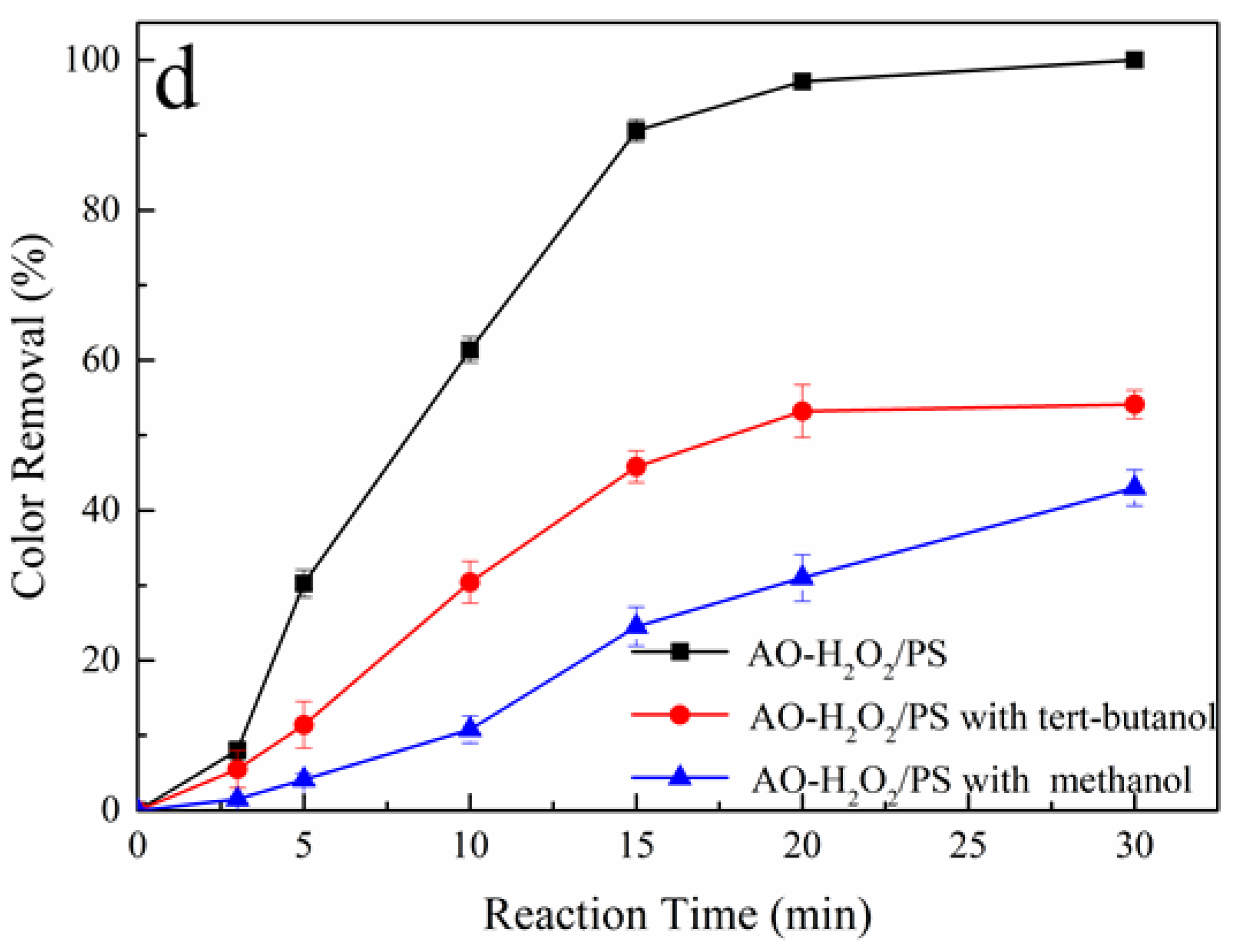
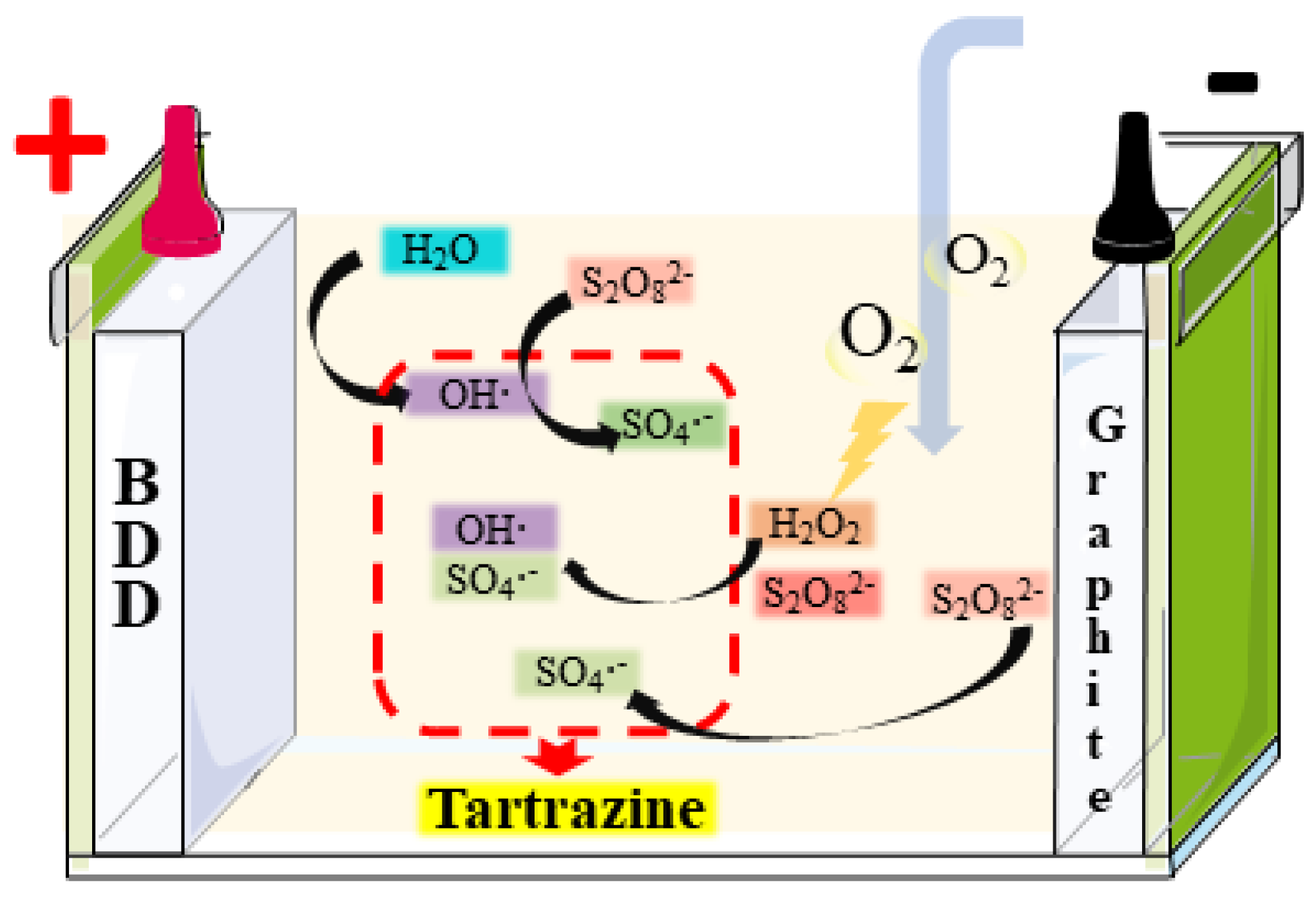
3.4. Oxidation Mechanism of Tartrazine in AO/PS and AO-H2O2/PS Process
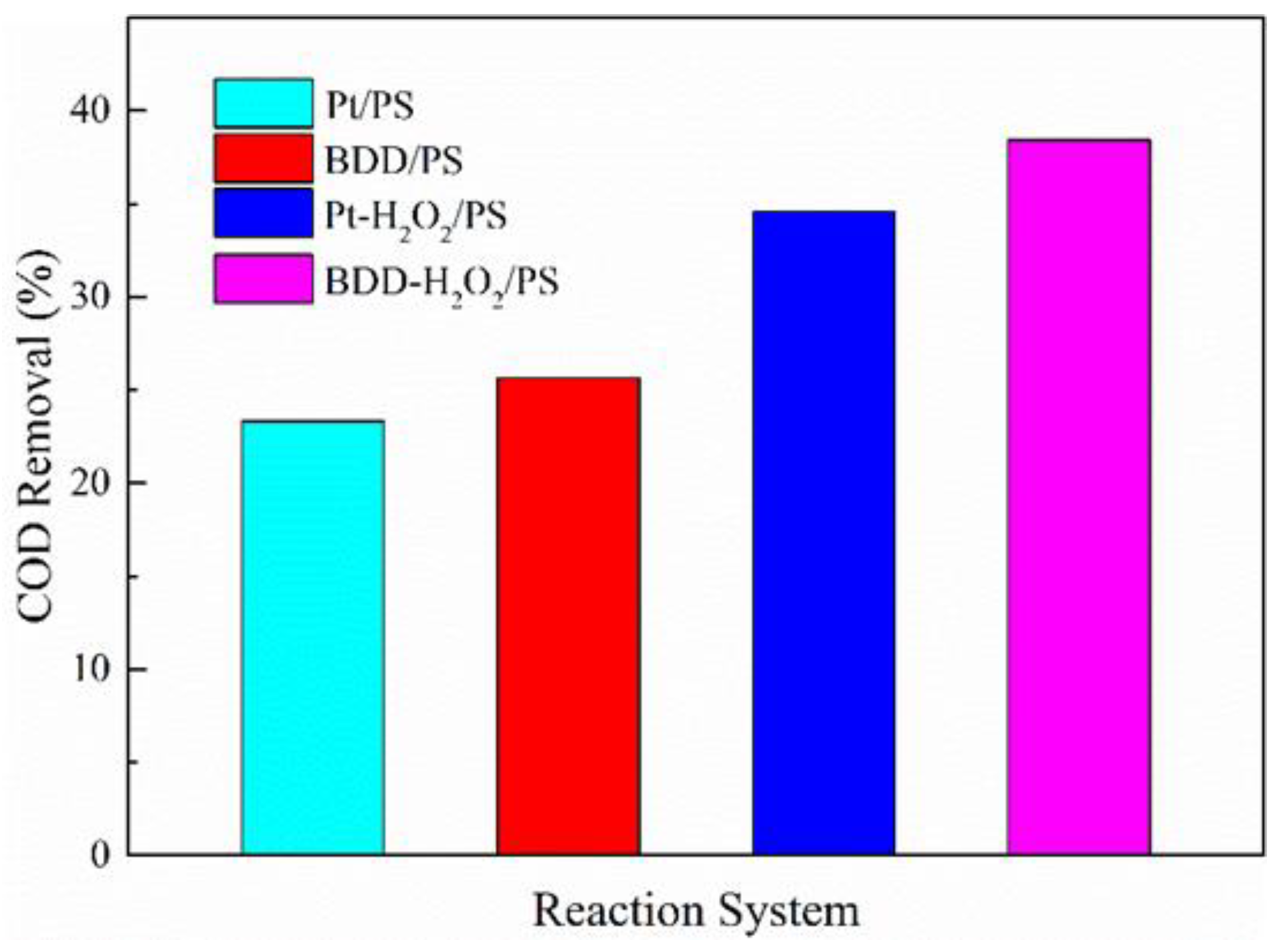
3.5. Economic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alderete, B.L.; da Silva, J.; Godoi, R.; da Silva, F.R.; Taffarel, S.R.; da Silva, L.P.; Garcia, A.L.H.; Júnior, H.M.; de Amorim, H.L.N.; Picada, J.N. Evaluation of toxicity and mutagenicity of a synthetic effluent containing azo dye after Advanced Oxidation Process treatment. Chemosphere 2020, 263, 128291. [Google Scholar] [CrossRef] [PubMed]
- Badvi, K.; Javanbakht, V. Enhanced photocatalytic degradation of dye contaminants with TiO2 immobilized on ZSM-5 zeolite modified with nickel nanoparticles. J. Clean. Prod. 2021, 280, 124518. [Google Scholar] [CrossRef]
- Balçik, U.; Chormey, D.S.; Ayyıldız, M.F.; Bakırdere, S. Liquid phase microextraction based sensitive analytical strategy for the determination of 22 hazardous aromatic amine products of azo dyes in wastewater and tap water samples by GC-MS system. Microchem. J. 2020, 155, 104712. [Google Scholar] [CrossRef]
- Hu, E.; Wu, X.; Shang, S.; Tao, X.; Jiang, S.; Gan, L. Catalytic ozonation of simulated textile dyeing wastewater using mesoporous carbon aerogel supported copper oxide catalyst. J. Clean. Prod. 2016, 112, 4710–4718. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, G.; Guo, L.; Dai, Q.; Ma, X. Electrochemical oxidation of Acid Orange 7 azo dye using a PbO2 electrode: Parameter optimization, reaction mechanism and toxicity evaluation. Chemosphere 2020, 241, 125010. [Google Scholar] [CrossRef]
- Wang, J.; Yao, J.; Wang, L.; Xue, Q.; Hu, Z.; Pan, B. Multivariate optimization of the pulse electrochemical oxidation for treating recalcitrant dye wastewater. Sep. Purif. Technol. 2020, 230, 115851. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, X.; Miao, J.; Zhang, R.; Zhang, J.; Zhou, M.; Lu, W. Enhanced performance of an Al-doped SnO2 anode for the electrocatalytic oxidation of organic pollutants in water. Mater. Today Commun. 2020, 24, 101164. [Google Scholar] [CrossRef]
- Zhu, K.; Qi, H.; Sun, X.; Sun, Z. Anodic oxidation of diuron using Co3O4/graphite composite electrode at low applied current. Electrochim. Acta 2019, 299, 853–862. [Google Scholar] [CrossRef]
- Tang, Y.; He, D.; Guo, Y.; Qu, W.; Shang, J.; Zhou, L.; Pan, R.; Dong, W. Electrochemical oxidative degradation of X-6G dye by boron-doped diamond anodes: Effect of operating parameters. Chemosphere 2020, 258, 127368. [Google Scholar] [CrossRef]
- Rueffer, M.; Bejan, D.; Bunce, N.J. Graphite: An active or an inactive anode? Electrochim. Acta 2011, 56, 2246–2253. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Liang, J.; Yue, L.; Li, T.; Luo, Y.; Liu, Q.; Lu, S.; Asiri, A.M.; Gong, Z.; et al. Anodic oxidation for the degradation of organic pollutants: Anode materials, operating conditions and mechanisms. A mini review. Electrochem. Commun. 2021, 123, 106912. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Rauf, M.; Luo, H.; Sun, X.; Jiang, Y. Gas diffusion electrodes for H2O2 production and their applications for electrochemical degradation of organic pollutants in water: A review. Sci. Total Environ. 2020, 759, 143459. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zheng, T.; Liu, Y.; Hou, D.; He, H.; Song, H.; Zhang, J.; Tian, S.; Zhang, W.; Wang, L.; et al. A cost-effective Electro-Fenton process with graphite felt electrode aeration for degradation of dimethyl phthalate: Enhanced generation of H2O2 and iron recycling that simultaneously regenerates the electrode. Chem. Eng. J. 2020, 394, 125033. [Google Scholar] [CrossRef]
- Qi, C.; Yu, G.; Huang, J.; Wang, B.; Wang, Y.; Deng, S. Activation of persulfate by modified drinking water treatment residuals for sulfamethoxazole degradation. Chem. Eng. J. 2018, 353, 490–498. [Google Scholar] [CrossRef]
- Ren, T.-L.; Ma, X.-W.; Wu, X.-Q.; Yuan, L.; Lai, Y.-L.; Tong, Z.-H. Degradation of imidazolium ionic liquids in a thermally activated persulfate system. Chem. Eng. J. 2021, 412, 128624. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cheng, Y.; Zeng, Y.; Luo, K.; He, D.; Pan, X. Rapid removal of organic micropollutants by heterogeneous peroxymonosulfate catalysis over a wide pH range: Performance, mechanism and economic analysis. Sep. Purif. Technol. 2020, 248, 117023. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Brillas, E.; Cabot, P.L.; Sirés, I. Simultaneous persulfate activation by electrogenerated H2O2 and anodic oxidation at a boron-doped diamond anode for the treatment of dye solutions. Sci. Total Environ. 2020, 747, 141541. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Cagnetta, G.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Nanoscale zero valent iron-activated persulfate coupled with Fenton oxidation process for typical pharmaceuticals and personal care products degradation. Sep. Purif. Technol. 2020, 239, 116534. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, Y.; Zhang, L.; Lu, D.; Lu, L.; Yang, X.; Cai, T. Degradation of Atrazine, Simazine and Ametryn in an arable soil using thermal-activated persulfate oxidation process: Optimization, kinetics, and degradation pathway. J. Hazard. Mater. 2020, 400, 123201. [Google Scholar] [CrossRef]
- Silveira, J.E.; Garcia-Costa, A.L.; Cardoso, T.O.; Zazo, J.A.; Casas, J.A. Indirect decolorization of azo dye Disperse Blue 3 by electro-activated persulfate. Electrochim. Acta 2017, 258, 927–932. [Google Scholar] [CrossRef]
- Zhang, W.; He, Y.; Li, C.; Hu, X.; Yang, S.; You, X.; Liang, W. Persulfate activation using Co/AC particle electrodes and synergistic effects on humic acid degradation. Appl. Catal. B Environ. 2021, 285, 119848. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, M.; Du, X.; Xu, X. Enhanced mechanism of 2,4-dichlorophenoxyacetic acid degradation by electrochemical activation of persulfate on Blue-TiO2 nanotubes anode. Sep. Purif. Technol. 2021, 254, 117560. [Google Scholar] [CrossRef]
- Ukundimana, Z.; Omwene, P.; Gengec, E.; Can, O.; Kobya, M. Electrooxidation as post treatment of ultrafiltration effluent in a landfill leachate MBR treatment plant: Effects of BDD, Pt and DSA anode types. Electrochim. Acta 2018, 286, 252–263. [Google Scholar] [CrossRef]
- El-Ghenymy, A.; Centellas, F.; Rodríguez, R.M.; Cabot, P.L.; Garrido, J.A.; Sirés, I.; Brillas, E. Comparative use of anodic oxidation, electro-Fenton and photoelectro-Fenton with Pt or boron-doped diamond anode to decolorize and mineralize Malachite Green oxalate dye. Electrochim. Acta 2015, 182, 247–256. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, M.; Pan, Y.; Lu, X. Degradation of 2,4-dichlorophenoxyacetic acid by anodic oxidation and electro-Fenton using BDD anode: Influencing factors and mechanism. Sep. Purif. Technol. 2019, 230, 115867. [Google Scholar] [CrossRef]
- Clematis, D.; Panizza, M. Electro-Fenton, solar photoelectro-Fenton and UVA photoelectro-Fenton: Degradation of Erythrosine B dye solution. Chemosphere 2020, 270, 129480. [Google Scholar] [CrossRef]
- Feng, H.; Chen, Z.; Wang, X.; Chen, S.; Crittenden, J. Electrochemical advanced oxidation for treating ultrafiltration effluent of a landfill leachate system: Impacts of organics and inorganics and economic evaluation. Chem. Eng. J. 2020, 413, 127492. [Google Scholar] [CrossRef]
- Chanikya, P.; Nidheesh, P.; Babu, D.S.; Gopinath, A.; Kumar, M.S. Treatment of dyeing wastewater by combined sulfate radical based electrochemical advanced oxidation and electrocoagulation processes. Sep. Purif. Technol. 2020, 254, 117570. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Y.; Chen, Z.; Duan, Y.; Lai, Y.; Fang, Q.; Wang, F.; Li, S. Performance of boron-doped graphene aerogel modified gas diffusion electrode for in-situ metal-free electrochemical advanced oxidation of Bisphenol A. Appl. Catal. B Environ. 2019, 255, 117784. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Huang, D.; Chen, N.; Zhu, C.; Fang, G.; Zhou, D. The overlooked oxidative dissolution of silver sulfide nanoparticles by thermal activation of persulfate: Processes, mechanisms, and influencing factors. Sci. Total Environ. 2020, 760, 144504. [Google Scholar] [CrossRef]
- Isarain-Chávez, E.; Baró, M.D.; Rossinyol, E.; Morales-Ortiz, U.; Sort, J.; Brillas, E.; Pellicer, E. Comparative electrochemical oxidation of methyl orange azo dye using Ti/Ir-Pb, Ti/Ir-Sn, Ti/Ru-Pb, Ti/Pt-Pd and Ti/RuO2 anodes. Electrochim. Acta 2017, 244, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Wei, T.; Xiao, K.; Deng, J.; Yu, G.; Deng, S.; Li, J.; Zhu, C.; Duan, H.; Zhuo, Q. Effective mineralization of anti-epilepsy drug carbamazepine in aqueous solution by simultaneously electro-generated H2O2/O3 process. Electrochim. Acta 2018, 290, 203–210. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, N.; Feng, C.; Chen, F.; Wang, H.; Kuang, P.; Feng, Z.; Liu, T.; Gao, Y.; Hu, W. Treatment of organic wastewater containing nitrogen and chlorine by combinatorial electrochemical system: Taking biologically treated landfill leachate treatment as an example. Chem. Eng. J. 2019, 364, 349–360. [Google Scholar] [CrossRef]



| Oxidation System | 0% PS | 25% PS | 50% PS | 100% PS |
|---|---|---|---|---|
| Color Removal (%) * | ||||
| Pt/PS | 71 | 94 | 63 | 64 |
| BDD/PS | 90 | 83 | 95 | 72 |
| Pt-H2O2/PS | 36 | 95 | 91 | 81 |
| BDD-H2O2/PS | 69 | 96 | 93 | 74 |
| Oxidation System | 6 mA cm−2 | 9 mA cm−2 | 12 mA cm−2 | 15 mA cm−2 |
|---|---|---|---|---|
| Color Removal (%) * | ||||
| Pt/PS | 51 | 76 | 97 | 96 |
| BDD/PS | 68 | 83 | 100 | 100 |
| Pt-H2O2/PS | 53 | 95 | 96 | 97 |
| BDD-H2O2/PS | 63 | 93 | 96 | 97 |
| Oxidation System | 45 °C | 45 °C | 45 °C |
|---|---|---|---|
| Color Removal (%) * | |||
| Pt/PS | 80 | 84 | 90 |
| BDD/PS | 94 | 97 | 98 |
| Pt-H2O2/PS | 80 | 81 | 82 |
| BDD-H2O2/PS | 90 | 91 | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Zhu, K.; Liu, Y.; Liu, Q.; Huang, L. Effect of Persulfate Activation by Electrogenerated H2O2 and Anodic Oxidation on the Color Removal of Dye Solutions at Pt and BDD Anodes. Int. J. Environ. Res. Public Health 2022, 19, 15688. https://doi.org/10.3390/ijerph192315688
Yao Y, Zhu K, Liu Y, Liu Q, Huang L. Effect of Persulfate Activation by Electrogenerated H2O2 and Anodic Oxidation on the Color Removal of Dye Solutions at Pt and BDD Anodes. International Journal of Environmental Research and Public Health. 2022; 19(23):15688. https://doi.org/10.3390/ijerph192315688
Chicago/Turabian StyleYao, Yifan, Kai Zhu, Yucan Liu, Qianjin Liu, and Lihua Huang. 2022. "Effect of Persulfate Activation by Electrogenerated H2O2 and Anodic Oxidation on the Color Removal of Dye Solutions at Pt and BDD Anodes" International Journal of Environmental Research and Public Health 19, no. 23: 15688. https://doi.org/10.3390/ijerph192315688
APA StyleYao, Y., Zhu, K., Liu, Y., Liu, Q., & Huang, L. (2022). Effect of Persulfate Activation by Electrogenerated H2O2 and Anodic Oxidation on the Color Removal of Dye Solutions at Pt and BDD Anodes. International Journal of Environmental Research and Public Health, 19(23), 15688. https://doi.org/10.3390/ijerph192315688







