Abstract
Conventional wastewater treatment technologies have difficulties in feasibly removing persistent organics. The photocatalytic oxidation of these contaminants offers an economical and environmentally friendly solution. In this study, TiO2 membranes and Ag/TiO2 membranes were prepared and used for the decomposition of dissolved formic acid in wastewater. The photochemical deposition of silver on a TiO2 membrane improved the decomposition rate. The rate doubled by depositing ca. 2.5 mg of Ag per 1 g of TiO2. The influence of salinity on formic acid decomposition was studied. The presence of inorganic salts reduced the treatment performance of the TiO2 membranes to half. Ag/TiO2 membranes had a larger reduction of ca. 40%. The performance was recovered by washing the membranes with water. The anion adsorption on the membrane surface likely caused the performance reduction.
1. Introduction
A large variety of persistent organic molecules are found in the wastewater from municipal and industrial activities of a city. Some of these organics are bio-accumulative and can be toxic, even in low concentrations. However, current technologies have difficulties in treating these dilute components in an economic and environmentally friendly manner. In addition, municipal wastewater contains salinity in the coastal area, which can change the properties of wastewater treatment technology [1,2,3]. For example, salts can hinder the growth of microorganisms and change the performance of activated sludge treatment [1]. Concentrations of 10 to 100 mmol/L NaCl in water changed the adhesion energy of humic substances and affected their removal by adsorption [3]. Coexisting NaCl in water is also reported to slightly reduce catalytic wet-air oxidation activity [2].
Catalytic wet-air oxidation decomposes various types of organics in wastewater that are difficult to degrade by biological treatment [4,5]. Immobilizing catalyst particles on membranes eliminate the process required to separate spent catalysts from the treated water. In addition, the configuration of catalytic membrane contactors facilitates oxygen supply to the reaction field, which enhances the decomposition rate of dissolved organics by catalytic oxidation [6,7]. For example, platinum-based catalytic membranes decomposed dissolved phenols in seawater under mild conditions, showing the potential for membranes to treat wastewater [6]. However, the catalytic performance needs to be improved to reduce the footprint of the membrane unit. As photocatalysts can decompose various types of organics in water [8], immobilizing these photocatalysts on membranes is a possible solution to improve the catalytic membrane performance.
Titanium dioxide (TiO2) is a commonly used photocatalyst as it is robust, nontoxic, and cost-effective. However, TiO2 requires UV light irradiation to activate due to the wide band gap energy of 3.2 and 3.0 eV for the anatase and rutile phases, respectively [9,10]. Large efforts have been made to narrow the band gap by modifying TiO2 with dye, noble metals, such as Au, Ag, and Pt, transition metals, such as Fe and Cu, and other materials [10]. Successful modifications made the TiO2-based catalysts visible-light active and the high performance of decomposing dissolved organics in water is reported [11,12].
Many studies evaluating photocatalytic activity use solutions prepared by dissolving a controlled amount of target organic compounds into distilled water. While salinity can exist in wastewater, its influence on photocatalytic activity is not fully understood. For example, Chen et al. reported a negative influence of anions, such as chloride, on the photocatalytic oxidation of dichloroethane, as anions inhibit the adsorption of dichloroethane to a TiO2 surface [13]. On the contrary, Makita et al. reported a positive influence of cations on the decomposition rate of dye [14].
In this study, the influence of water salinity was examined using TiO2 and Ag/TiO2 membranes. Formic acid was used as a model organic compound in water as its degradation process is simple and easy to follow [15,16]. Salinity in municipal wastewater can increase in the coastal area due to the infiltration of seawater. To simulate the seawater contamination, sodium chloride (NaCl) and magnesium sulfate (MgSO4) were used as salts. Magnesium chloride (MgCl2) and potassium sulfate (K2SO4) were also used for comparison.
2. Materials and Methods
Porous flat discs were prepared using kaolin (ER, Caobar S.A., Guadalajara, Spain), alumina (AR12B5, Aluminium Pechiney, Salindres, France), potato starch (Sigma Aldrich Inc., St. Louis, MO, USA) and polyvinyl alcohol (PVA, Mowiol 4-88, Sigma Aldrich Inc., USA) as a ligand. After sintering the supports at 1673 K for four hours, XRD analysis identified mullite, corundum, and cristobalite (Figure S1). TiO2 particles (P25, Evonik Industries) were applied to the surface of the disc supports, having a 47 mm diameter. A detailed procedure will be found elsewhere [15]. Then, silver was photochemically applied to some of the prepared TiO2 membranes using a silver acetate solution of 0.01–1 mmol/L. The concentration of silver in the immersed solution was measured by inductively coupled plasma (ICP, SII Nano Technology Co. Ltd., Tokyo, Japan) before and after applying UV light to the membrane. The amount of silver deposited on the TiO2 membranes was calculated from the concentration decrease in silver ions in the solution and the weight of the solution used. Three pieces of black lamps (Toshiba, maximum light emission at 352 nm) were used as the UV source and the light intensity was adjusted to 3.3 mW/cm2 by changing the distance between the membrane and the lamps. The light strength was measured by a photometer (C10427H102428, Hamamatsu Photonics, Tsukuba, Japan). Prepared TiO2 and Ag/TiO2 membranes were analyzed by scanning electron microscope (FE-SEM, JSM-633F, JSM-7600FG, JEOL Ltd., Tokyo, Japan) and X-ray diffraction (XRD, XRD-6100, SHIMADZU Co., Kyoto, Japan) with Cu-Kα radiation.
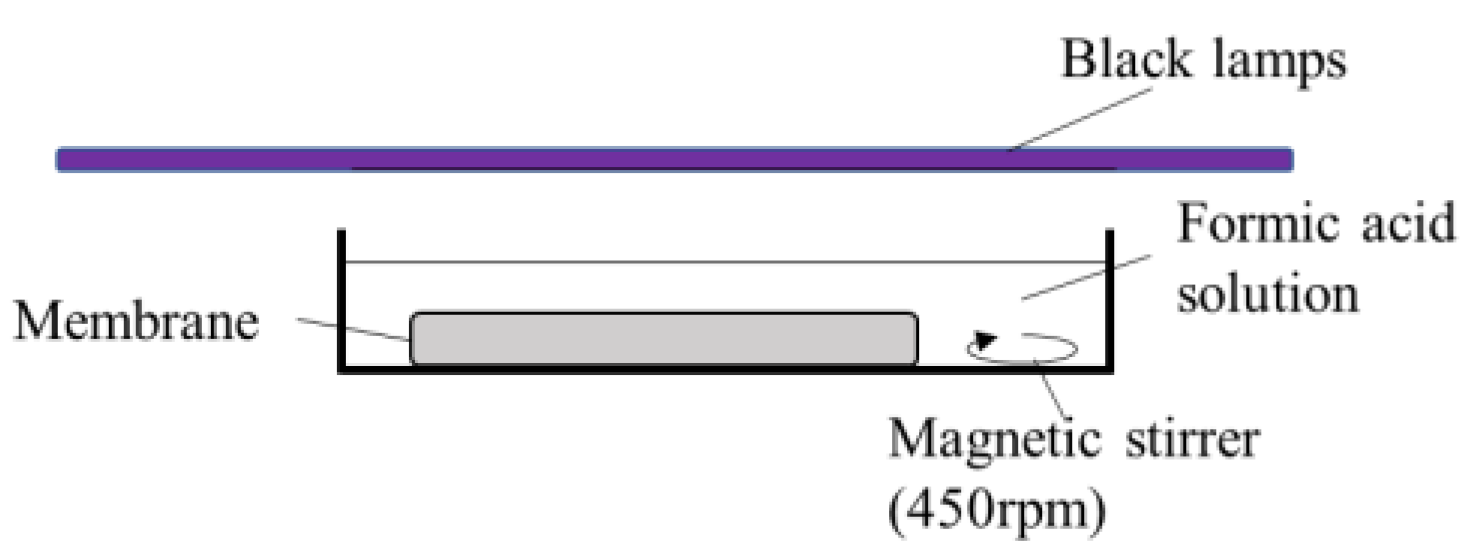
The photocatalytic activity of the obtained TiO2 and Ag/TiO2 membranes was evaluated by the decomposition of formic acid (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) in water. Formic acid was used in this study. The flat disc-shaped membrane was soaked in formic acid solution and kept under dark conditions for 20 min before applying UV light with a light intensity of 3.3 mW/cm2 at room temperature. This light intensity was used to simulate the UVA part of the sunlight [17]. The same black lamps being used in the silver deposition were used as the UV light source in the photocatalytic activity tests. Figure 1 illustrates the experimental setup. Humidity was not controlled nor measured. The starting concentration of the formic acid solution was adjusted to 200 mg/L, a concentration similar to other studies [15]. The concentration of the formic acid solutions was measured using a UV–Vis spectrophotometer UV-1800 (Shimadzu, Columbia, MD, USA) at a 205.6 nm wavelength. Calibration curves to determine the formic acid concentration were prepared for each salt concentration.
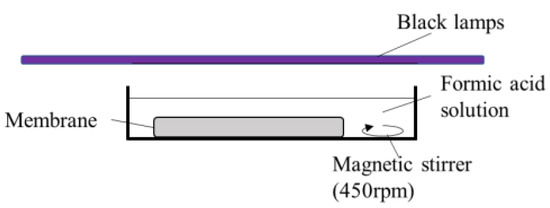
Figure 1.
The experimental setup for photocatalytic formic acid decomposition.
Inorganic salts, NaCl, MgSO4, MgCl2, and K2SO4, were purchased from Fujifilm Wako Pure Chemical Corporation, Japan, and dissolved into the formic acid solution at the concentration of 0.6, 6, 60 mmol/L for NaCl, MgSO4 and K2SO4, and 0.3, 3, 30 mmol/L for MgCl2. The maximum concentration of NaCl studied was about 1/10 of that in seawater. After each test, the membranes were washed with water.
3. Results and Discussion
3.1. Influence of Silver Deposition
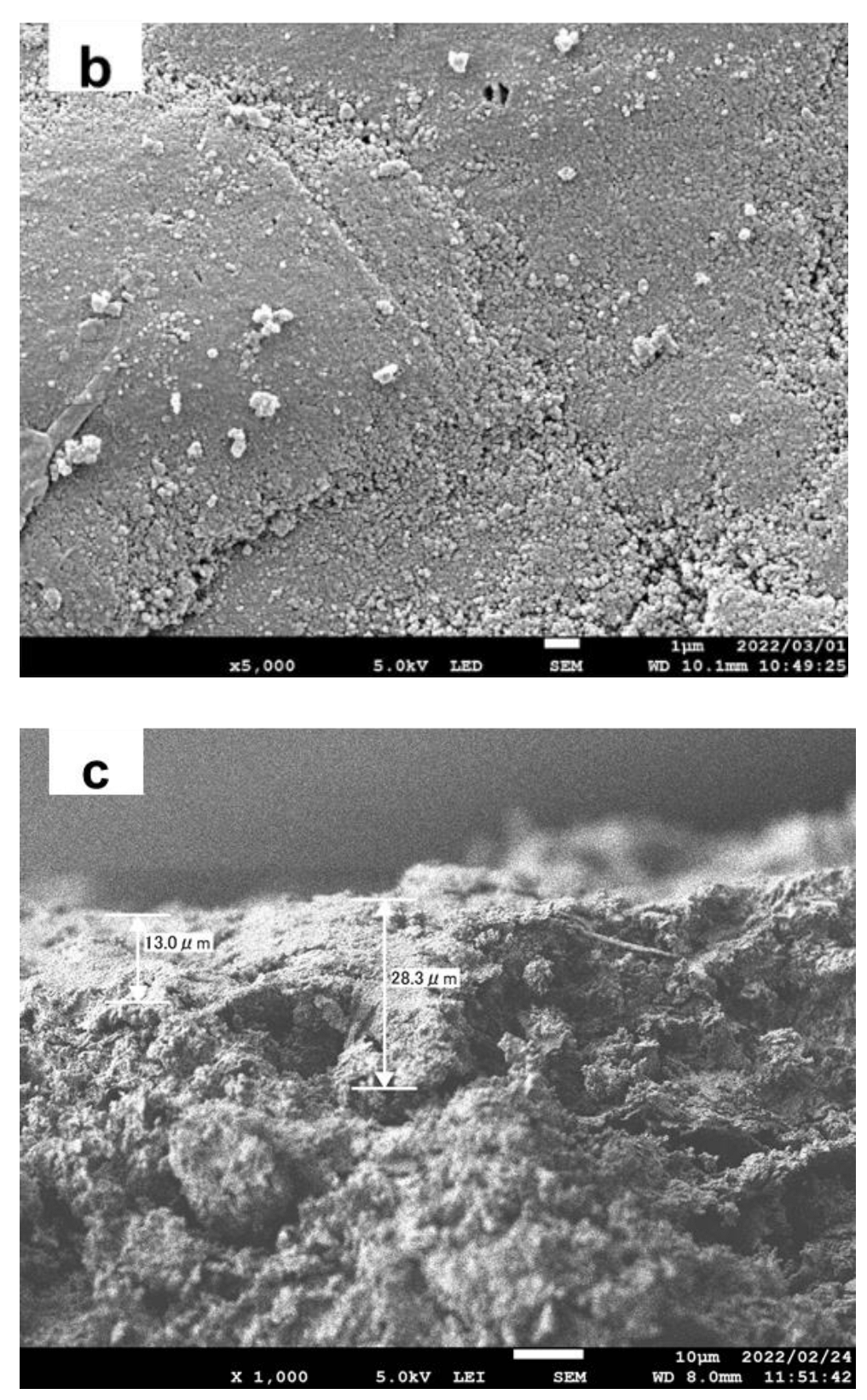
Figure 2 shows the membrane morphology. The surface of the porous ceramic disc (Figure 2a) was completely covered with TiO2 particles (Figure 2b). The thickness of the TiO2 layer was about 10–30 μm (Figure 2c). The variation is due to the large pores in the ceramic disc, which were plugged by the TiO2 particles. Element mapping of the cross-sectional view of the TiO2 membrane is shown in Figure S2. Al and Si mapping images show a void in the flat support. The Ti mapping showed that such voids can be filled with TiO2 particles applied to the surface. As a result, the surface of the TiO2 membrane became smoother compared to the support surface (Figure 2a,b). The surface morphology of the AgTiO2 membrane was similar to that of the TiO2 membrane. No peaks relating to Ag or AgOx were found in the XRD pattern, probably due to its small amount and size [18] (Figure S1). X-ray photoelectron spectroscopy (XPS) analyses showed that silver was deposited as Ag0, Ag+, and Ag2+ (Figure S3).

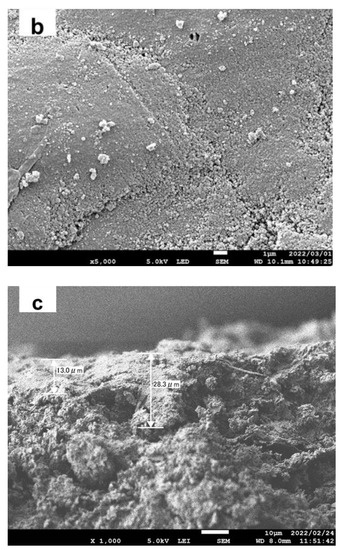
Figure 2.
Membrane morphologies (a) surface view of the porous ceramic disc, (b) surface view of the TiO2 membrane, (c) cross-sectional view of the TiO2 membrane.
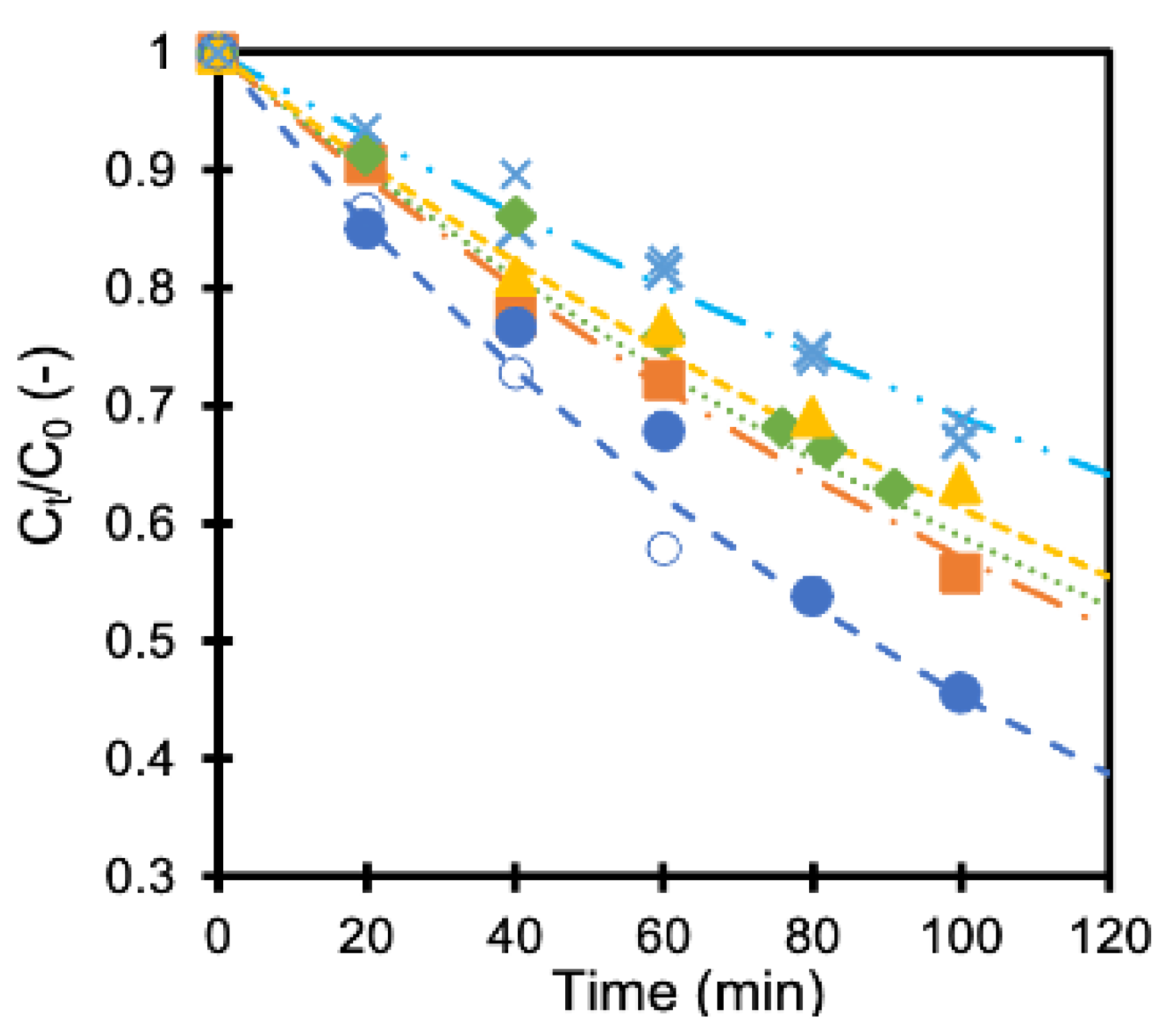
The concentration of formic acid did not change in the dark, showing little influence on the adsorption of formic acid on the membranes. On the contrary, the concentration decreased when UV light was irradiated, as shown in Figure 3. The concentration at time t (Ct) was normalized with the initial concentration (C0) in the figure. The results obtained with a TiO2 membrane and four pieces of Ag/TiO2 membrane having different deposition amounts of silver are shown in the same figure. The formic acid concentration became about 75% and 50% compared to the initial concentration after 80 min with TiO2 membrane and Ag/TiO2 (AgT2) membrane, respectively. Silver deposition improved the photocatalytic activity of the membrane. The membranes showed reproducible performance, as indicated with smaller keys.
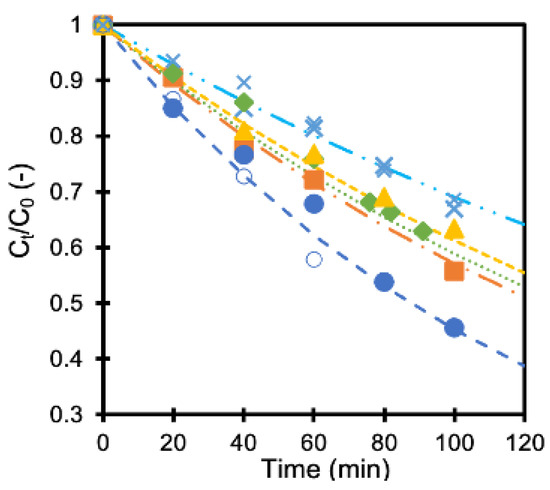
Figure 3.
Normalized formic acid concentration as a function of time (x: TiO2 membranes, x: TiO2 membranes 2nd run, ■ AgT1 membrane, ● AgT2 membrane, ○ AgT2 membrane 2nd run, ◆ AgT3 membrane, ▲ AgT4 membrane).
The concentration change was fitted (lines in Figure 3) with the first-order equation (Equation (1)) as reported earlier [16].
where k is a rate constant, and C0,t are the formic acid concentrations at the start and at a time, t, respectively. The calculated rate constant values are summarized in Table 1. Applying small amounts of silver to the TiO2 membranes improved the formic acid decomposition rate as reported earlier, for it reduces the recombination of an electron-hole pair that facilitates the formation of oxidants [16,19]. However, an optimum amount of silver maximizes the rate constant. An excessive amount of silver may act as the center for electron-hole recombination, which is considered to be one of the reasons for its negative influence [20].
Ct/C0 = e−kt

Table 1.
Influence of silver on the rate constant values obtained in formic acid decomposition.
3.2. Effect of Salinity on the Photocatalytic Performance
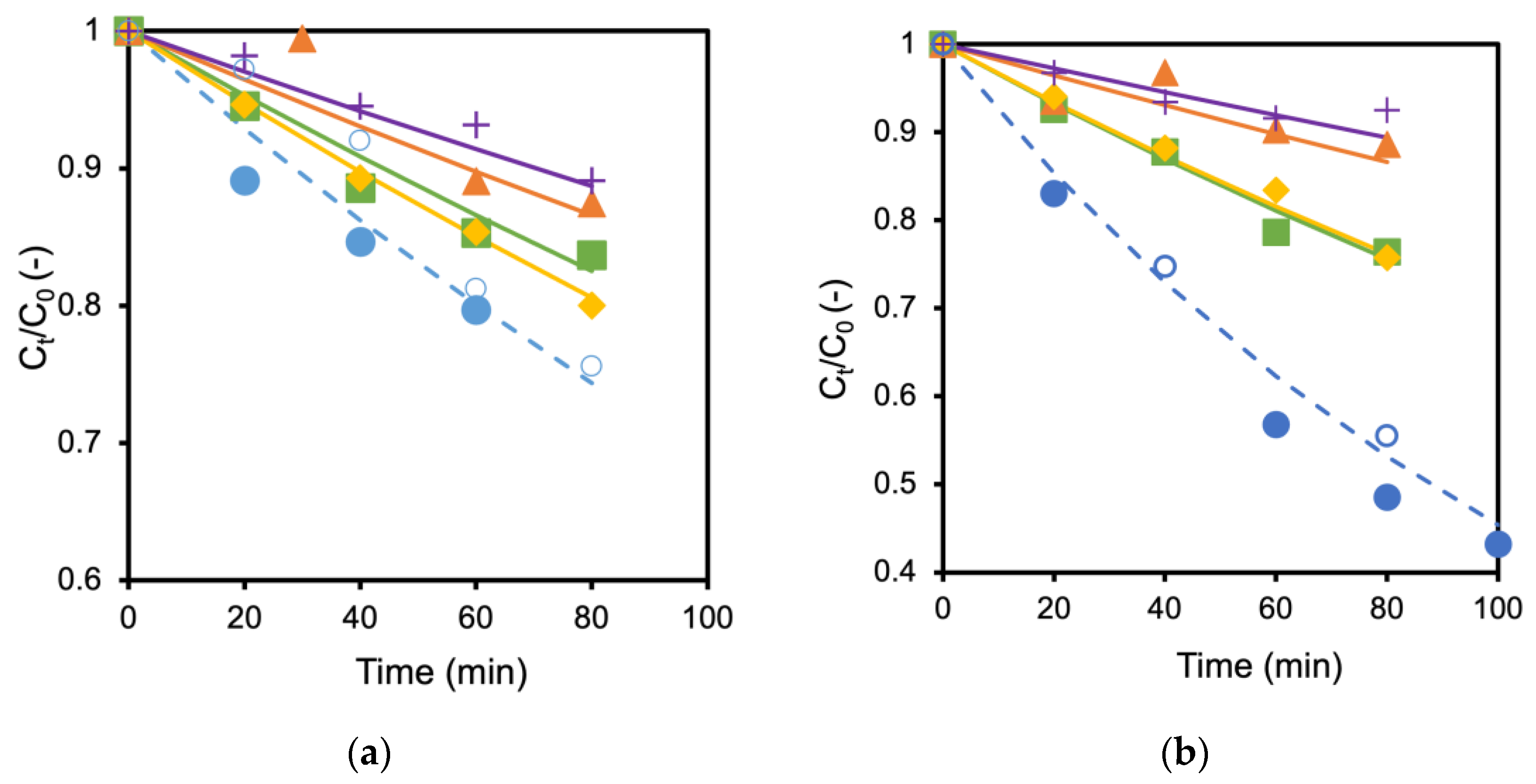
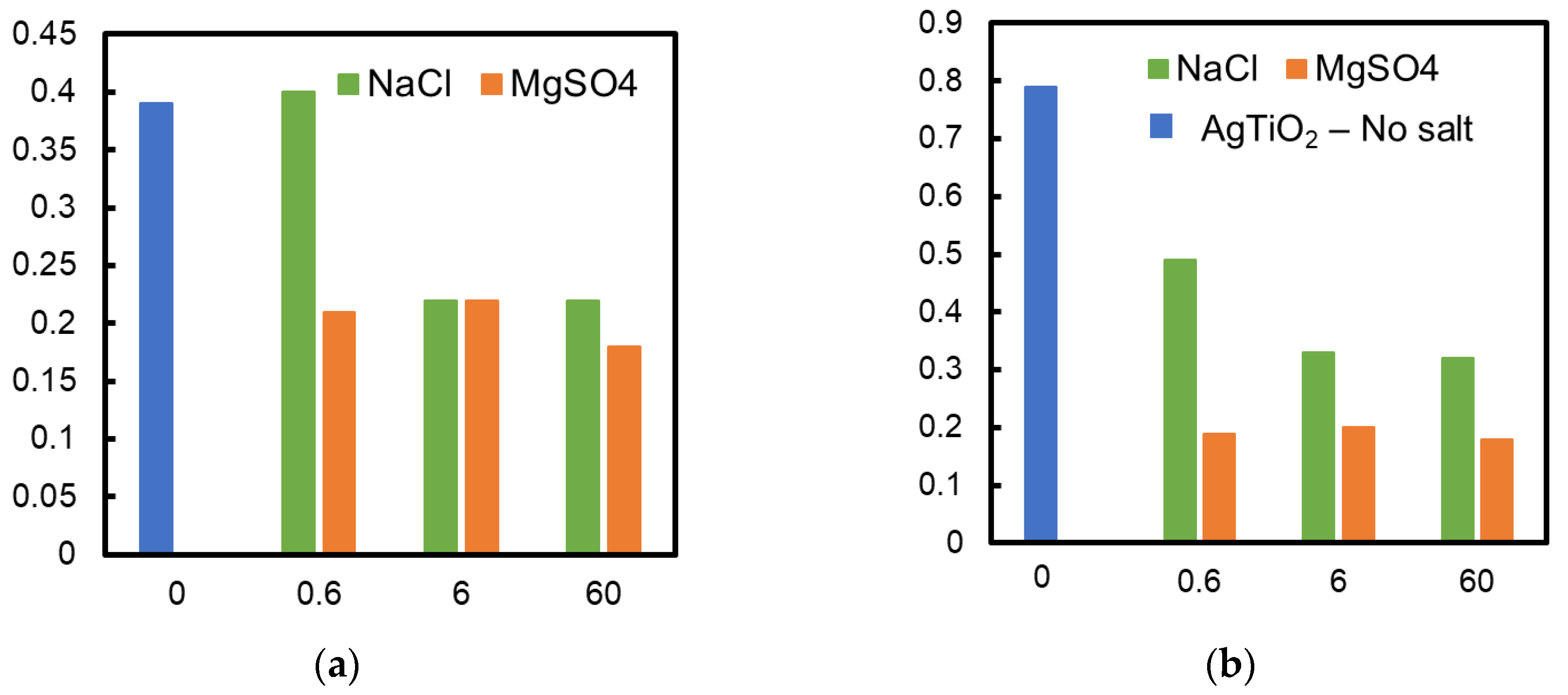
The TiO2 membrane and the Ag/TiO2 (AgT2) membrane showing the fastest decomposition rate were used to examine the influence of coexisting salts. Figure 4a,b show the influence of adding different types of salts to the formic acid solution in which TiO2 and Ag/TiO2 membranes were immersed, respectively. The decomposition of formic acid became slower when the salts were added to the solutions. For example, the decomposition rate became about half when 60 mmol/L NaCl coexisted in the formic acid solution (Figure 4a). Adding 60 mmol/L NaCl and 30 mmol/L MgCl2 to the formic acid solutions caused almost the same reduction in the decomposition rate for both TiO2 and Ag/TiO2 membranes. Adding MgSO4 and K2SO4 further hindered the formic acid decomposition. Interestingly, the performances were recovered by washing the membranes with water (open keys in the figure as control). These results suggest that anions, SO42− and Cl−, inhibit formic acid decomposition. A similar reduction by the inorganic salts was observed with TiO2 and Ag/TiO2 membranes prepared on alumina supports. Consequently, the support materials seem to have a negligible influence on the effect of salts.
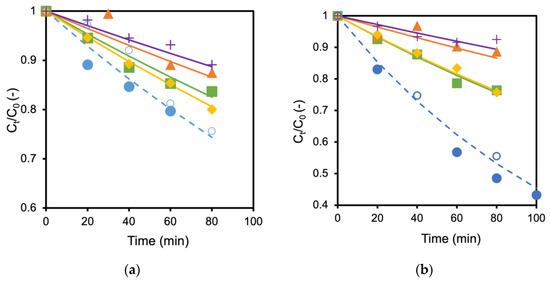
Figure 4.
Influence of different salts toward formic acid decomposition; (a) TiO2 membrane, (b) Ag/TiO2 membrane; ● No salt, ■ with 60 mmol/L NaCl, ◆ with 30 mmol/L MgCl2, ▲ with 60 mmol/L MgSO4 and + with 60 mmol/L K2SO4, ○ membrane performance after washing (no salt).
Figure 5 shows the influence of NaCl and MgSO4 concentrations on the rate constant of formic acid decomposition. Adding MgSO4 to the formic acid solution reduced the photocatalytic activity of both TiO2 and Ag/TiO2 membranes. The MgSO4 concentration did not affect the decomposition rate. Both TiO2 and Ag/TiO2 membranes showed almost the same rate constant when MgSO4 was added, showing no relevant enhancement of depositing silver to the TiO2 membrane. A NaCl concentration greater than 6 mmol/L in the solution hindered the decomposition of formic acid by the TiO2 membrane to the same degree as MgSO4. On the contrary, NaCl addition showed less influence on the Ag/TiO2 membrane performance. The decomposition rate with the latter was higher compared to that with the TiO2 membrane when membranes were applied in solutions of the same NaCl concentration.
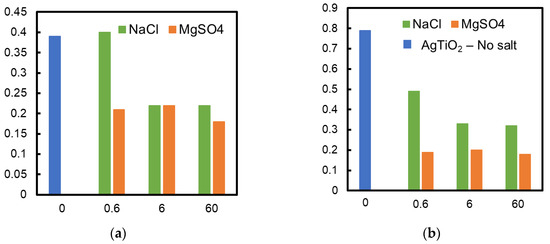
Figure 5.
Influence of salt concentration on the rate constant (a) TiO2 and (b) Ag/TiO2 membrane; results obtained without any salt addition and addition of NaCl and MgSO4 at different concentrations are shown in the figure.
Since chloride enhances silver dissolution [21], this process under experimental conditions was checked. Membranes were prepared under the same conditions as for the AgT2 membrane in Table 1. The membranes were soaked in a 40 g solution for one hour and the concentration of silver was measured by ICP. Silver dissolution was enhanced about 8 times in the NaCl-containing solution under dark conditions. The dissolution was almost negligible under UV light (Table 2).

Table 2.
Silver dissolution from Ag/TiO2 membrane in different solutions.
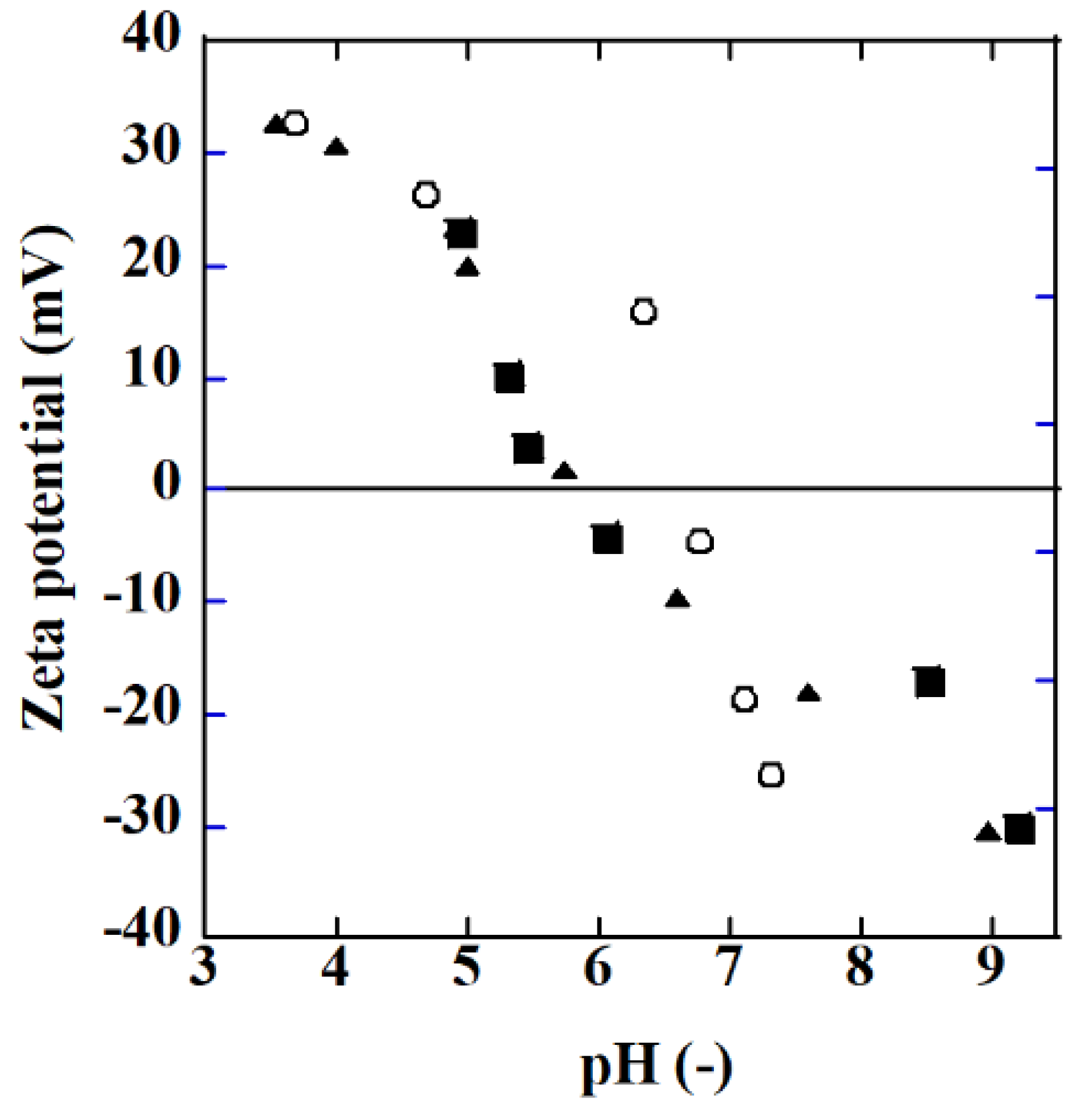
Figure 6 shows the zeta potential of TiO2 (P25) particles and Ag/TiO2 particles measured using a Zetasizer Ultra (Malvern Instruments Ltd., Malvern, UK). HNO3 and NaOH were used to adjust the pH of the solution. The Ag/TiO2 particles were prepared in the same manner as for membrane preparation. The amount of silver on TiO2 particles, 0.89 and 8.9 mg Ag/g TiO2, were comparable to the membranes. For example, the AgT2 membrane in Table 2 had a silver amount of ca. 2.5 mg Ag/g TiO2.
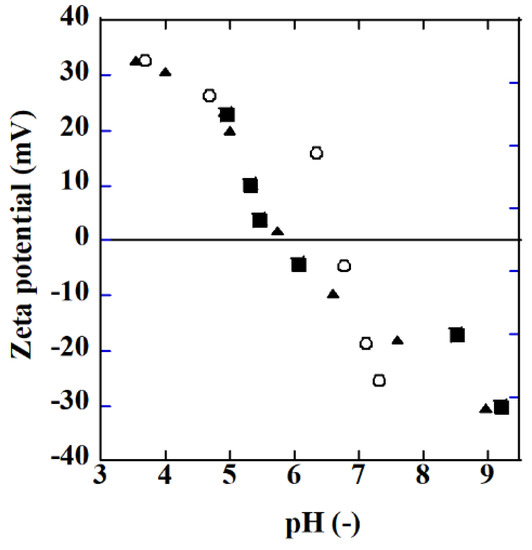
Figure 6.
Zeta potential as a function of pH (○: TiO2 (P25), ■: Ag/TiO2 (0.89 mg Ag/g TiO2), ▲: Ag/TiO2 (8.9 mg Ag/g TiO2).
The isoelectric point (IEP) of TiO2 was about 6.5, which is a value reported similarly in [22]. The IEP value became smaller with silver deposition on TiO2. The difference in the silver amount did not affect the IEP. The pH of the formic acid solution used to evaluate the photocatalytic property of the membranes changed from ca. 3.1 to 3.3 during the decomposition of the acid. Accordingly, the surface of both the TiO2 and Ag/TiO2 membranes was positively charged when immersed in the formic acid solution. The SO42− and Cl− anions coexisting in the solution can adsorb on the membrane surface and may hinder the photocatalytic reactions by hindering the adsorption of hydroxide to the surface which reduces the formation of oxidants, for example.
4. Conclusions
Ag/TiO2 membranes were prepared by the photochemical deposition of silver on TiO2 membranes. A small amount of silver deposition improved the photocatalytic decomposition rate of formic acid dissolved in wastewater. The excessive addition of silver reduced the decomposition rate. The largest enhancement was with 2.5 mg Ag/g TiO2. Salinity in water reduced the decomposition property of both the TiO2 and Ag/TiO2 membranes, but the membrane performance recovered by washing the membrane with water. The addition of NaCl influenced the Ag/TiO2 membranes less than the TiO2 membranes. The reduction in oxidation performance can be attributed to the anion adsorption on the membrane surface, which is positively charged in a formic acid solution. Ag deposition on TiO2 shifts the IEP value to lower pH, which may reduce the anion influence at around a pH of 6. The zeta potential cannot explain the different influences of SO42− and Cl− on the Ag/TiO2 and TiO2 membranes and further study is required to understand this mechanism. Even though the decomposition rate became about half when using the coexisting salts, photocatalytic membranes decomposed dissolved formic acid. The results suggest a wider application potential of photocatalytic membranes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192315736/s1. Figure S1: XRD patterns (a) porous ceramic disk, (b) TiO2 membrane and (c) AgTiO2 membrane; Figure S2: SEM-EDS images of AgTiO2 membrane (cross sectional view) (a) cross sectional image, (b) Al mapping, (c) Si mapping, (d) Ti mapping; Figure S3: XPS of TiO2 membrane and AgTiO2 membrane (a) wide scan spectra, (b) O1s spectra, (c) Ti2p spectra, and (d) Ag3d spectrum of AgTiO2 membrane with fitting curves.
Author Contributions
Conceptualization, I.K.; validation, A.N.C.A.R., S.Y., H.B. and I.K.; investigation, A.N.C.A.R., S.Y., H.B. and I.K.; resources, S.M. and I.K.; data curation, A.N.C.A.R. and I.K.; writing—original draft preparation, A.N.C.A.R. and I.K.; writing—review and editing, I.K., S.M., T.I. and Y.-T.H.; visualization, A.N.C.A.R. and I.K.; project administration, I.K.; funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Science and Technology Agency, JST SICORP, grant number JPMJSC18C5, Japan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of salinity on microorganisms in activated sludge processes: A review. Int. Biodeterior. Biodegrad. 2016, 119, 520–527. [Google Scholar] [CrossRef]
- Béziat, J.-C.; Besson, M.; Gallezot, P.; Durécu, S. Catalytic Wet Air Oxidation of Carboxylic Acids on TiO2-Supported Ruthenium Catalysts. J. Catal. 1999, 182, 129–135. [Google Scholar] [CrossRef]
- Xie, L.; Lu, Q.; Mao, X.; Wang, J.; Han, L.; Hu, J.; Lu, Q.; Wang, Y.; Zeng, H. Probing the intermolecular interaction mechanisms between humic acid and different substrates with implications for its adsorption and removal in water treatment. Water Res. 2020, 176, 115766. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Ovejero, G.; Romero, M.; Díaz, C.; Barreiro, M.; García, J. Catalytic wet air oxidation of textile industrial wastewater using metal supported on carbon nanofibers. J. Supercrit. Fluids 2008, 46, 163–172. [Google Scholar] [CrossRef]
- Zou, L.Y.; Li, Y.; Hung, Y.-T. Wet Air Oxidation for Waste Treatment. In Advanced Physiochemical Treatment Technologies; Wang, L.K., Hung, Y.-T., Shammas, N.K., Eds.; Handbook of Environmental Engineering; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Kumakiri, I.; Hokstad, J.; Peters, T.A.; Melbye, A.G.; Ræder, H. Oxidation of aromatic components in water and seawater by a catalytic membrane process. J. Pet. Sci. Eng. 2011, 79, 37–44. [Google Scholar] [CrossRef]
- Iojoiu, E.E.; Walmsley, J.C.; Raeder, H.; Miachon, S.; Dalmon, J.-A. Catalytic membrane structure influence on the pressure effects in an interfacial contactor catalytic membrane reactor applied to wet air oxidation. Catal. Today 2005, 104, 329–335. [Google Scholar] [CrossRef]
- Furtado, R.X.D.S.; Sabatini, C.A.; Zaiat, M.; Azevedo, E.B. Perfluorooctane sulfonic acid (PFOS) degradation by optimized heterogeneous photocatalysis (TiO2/UV) using the response surface methodology (RSM). J. Water Process. Eng. 2021, 41, 101986. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar]
- Khdary, N.H.; Alkhuraiji, W.S.; Sakthivel, T.S.; Khdary, D.N.; Salam, M.A.; Alshihri, S.; Al-Mayman, S.I.; Seal, S. Synthesis of Superior Visible-Light-Driven Nanophotocatalyst Using High Surface Area TiO2 Nanoparticles Decorated with CuxO Particles. Catalysts 2020, 10, 872. [Google Scholar] [CrossRef]
- Sanzone, G.; Zimbone, M.; Cacciato, G.; Ruffino, F.; Carles, R.; Privitera, V.; Grimaldi, M. Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattices Microstruct. 2018, 123, 394–402. [Google Scholar] [CrossRef]
- Chen, H.; Zahraa, O.; Bouchy, M. Inhibition of the adsorption and photocatalytic degradation of an organic contaminant in an aqueous suspension of TiO2 by inorganic ions. J. Photochem. Photobiol. A Chem. 1997, 108, 37–44. [Google Scholar] [CrossRef]
- Makita, M.; Harata, A. Photocatalytic decolorization of rhodamine B dye as a model of dissolved organic compounds: Influence of dissolved inorganic chloride salts in seawater of the Sea of Japan. Chem. Eng. Process. Process Intensif. 2008, 47, 859–863. [Google Scholar] [CrossRef]
- Kumakiri, I.; Diplas, S.; Simon, C.; Nowak, P. Photocatalytic Membrane Contactors for Water Treatment. Ind. Eng. Chem. Res. 2011, 50, 6000–6008. [Google Scholar] [CrossRef]
- Pipelzadeh, E.; Derakhshan, M.V.; Babaluo, A.A.; Haghighi, M.; Tavakoli, A. Formic Acid Decomposition Using Synthesized Ag/TiO2 Nanocomposite in Ethanol-Water Media Under Illumination of Near UV Light. arXiv 2018, arXiv:physics/1811.12508. [Google Scholar]
- Tran, T.T.H.; Bui, T.T.H.; Nguyen, T.L.; Man, H.N.; Tran, T.K.C. Phase-pure brookite TiO2 as a highly active photocatalyst for the degradation of pharmaceutical pollutants. J. Electron. Mater. 2019, 48, 7846–7861. [Google Scholar]
- Kumakiri, I.; Murasaki, K.; Yamada, S.; Azzah Nazihah, C.A.R.; Ishii, H. A Greener Procedure to Prepare TiO2 Membranes for Photocatalytic Water Treatment Applications. J. Membr. Sci. Res. 2022, 8. in progress. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef] [PubMed]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 2007, 189, 258–263. [Google Scholar] [CrossRef]
- Levard, C.; Mitra, S.; Yang, T.; Jew, A.D.; Badireddy, A.R.; Lowry, G.V.; Brown, G.E. Effect of Chloride on the Dissolution Rate of Silver Nanoparticles and Toxicity to E. coli. Environ. Sci. Technol. 2013, 47, 5738–5745. [Google Scholar] [CrossRef]
- Lee, H.-S.; Hur, T.; Kim, S.; Kim, J.-H.; Lee, H.-I. Effects of pH and surface modification of TiO2 with SiOx on the photocatalytic degradation of a pyrimidine derivative. Catal. Today 2003, 84, 173–180. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).