Sexual Function and Quality of Life in Brazilian Transgender Women Following Gender-Affirming Surgery: A Cross-Sectional Study
Abstract
:1. Introduction
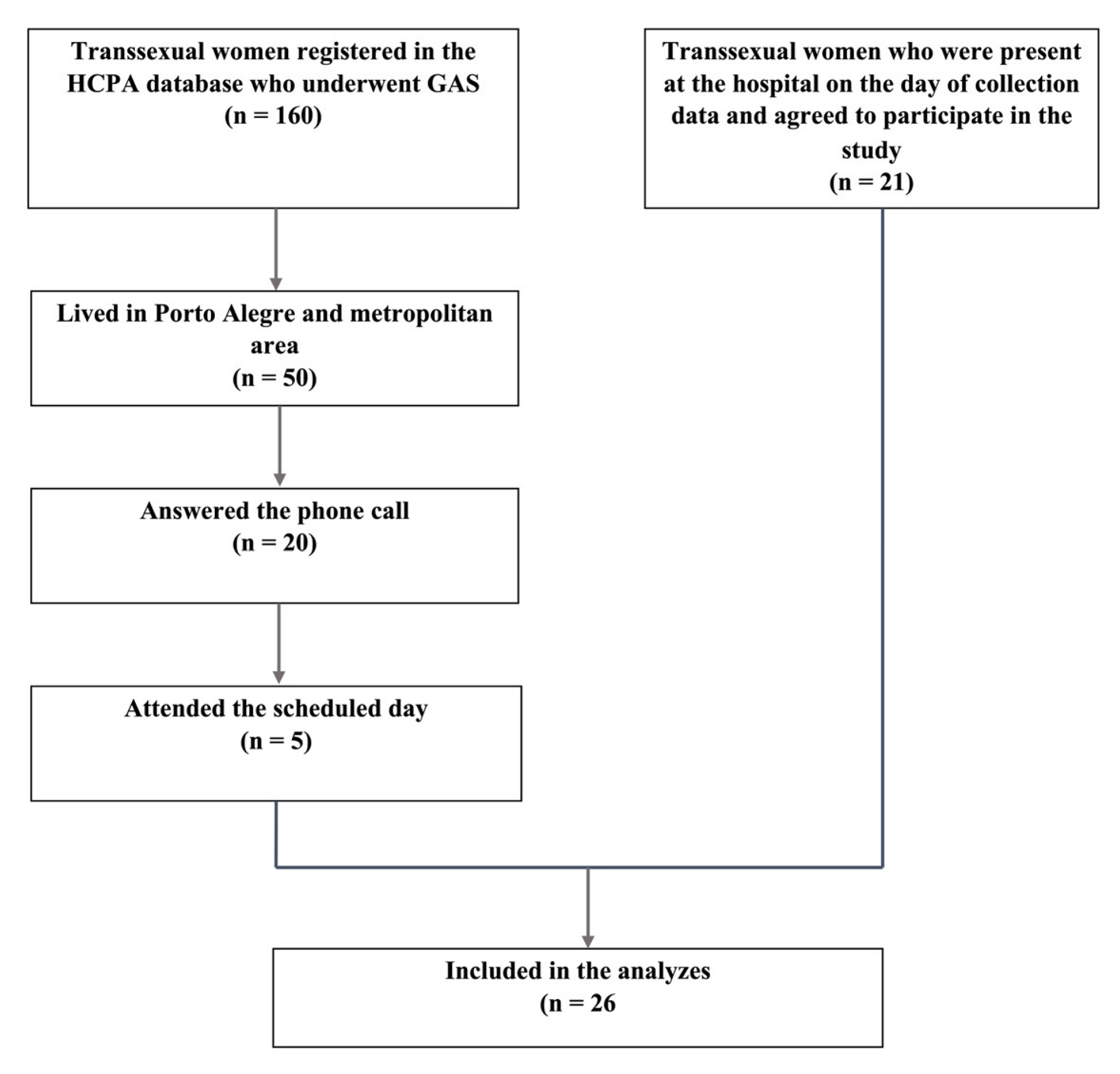
2. Materials and Methods
2.1. Study Design
2.2. Gender Identity Transdisciplinary Program (PROTIG)
2.3. Variables
2.3.1. Female Sexual Function Index (FSFI)
2.3.2. International Consultation on Incontinence Questionnaire (ICIQ-SF)
2.3.3. SF-36 Health Survey (SF-36)
2.4. Statistical Analysis
3. Results
3.1. Sexual Function
3.2. Urinary Incontinence
3.3. Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder, 5th ed.; (DSM-V); American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Lobato, M.I.; Koff, W.J.; Manenti, C.; da Fonseca Seger, D.; Salvador, J.; da Graca Borges Fortes, M.; Petry, A.R.; Silveira, E.; Henriques, A.A. Follow-up of sex reassignment surgery in transsexuals: A Brazilian cohort. Arch. Sex. Behav. 2006, 35, 711–715. [Google Scholar] [CrossRef]
- Oles, N.; Darrach, H.; Landford, W.; Garza, M.; Twose, C.; Park, C.S.; Tran, P.; Schechter, L.S.; Lau, B.; Coon, D. Gender Affirming Surgery: A Comprehensive, Systematic Review of All Peer-reviewed Literature and Methods of Assessing Patient-centered Outcomes (Part 2: Genital Reconstruction). Ann. Surg. 2022, 275, e67–e74. [Google Scholar] [CrossRef]
- Javier, C.; Crimston, C.R.; Barlow, F.K. Surgical satisfaction and quality of life outcomes reported by transgender men and women at least one year post gender-affirming surgery: A systematic literature review. Int. J. Transgend. Health 2022, 23, 255–273. [Google Scholar]
- Safa, B.; Lin, W.C.; Salim, A.M.; Deschamps-Braly, J.C.; Poh, M.M. Current Concepts in Feminizing Gender Surgery. Plast. Reconstr. Surg. 2019, 143, 1081e–1091e. [Google Scholar] [CrossRef] [PubMed]
- Brasil, A.P.A.; Abdo, C.H.N. Transtornos sexuais dolorosos femininos. Diagn. Tratamento 2016, 21, 89–92. [Google Scholar]
- Caruso, S.; Bandiera, S.; Cavallaro, A.; Cianci, S.; Vitale, S.G.; Rugolo, S. Quality of life and sexual changes after double transobturator tension-free approach to treat severe cystocele. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 151, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Khajehei, M.; Doherty, M.; Tilley, P.J. An update on sexual function and dysfunction in women. Arch. Womens Ment. Health 2015, 18, 423–433. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Brazilian Ministry of Health Portaria n°2803: Redefine e amplia o Processo Transexualizador no Sistema Único de Saúde (SUS). Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2013/prt2803_19_11_2013.html (accessed on 12 May 2022).
- Brazilian Ministry of Health Portaria n°170. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/sas/2008/prt0170_20_03_2008.html (accessed on 12 May 2022).
- Brazilian Ministry of Health Portaria n°457. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/sas/2008/prt0457_19_08_2008.html (accessed on 12 May 2022).
- World Health Organization ICD-11: International classification of diseases (11th revision). Available online: https://icd.who.int/ (accessed on 16 April 2022).
- Thiel Rdo, R.; Dambros, M.; Palma, P.C.; Thiel, M.; Riccetto, C.L.; Ramos Mde, F. Translation into Portuguese, cross-national adaptation and validation of the Female Sexual Function Index. Rev. Bras. Ginecol. Obstet. 2008, 30, 504–510. [Google Scholar]
- Ciconelli, R.; Ferraz, M.; Santos, W.; Meinao, I.; Quaresma, M. Brazilian-Portuguese version of the SF-36. A reliable and valid quality of life outcome measure. Rev. Bras. Reumatol. 1999, 39, 143–150. [Google Scholar]
- Tamanini, J.T.; Dambros, M.; D’Ancona, C.A.; Palma, P.C.; Rodrigues Netto, N., Jr. Validation of the “International Consultation on Incontinence Questionnaire—Short Form” (ICIQ-SF) for Portuguese. Rev. Saude Publica 2004, 38, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Rosen, R.; Brown, C.; Heiman, J.; Leiblum, S.; Meston, C.; Shabsigh, R.; Ferguson, D.; D’Agostino, R., Jr. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef]
- Wiegel, M.; Meston, C.; Rosen, R. The female sexual function index (FSFI): Cross-validation and development of clinical cut-off scores. J. Sex Marital Ther. 2005, 31, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Riccetto, C.; Palma, P.; Herrmamm, V.; Dambros, M.; Thiel, M.; Tamanini, J.T.N.; Netto, N.R. 1315: Is There Correlation between Urodynamic Findings and International Consultation on Incontinence Questionnaire—Short form (ICIQ-SF) Score? J. Urol. 2005, 173, 357. [Google Scholar] [CrossRef]
- Buncamper, M.E.; van der Sluis, W.B.; van der Pas, R.S.D.; Ozer, M.; Smit, J.M.; Witte, B.I.; Bouman, M.B.; Mullender, M.G. Surgical Outcome after Penile Inversion Vaginoplasty: A Retrospective Study of 475 Transgender Women. Plast Reconstr. Surg. 2016, 138, 999–1007. [Google Scholar] [CrossRef]
- Papadopulos, N.A.; Lelle, J.D.; Zavlin, D.; Herschbach, P.; Henrich, G.; Kovacs, L.; Ehrenberger, B.; Kluger, A.K.; Machens, H.G.; Schaff, J. Quality of Life and Patient Satisfaction Following Male-to-Female Sex Reassignment Surgery. J. Sex. Med. 2017, 14, 721–730. [Google Scholar] [CrossRef]
- Buncamper, M.E.; Honselaar, J.S.; Bouman, M.B.; Ozer, M.; Kreukels, B.P.; Mullender, M.G. Aesthetic and Functional Outcomes of Neovaginoplasty Using Penile Skin in Male-to-Female Transsexuals. J. Sex. Med. 2015, 12, 1626–1634. [Google Scholar] [CrossRef]
- Heylens, G.; Verroken, C.; De Cock, S.; T’Sjoen, G.; De Cuypere, G. Effects of different steps in gender reassignment therapy on psychopathology: A prospective study of persons with a gender identity disorder. J. Sex. Med. 2014, 11, 119–126. [Google Scholar] [CrossRef]
- Lief, H.I.; Hubschman, L. Orgasm in the postoperative transsexual. Arch. Sex. Behav. 1993, 22, 145–155. [Google Scholar] [CrossRef]
- Castellano, E.; Crespi, C.; Dell’Aquila, C.; Rosato, R.; Catalano, C.; Mineccia, V.; Motta, G.; Botto, E.; Manieri, C. Quality of life and hormones after sex reassignment surgery. J. Endocrinol. Invest. 2015, 38, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Bodmer, C.; Stadlmayr, W.; Kuhn, P.; Mueller, M.D.; Birkhauser, M. Quality of life 15 years after sex reassignment surgery for transsexualism. Fertil. Steril. 2009, 92, 1685–1689.e3. [Google Scholar] [CrossRef] [PubMed]
- van de Grift, T.C.; Elaut, E.; Cerwenka, S.C.; Cohen-Kettenis, P.T.; Kreukels, B.P.C. Surgical Satisfaction, Quality of Life, and Their Association After Gender-Affirming Surgery: A Follow-up Study. J. Sex. Marital Ther. 2018, 44, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Salvador, J.; Massuda, R.; Andreazza, T.; Koff, W.J.; Silveira, E.; Kreische, F.; de Souza, L.; de Oliveira, M.H.; Rosito, T.; Fernandes, B.S.; et al. Minimum 2-year follow up of sex reassignment surgery in Brazilian male-to-female transsexuals. Psychiatry Clin. Neurosci. 2012, 66, 371–372. [Google Scholar] [CrossRef]
- Cardoso da Silva, D.; Schwarz, K.; Fontanari, A.M.; Costa, A.B.; Massuda, R.; Henriques, A.A.; Salvador, J.; Silveira, E.; Elias Rosito, T.; Lobato, M.I. WHOQOL-100 Before and After Sex Reassignment Surgery in Brazilian Male-to-Female Transsexual Individuals. J. Sex. Med. 2016, 13, 988–993. [Google Scholar] [CrossRef]
- Kuhn, A.; Hiltebrand, R.; Birkhauser, M. Do transsexuals have micturition disorders? Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 131, 226–230. [Google Scholar] [CrossRef]
- Lawrence, A.A. Factors associated with satisfaction or regret following male-to-female sex reassignment surgery. Arch. Sex. Behav. 2003, 32, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Monstrey, S.; Hoebeke, P.; Dhont, M.; Cuypere, G.D.; Rubens, R.; Moerman, M.; Hamdi, M.; Landuyt, K.V.; Blondeel, P. Surgical Therapy in Transsexual Patients: A Multidisciplinary Approach. Acta Chir. Belg. 2020, 101, 200–209. [Google Scholar] [CrossRef]
- Vedovo, F.; Di Blas, L.; Perin, C.; Pavan, N.; Zatta, M.; Bucci, S.; Morelli, G.; Cocci, A.; Delle Rose, A.; Caroassai Grisanti, S.; et al. Operated Male-to-Female Sexual Function Index: Validity of the First Questionnaire Developed to Assess Sexual Function after Male-to-Female Gender Affirming Surgery. J. Urol. 2020, 204, 115–120. [Google Scholar] [CrossRef]
| Variables | n (%) | Mean (SD) |
|---|---|---|
| Age | - | 39.9 (10.41) |
| Weight (kg) | - | 74.23 (10.82) |
| Height (m) | - | 1.71 (5.61) |
| Smoking | ||
| Cigarettes/day | - | 1.69 (6.50) |
| Days after surgery, they resumed smoking | - | 40.87 (63.49) |
| Chronic Diseases | ||
| HIV | 5 (19) | - |
| DM and SAH | 1 (3.8) | - |
| Other unspecified diseases | 1 (3.8) | - |
| Elapsed time of GAS (months) | - | 54 (56.40) |
| Number of genital surgeries | - | 1.88 (2.78) |
| Treatment/postoperative care | ||
| Hygiene | 13 (50) | - |
| Use of a vaginal mold | 3 (11.5) | - |
| Hygiene and use of vaginal mold | 10 (38.5) | - |
| Reasons for dissatisfaction with the result of the GAS | ||
| Large clitoris | 1 (3.8) | - |
| Recurrent fistulas | 1 (3.8) | - |
| No depth in the neovagina | 2 (7.7) | - |
| Domain | Mean (SD) | Mean (SD) | p-Value | Mean (SD) | p-Value |
|---|---|---|---|---|---|
| Desire | 3.85 (1.42) | 4.11 (1.20) | 0.198 | 3.17 (1.82) | 0.167 |
| Arousal | 3.33(2.14) | 4.50 (0.98) | <0.001 | 0.17 (0.45) | <0.001 |
| Lubrication | 2.94 (2.13) | 4.03 (1.31) | <0.001 | 0.00 (0.00) | 0.002 |
| Orgasm | 3.25 (2.13) | 4.34 (1.19) | <0.001 | 0.29 (0.76) | <0.001 |
| Satisfaction | 4.97(1.20) | 5.28 (0.59) | <0.020 | 2.00 (1.70) | * |
| Pain | 3.23(2.46) | 4.42 (1.68) | <0.001 | 0.00 (0.00) | 0.003 |
| Total | 21.58 (11.47) | 26.67 (6.96) | 0.023 | 5.63 (4.73) | * |
| Degree of Incontinence | Score | |
|---|---|---|
| n (%) | ||
| Absence of Urinary Incontinence | 22 | 84.6 |
| Mild Urinary Incontinence (score 1–5) | 1 | 3.8 |
| Moderate Urinary Incontinence (score 6–12) | 2 | 7.7 |
| Severe Urinary Incontinence (score 13–18) | 1 | 3.8 |
| Very Severe Urinary Incontinence (score 19–21) | 0 | 0 |
| Total | 26 | 100.0 |
| Domains | Mean | SD | Minimal | Maximum |
|---|---|---|---|---|
| Physical functioning | 93.65 | 11.88 | 45.00 | 100.00 |
| Role—physical | 81.73 | 34.32 | 0.00 | 100.00 |
| Bodily pain | 73.35 | 25.40 | 10.00 | 100.00 |
| General health | 78.31 | 13.88 | 52.00 | 100.00 |
| Vitality | 72.88 | 22.77 | 15.00 | 100.00 |
| Social functioning | 85.10 | 24.75 | 0.00 | 100.00 |
| Role—emotional | 85.90 | 30.07 | 0.00 | 100.00 |
| Mental health | 82.77 | 14.26 | 52.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro Petry Jardim, L.M.; Cerentini, T.M.; Lobato, M.I.R.; Costa, Â.B.; Cardoso da Silva, D.; Schwarz, K.; Vaitses Fontanari, A.M.; Schneider, M.A.; Rosito, T.E.; La Rosa, V.L.; et al. Sexual Function and Quality of Life in Brazilian Transgender Women Following Gender-Affirming Surgery: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 15773. https://doi.org/10.3390/ijerph192315773
Monteiro Petry Jardim LM, Cerentini TM, Lobato MIR, Costa ÂB, Cardoso da Silva D, Schwarz K, Vaitses Fontanari AM, Schneider MA, Rosito TE, La Rosa VL, et al. Sexual Function and Quality of Life in Brazilian Transgender Women Following Gender-Affirming Surgery: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(23):15773. https://doi.org/10.3390/ijerph192315773
Chicago/Turabian StyleMonteiro Petry Jardim, Lísia Maya, Taís Marques Cerentini, Maria Inês Rodrigues Lobato, Ângelo Brandelli Costa, Dhiordan Cardoso da Silva, Karine Schwarz, Anna Martha Vaitses Fontanari, Maiko Abel Schneider, Tiago Elias Rosito, Valentina Lucia La Rosa, and et al. 2022. "Sexual Function and Quality of Life in Brazilian Transgender Women Following Gender-Affirming Surgery: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 23: 15773. https://doi.org/10.3390/ijerph192315773
APA StyleMonteiro Petry Jardim, L. M., Cerentini, T. M., Lobato, M. I. R., Costa, Â. B., Cardoso da Silva, D., Schwarz, K., Vaitses Fontanari, A. M., Schneider, M. A., Rosito, T. E., La Rosa, V. L., Commodari, E., & Viana da Rosa, P. (2022). Sexual Function and Quality of Life in Brazilian Transgender Women Following Gender-Affirming Surgery: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(23), 15773. https://doi.org/10.3390/ijerph192315773







