Chemical Composition of Atmospheric Air in Nemoral Scots Pine Forests and Submountainous Beech Forests: The Potential Region for the Introduction of Forest Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Sampling Area and Location of Sampling Points
2.2. Collection of Organic Compound Samples and Screening Measurements of Ozone and Atmospheric Aerosol
2.3. Laboratory Analysis—Analytical Procedure, Extraction/Liberation, and Final Determination Conditions
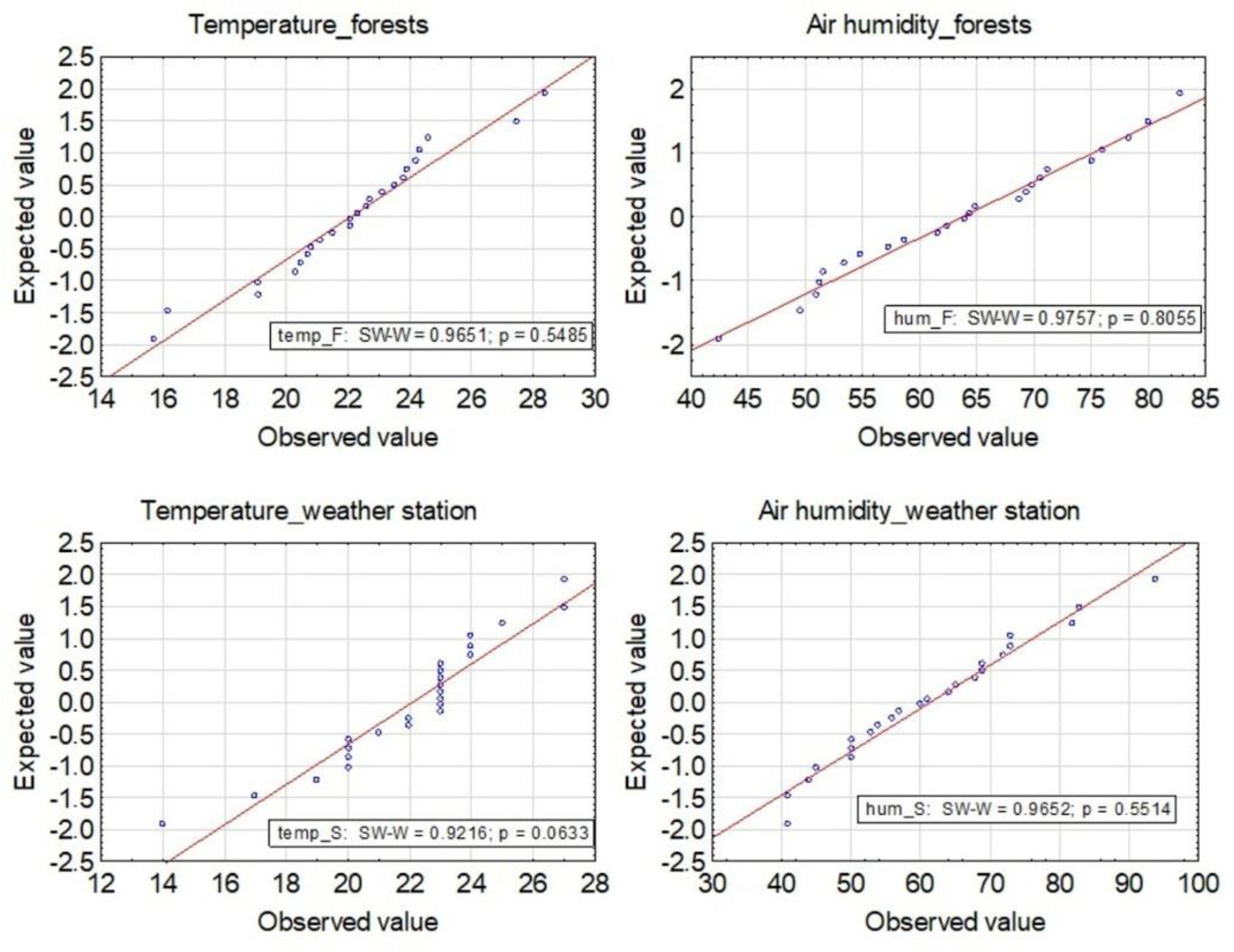
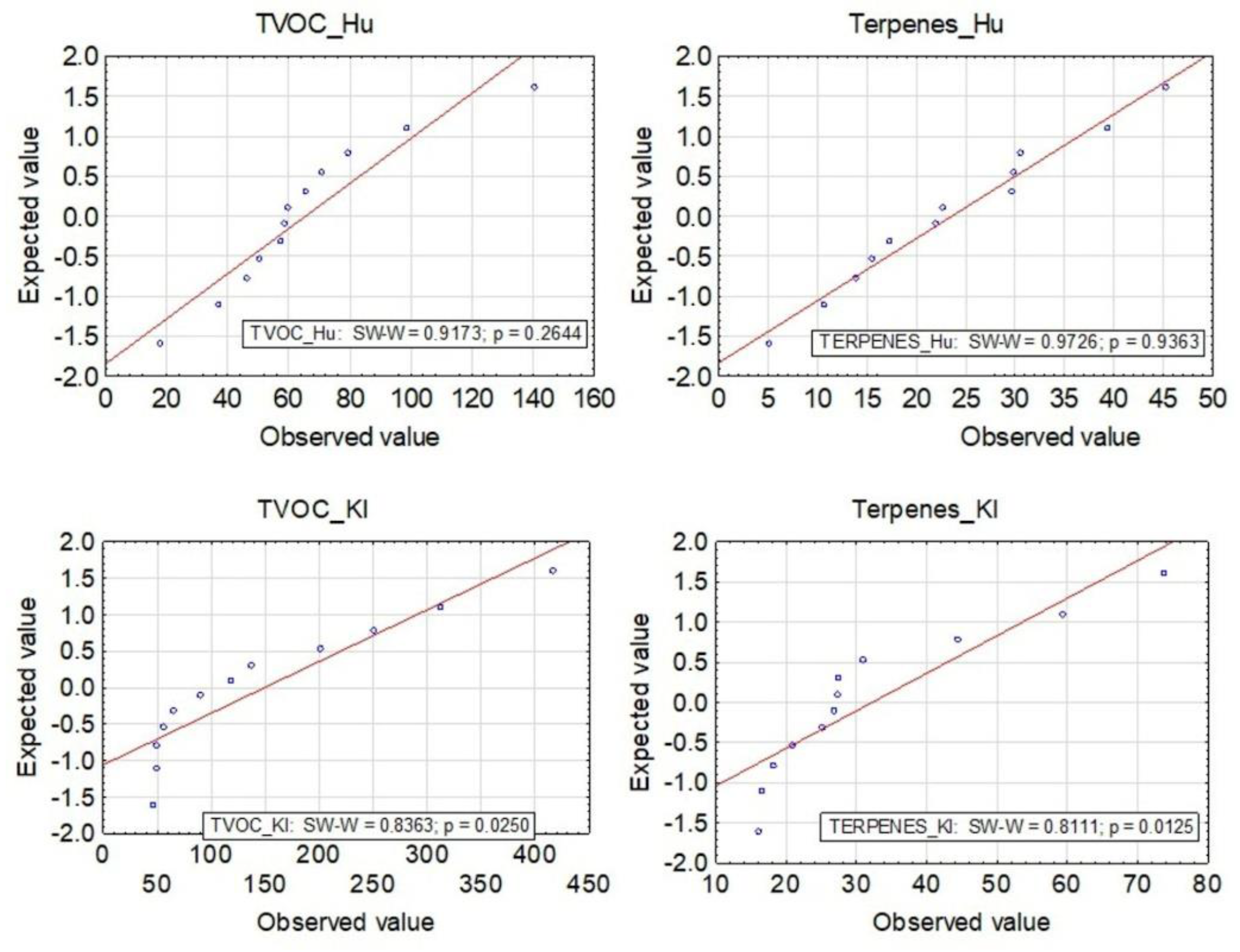
2.4. Statistical Analysis
3. Results and Discussion
3.1. Meteorological Conditions over the Sampling Areas
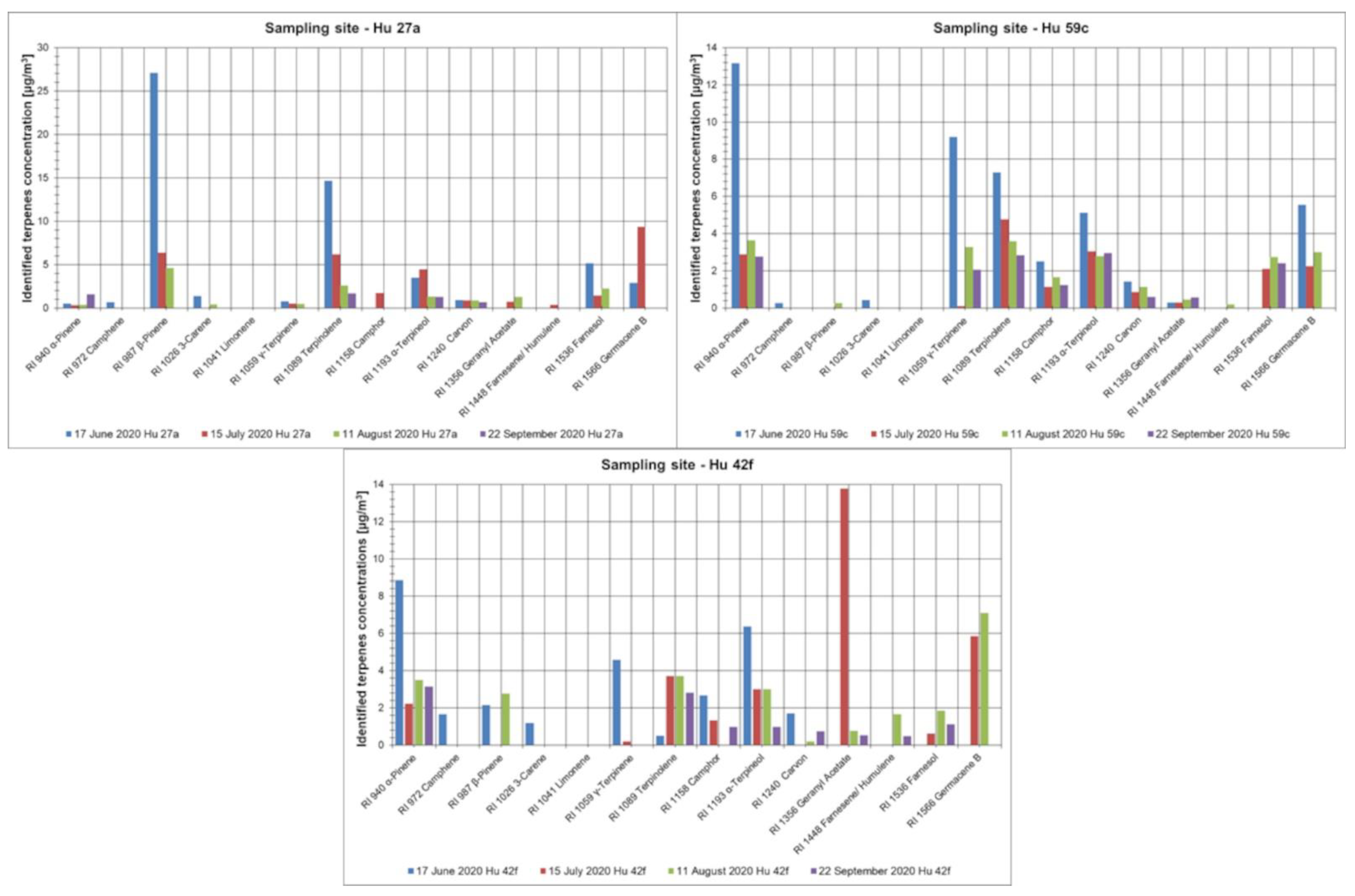
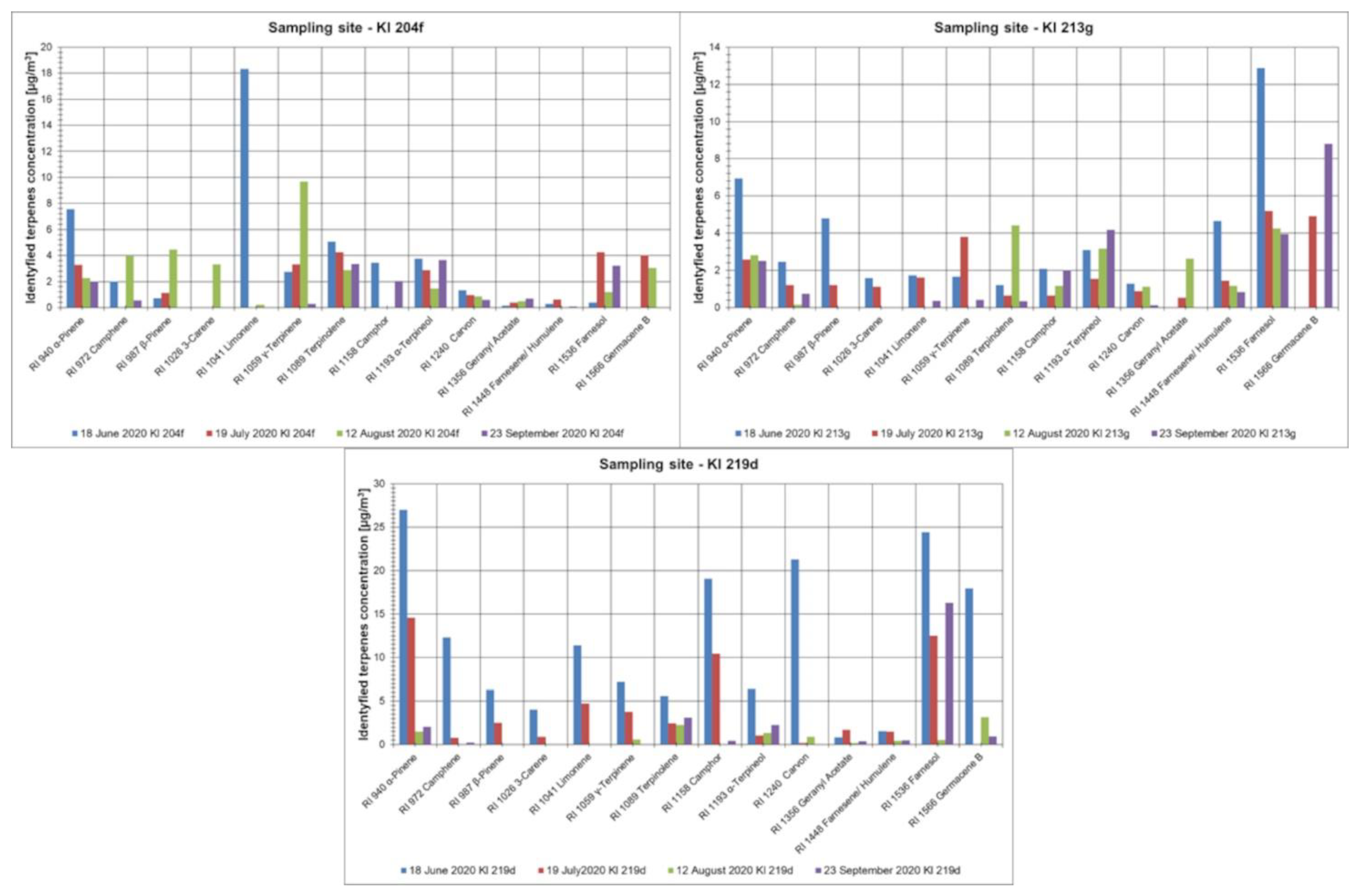
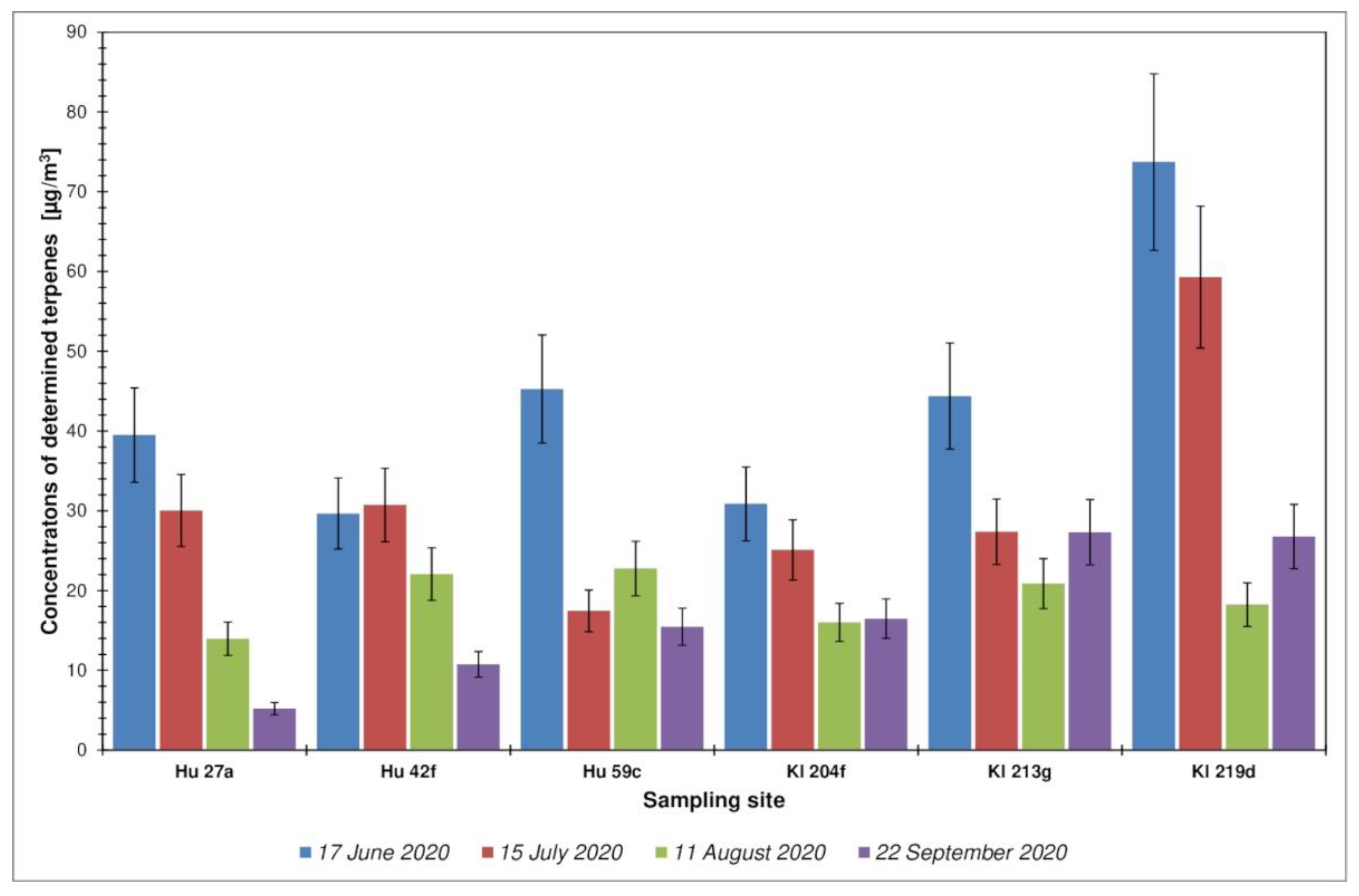
3.2. Representatives of Volatile Organic Compounds Determined in the Investigated Forest Areas
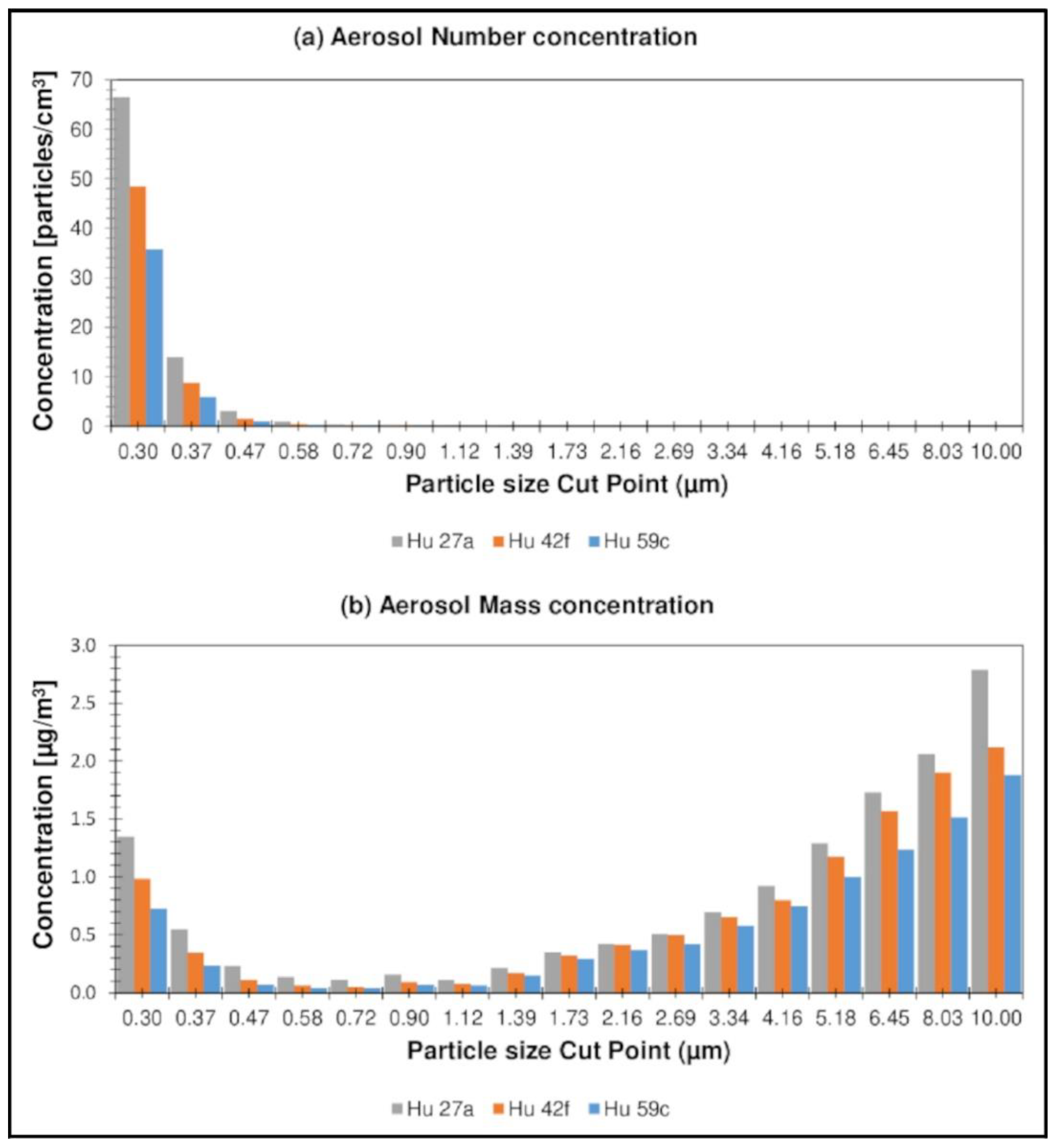
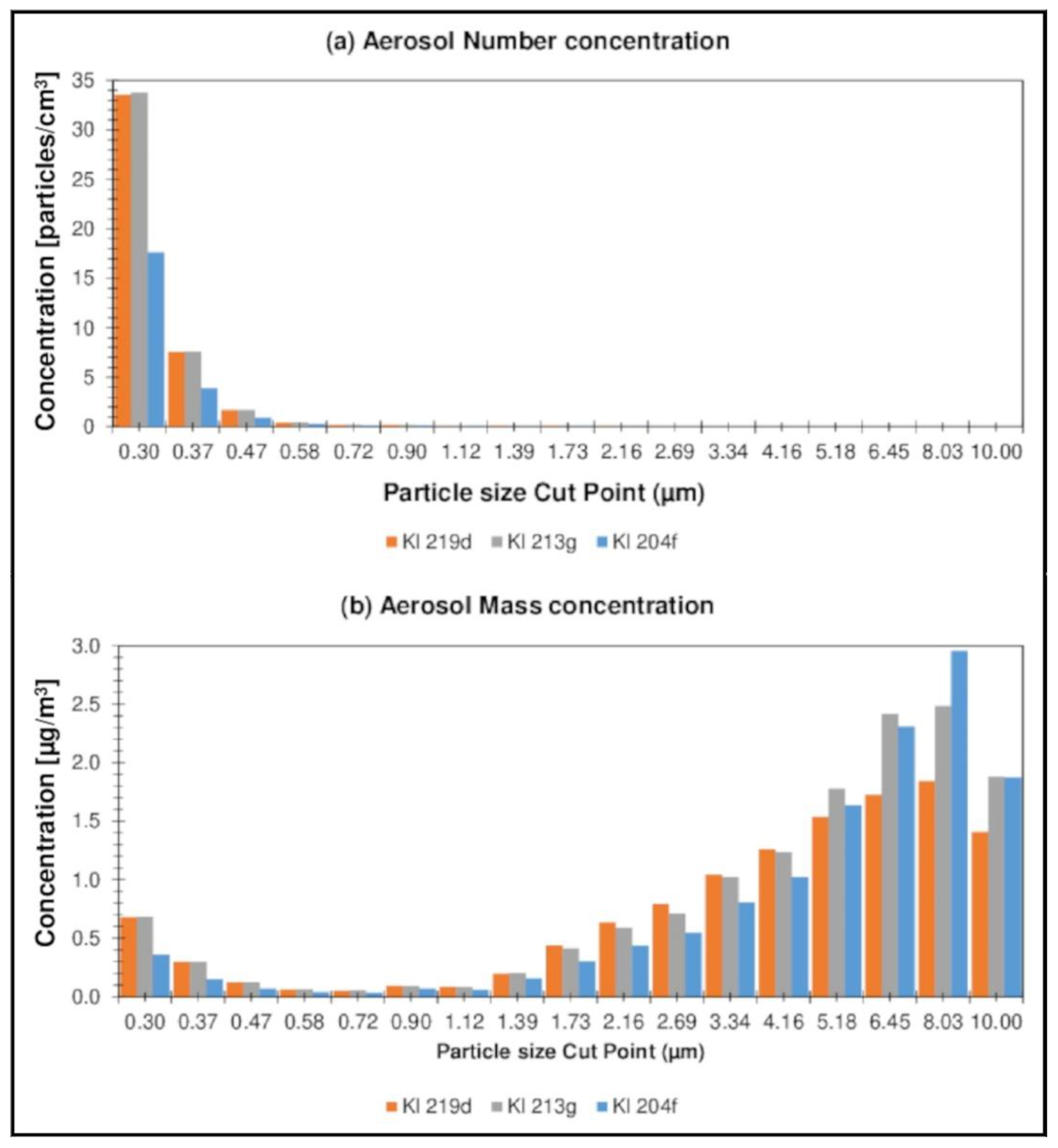
3.3. Aerosol Concentration in Forest Air
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Equipment Type Employed to Perform Analytes Thermal Extraction | ||
|---|---|---|
| System Acronym | TD-GC-FID | TD-GC-MS |
| Type of thermal desorption unit | Markes’ Series 2 Thermal Desorption System; UNITY/TD-100 | Markes Unity v.2 |
| Analyte sampling device | Sorption tubes filled with Tenax TA (O.D. × L ¼ in. × 3 ½ in.) | |
| 1st stage of thermal desorption process—working conditions | ||
| Heating temperature | 290 °C | |
| Inert gas (He) flow rate during the desorption stage | 50 mL·min−1 | |
| Heating time | 15 min | |
| Microtrap composition and temperature | Microtrap dedicated to measuring monoterpene compounds filled with Tenax TA and Carbotrap; 0 °C | |
| 2nd stage of thermal desorption process—working conditions | ||
| Microtrap temperature (ballistic heating mode) | 300 °C | |
| Microtrap heating time | 5 min | |
| Flow rate of the inert gas (He) through the microtrap to the chromatographic column | 2.0 mL·min−1 | 1.0 mL·min−1 |
| Final determination system working parameters | ||
| Type of applied gas chromatography | Agilent 7820A GC | Agilent Technologies 6890 |
| Detector | Flame ionization detector, detector temp. 280 °C | Mass spectrometer (5873 Network Mass Selective Detector, Agilent Technologies); transmission line temp. of GC-MS: 285 °C; ion source temp.: 230 °C; quadrupole mass analyzer temp.: 150 °C |
| TD-GC transfer line temperature | 160 °C | |
| Capillary column type | DB-1 (J&W, USA); 30 m × 320 µm × 5 μm | HP-1 ms (J&W, USA); 30 m × 0.25 µm × 1 μm |
| Helium gas (flow rate) | 2.0 mL·min−1 | 1.0 mL·min−1 |
| Oven temperature program | 50 °C for 1 min; 7 °C·min−1 up to 260 °C and held for 6 min | |
| Data processing system | OpenLAB CDS ChemStation Workstation VL | Chemstation |
| Retention Index/Chemical Name | Scent/Odor Quality | Chemical Structure | Formula |
|---|---|---|---|
| RI 950 α-Pinene | fir needle-like, resin-like | Bicyclic | C10H16 |
| RI 972 Camphene | terpene-like, woody type | Bicyclic | C10H16 |
| RI 987 β-Pinene | woody-green pine-like | Bicyclic | C10H16 |
| RI 1026 3-Carene | terpene-like | Bicyclic | C10H16 |
| RI 1041 Limonene | citrus-like | Cyclic, | C10H16 |
| RI 1059 γ-Terpinene | petrol-like | Cyclic, | C10H16 |
| RI 1089 Terpinolene | pine-like | Cyclic | C10H16 |
| RI 1160 Camphor | camphor-like | Ketone, cyclic | C10H16 |
| RI 1193 α-Terpineol | flowery, citrus-like, pleasant odor similar to lilac | Alcohol, cyclic | C10H18O |
| RI 1240 Carvone | mint-like, caraway-like | Ketone | C10H14O |
| RI 1356 Geranyl Acetate | pleasant floral or fruity rose aroma | Ester, linear | C12H20O2 |
| RI 1448 Farnesene/Humulene | sweet, flowery/balsamic vinegar-like/woody type | Linear/cyclic, sesquiterpene | C15H24 |
| RI 1536 Nerolidol/Farnesol | citrus-like, flowery | Alcohol, linear, sesquiterpene | C15H26O |
| RI 1566 Germacene B | woody type odor | Cyclic, sesquiterpene | C15H24 |
References
- Dudek, T. Needs of the local population related to development of forests for recreational purposes: Example of south-eastern Poland. J. For. Sci. 2016, 62, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Lee, D.-J. Nature experience, recreation activity and health benefits of visitors in mountain and urban forests in Vienna, Zurich and Freiburg. J. Mt. Sci. 2015, 12, 1551–1561. [Google Scholar] [CrossRef]
- Neuvonen, M.; Sievänen, T.; Tönnes, S.; Koskela, T. Access to green areas and the frequency of visits—A case study in Helsinki. Urban For. Urban Green. 2007, 6, 235–247. [Google Scholar] [CrossRef]
- Dudek, T. Recreational potential of Rzeszów suburban forests versus the demand for spending leisure time in forests among the residents of the Podkarpackie Province. Sylwan 2016, 160, 169–176. [Google Scholar] [CrossRef]
- Chen, B.; Nakama, Y. Thirty years of forest tourism in China. J. For. Res. 2013, 18, 285–292. [Google Scholar] [CrossRef]
- Jankovska, I.; Donis, J.; Straupe, I.; Panagopoulos, T.; Kupfere, L. Assessment of forest recreation accessibility in Latvia. Fresen. Environ. Bull. 2013, 22, 2145–2151. [Google Scholar]
- Annerstedt, M.; Norman, J.; Boman, M.; Mattsson, L.; Grahn, P.; Währborg, P. Finding stress relief in a forest. In Broadleaves Forest in Southern Sweden: Managament for Multipl Goals; Löf, M., Brunet, J., Mattsson, L., Nylinder, M., Eds.; Oikos Editorial Office: Lund, Sweden, 2010; Volume 53, pp. 33–42. [Google Scholar]
- Kuo, M. How might contact with nature promote human health? Promising mechanisms and a possible central pathway. Front. Psychol. 2015, 6, 1093. [Google Scholar] [CrossRef] [Green Version]
- Van Den Berg, A.E.; Maas, J.; Verheij, R.A.; Groenewegen, P.P. Green space as a buffer between stressful life events and health. Soc. Sci. Med. 2010, 70, 1203–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laumann, K.; Gärling, T.; Stormark, K.M. Selective attention and heart rate responses to natural and urban environments. J. Environ. Psychol. 2003, 23, 125–134. [Google Scholar] [CrossRef]
- Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-Yoku (Forest Bathing) and Nature Therapy: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajoo, K.S.; Karam, D.S.; Aziz, N.A.A. Developing an effective forest therapy program to manage academic stress in conservative societies: A multi-disciplinary approach. Urban For. Urban Green. 2019, 43, 126353. [Google Scholar] [CrossRef]
- Irvine, K.N.; Warber, S.L. Greening healthcare: Practicing as if the natural environment really mattered. Altern. Ther. Health Med. 2002, 8, 76. [Google Scholar]
- Lee, I.; Choi, H.; Bang, K.-S.; Kim, S.; Song, M.; Lee, B. Effects of Forest Therapy on Depressive Symptoms among Adults: A Systematic Review. Int. J. Environ. Res. Public Health 2017, 14, 321. [Google Scholar] [CrossRef] [Green Version]
- Bielinis, E.; Takayama, N.; Boiko, S.; Omelan, A.; Bielinis, L. The effect of winter forest bathing on psychological relaxation of young Polish adults. Urban For. Urban Green. 2018, 29, 276–283. [Google Scholar] [CrossRef]
- Bielinis, E.; Janeczko, E.; Takayama, N.; Zawadzka, A.; Słupska, A.; Piętka, S.; Lipponen, M.; Bielinis, L. The effects of viewing a winter forest landscape with the ground and trees covered in snow on the psychological relaxation of young Finnish adults: A pilot study. PLoS ONE 2021, 16, e0244799. [Google Scholar] [CrossRef]
- Brown, S.C.; Lombard, J.; Wang, K.; Byrne, M.M.; Toro, M.; Plater-Zyberk, E.; Feaster, D.J.; Kardys, J.; Nardi, M.I.; Perez-Gomez, G.; et al. Neighborhood Greenness and Chronic Health Conditions in Medicare Beneficiaries. Am. J. Prev. Med. 2016, 51, 78–89. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Banay, R.F.; Hart, J.E.; Laden, F. A Review of the Health Benefits of Greenness. Curr. Epidemiol. Rep. 2015, 2, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, G.-X.; Cao, Y.-B.; Lan, X.-G.; He, Z.-H.; Chen, Z.-M.; Wang, Y.-Z.; Hu, X.-L.; Lv, Y.-D.; Wang, G.-F.; Yan, J. Therapeutic effect of forest bathing on human hypertension in the elderly. J. Cardiol. 2012, 60, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.-J.; Tsunetsugu, Y.; Kasetani, T.; Morikawa, T.; Kagawa, T.; Miyazaki, Y. Physiological effects of forest recreation in a young conifer forest in Hinokage Town, Japan. Silva Fenn. 2009, 43, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.-P.; Lin, C.-M.; Tsai, M.-J.; Tsai, Y.-C.; Chen, C.-Y. Effects of Short Forest Bathing Program on Autonomic Nervous System Activity and Mood States in Middle-Aged and Elderly Individuals. Int. J. Environ. Res. Public Health 2017, 14, 897. [Google Scholar] [CrossRef] [Green Version]
- Astell-Burt, T.; Feng, X.; Kolt, G.S. Is Neighborhood Green Space Associated With a Lower Risk of Type 2 Diabetes? Evidence From 267,072 Australians. Diabetes Care 2013, 37, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, J.; Verheij, R.A.; de Vries, S.; Spreeuwenberg, P.; Schellevis, F.G.; Groenewegen, P.P. Morbidity is related to a green living environment. J. Epidemiol. Community Health 2009, 63, 967–973. [Google Scholar] [CrossRef]
- De Keijzer, C.; Basagaña, X.; Tonne, C.; Valentín, A.; Alonso, J.; Antó, J.M.; Nieuwenhuijsen, M.J.; Kivimäki, M.; Singh-Manoux, A.; Sunyer, J.; et al. Long-term exposure to greenspace and metabolic syndrome: A Whitehall II study. Environ. Pollut. 2019, 255, 113231. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a Plant-Derived Monoterpene, Reduces Plasma Cholesterol and Triglycerides in Hyperlipidemic Rats Independently of HMG-CoA Reductase Activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.-J.; Li, C.-Y.; Pan, W.-C.; Yao, T.-C.; Su, H.-J.; Wu, C.-D.; Chern, Y.-R.; Spengler, J.D. The Effect of Surrounding Greenness on Type 2 Diabetes Mellitus: A Nationwide Population-Based Cohort in Taiwan. Int. J. Environ. Res. Public Health 2020, 18, 267. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Lim, Y.-R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.-S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef]
- Gould, M.N. Cancer chemoprevention and therapy by monoterpenes. Environ. Health Perspect. 1997, 105, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Bardon, S.; Foussard, V.; Fournel, S.; Loubat, A. Monoterpenes inhibit proliferation of human colon cancer cells by modulating cell cycle-related protein expression. Cancer Lett. 2002, 181, 187–194. [Google Scholar] [CrossRef]
- Crowell, P.L.; Ayoubi, A.S.; Burke, Y.D. Antitumorigenic Effects of Limonene and Perillyl Alcohol Against Pancreatic and Breast Cancer. Diet. Phytochem. Cancer Prev. Treat. 1996, 401, 131–136. [Google Scholar] [CrossRef]
- Gupta, A.; Myrdal, P.B. Development of a perillyl alcohol topical cream formulation. Int. J. Pharm. 2003, 269, 373–383. [Google Scholar] [CrossRef]
- Stark, M.; Burke, Y.D.; McKinzie, J.H.; Ayoubi, A.; Crowell, P.L. Chemotherapy of pancreatic cancer with the monoterpene perillyl alcohol. Cancer Lett. 1995, 96, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.; Matsuo, A.L.; Travassos, L.R.; et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Wolkoff, P. Indoor air chemistry: Terpene reaction products and airway effects. Int. J. Hyg. Environ. Health 2020, 225, 113439. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Pollut. 2011, 159, 2078–2087. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Janson, R. Monoterpene concentrations in and above a forest of scots pine. J. Atmos. Chem. 1992, 14, 385–394. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Bartolini, G.; Zabini, F. Temporal and Spatial Variability of Volatile Organic Compounds in the Forest Atmosphere. Int. J. Environ. Res. Public Health 2019, 16, 4915. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Park, S.H.; Lee, H.J.; Kang, J.W.; Lee, K.M.; Yeon, P.S. Study on NVOCs Concentration Characteristics by Season, Time and Climatic Factors: Focused on Pinus densiflora Forest in National Center for Forest Therapy. J. People Plants Environ. 2018, 21, 403–409. [Google Scholar] [CrossRef]
- Yu, J.; Griffin, R.J.; Cocker, D.R.; Flagan, R.C.; Seinfeld, J.H.; Blanchard, P. Observation of gaseous and particulate products of monoterpene oxidation in forest atmospheres. Geophys. Res. Lett. 1999, 26, 1145–1148. [Google Scholar] [CrossRef] [Green Version]
- Dudek, T.; Kasprzyk, I.; Dulska-Jeż, A. Forest as a place for recreation but also the source of allergenic plant pollen: To come or avoid? Forstwiss. Cent. 2018, 137, 849–862. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.C.; Yoo, Y.; Park, S.; Park, C.W. The clinical effects of forest camp on children with allergic diseases. ISEE Conf. Abstr. 2013, 2013, 3391. [Google Scholar] [CrossRef]
- National Bureau of Statistics of the Republic of Poland. Statistical Yearbook of Forestry; Statistics Poland: Warszawa, Poland, 2019; ISSN 2657-3199.
- Tomczyk, A.M.; Szyga-Pluta, K. Growing seasons in Poland in the period 1971–2010. Przeg. Geogr. 2016, 88, 75–86. [Google Scholar] [CrossRef]
- Forest Management Plan 2010, Plan for the Głogów Forest District Forests for 2010–2019. Available online: https://www.gov.pl/web/nadlesnictwo-glogow/plan-urzadzania-lasu (accessed on 25 October 2022).
- Dudek, T. Recreational potential as an indicator of accessibility control in protected mountain forest areas. J. Mt. Sci. 2017, 14, 1419–1427. [Google Scholar] [CrossRef]
- van Den Dol, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Zhang, X. A rapid and novel method for predicting nicotine alkaloids in tobacco through electronic nose and partial least-squares regression analysis. Anal. Methods 2016, 8, 1609–1617. [Google Scholar] [CrossRef]
- Marć, M.; Namiesnik, J.; Zabiegała, B. BTEX concentration levels in urban air in the area of the Tri-City agglomeration (Gdansk, Gdynia, Sopot), Poland. Air Qual. Atmos. Health 2014, 7, 489–504. [Google Scholar] [CrossRef]
- Marć, M.; Zabiegała, B.; Namiesnik, J. Application of passive sampling technique in monitoring research on quality of atmospheric air in the area of Tczew, Poland. Int. J. Environ. Anal. Chem. 2013, 94, 151–167. [Google Scholar] [CrossRef]
- Zabiegała, B.; Urbanowicz, M.; Szymanska, K.; Namiesnik, J. Application of Passive Sampling Technique for Monitoring of BTEX Concentration in Urban Air: Field Comparison of Different Types of Passive Samplers. J. Chromatogr. Sci. 2010, 48, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.J.; Johannes, A.; Duivenbode, R.V.; Duyzer, J.H.; Verhagen, H.L. The determination of terpenes in forest air. Atmos. Environ. 1994, 28, 2413–2419. [Google Scholar] [CrossRef]
- Bottoni, M.; Milani, F.; Mozzo, M.; Kolloffel, D.R.; Papini, A.; Fratini, F.; Maggi, F.; Santagostini, L. Sub-Tissue Localization of Phytochemicals in Cinnamomum camphora (L.) J. Presl. Growing in Northern Italy. Plants 2021, 10, 1008. [Google Scholar] [CrossRef]
- Khan, M.; Al-Saleem, M.S.; Alkhathlan, H.Z. A detailed study on chemical characterization of essential oil components of two Plectranthus species grown in Saudi Arabia. J. Saudi Chem. Soc. 2016, 20, 711–721. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Zenkevich, I.G.; Ioffe, B.V. Volatile organic compounds in the atmosphere of forests. Atmos. Environ. 1985, 19, 1–8. [Google Scholar] [CrossRef]
- Llusià, J.; Pen~Uelas, J. Seasonal patterns of terpene content and emission from seven Mediterranean woody species in field conditions. Am. J. Bot. 2000, 87, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staudt, M.; Bourgeois, I.; Al Halabi, R.; Song, W.; Williams, J. New insights into the parametrization of temperature and light responses of mono—and sesquiterpene emissions from Aleppo pine and rosemary. Atmos. Environ. 2017, 152, 212–221. [Google Scholar] [CrossRef]
- Brocco, D.; Fratarcangeli, R.; Lepore, L.; Petricca, M.; Ventrone, I. Determination of aromatic hydrocarbons in urban air of Rome. Atmos. Environ. 1997, 31, 557–566. [Google Scholar] [CrossRef]
- Hellén, H.; Hakola, H.; Laurila, T.; Hiltunen, V.; Koskentalo, T. Aromatic hydrocarbon and methyl tert-butyl ether measurements in ambient air of Helsinki (Finland) using diffusive samplers. Sci. Total Environ. 2002, 298, 55–64. [Google Scholar] [CrossRef]
- Kerchich, Y.; Kerbachi, R.; Khatraoui, H. Ambient air levels of aromatic organic compounds BTEX in the urban area of Algiers. Asian J. Chem. 2010, 23, 323–330. [Google Scholar]
- Monod, A.; Sive, B.C.; Avino, P.; Chen, T.; Blake, D.R.; Rowland, F.S. Monoaromatic compounds in ambient air of various cities: A focus on correlations between the xylenes and ethylbenzene. Atmos. Environ. 2001, 35, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Pankow, J.F.; Luo, W.; Bender, A.D.; Isabelle, L.M.; Hollingsworth, J.S.; Chen, C.; Asher, E.W.; Zogorski, J.S. Concentrations and co-occurrence correlations of 88 volatile organic compounds (VOCs) in the ambient air of 13 semi-rural to urban locations in the United States. Atmos. Environ. 2003, 37, 5023–5046. [Google Scholar] [CrossRef]
- Marć, M.; Bielawska, M.; Simeonov, V.; Namiesnik, J.; Zabiegała, B. The effect of anthropogenic activity on BTEX, NO2, SO2, and CO concentrations in urban air of the spa city of Sopot and medium-industrialized city of Tczew located in North Poland. Environ. Res. 2016, 147, 513–524. [Google Scholar] [CrossRef]
- Cerón-Bretón, J.G.; Cerón-Bretón, R.M.; Kahl, J.D.W.; Ramírez-Lara, E.; Guarnaccia, C.; Aguilar-Ucán, C.A.; Montalvo-Romero, C.; Anguebes-Franseschi, F.; López-Chuken, U. Diurnal and seasonal variation of BTEX in the air of Monterrey, Mexico: Preliminary study of sources and photochemical ozone pollution. Air Qual. Atmos. Health 2014, 8, 469–482. [Google Scholar] [CrossRef]
- Andrews, E.; Saxena, P.; Musarra, S.; Hildemann, L.; Koutrakis, P.; McMurry, P.; Olmez, I.; White, W. Concentration and Composition of Atmospheric Aerosols from the 1995 SEAVS Experiment and a Review of the Closure between Chemical and Gravimetric Measurements. J. Air Waste Manag. Assoc. 2000, 50, 648–664. [Google Scholar] [CrossRef] [PubMed]
- Berico, M.; Luciani, A.; Formignani, M. Atmospheric aerosol in an urban area—Measurements of TSP and PM10 standards and pulmonary deposition assessments. Atmos. Environ. 1997, 31, 3659–3665. [Google Scholar] [CrossRef]
- Pietrzak-Zawadka, J.; Zawadka, J. Forest therapy as a form of medical tourism. Ekon. I Sr. 2015, 4, 199–209. [Google Scholar]
- Tsunetsugu, Y.; Park, B.-J.; Miyazaki, Y. Trends in research related to “Shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ. Health Prev. Med. 2010, 15, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumitomo, K.; Akutsu, H.; Fukuyama, S.; Minoshima, A.; Kukita, S.; Yamamura, Y.; Sato, Y.; Hayasaka, T.; Osanai, S.; Funakoshi, H.; et al. Conifer-Derived Monoterpenes and Forest Walking. Mass Spectrom. 2015, 4, A0042. [Google Scholar] [CrossRef]
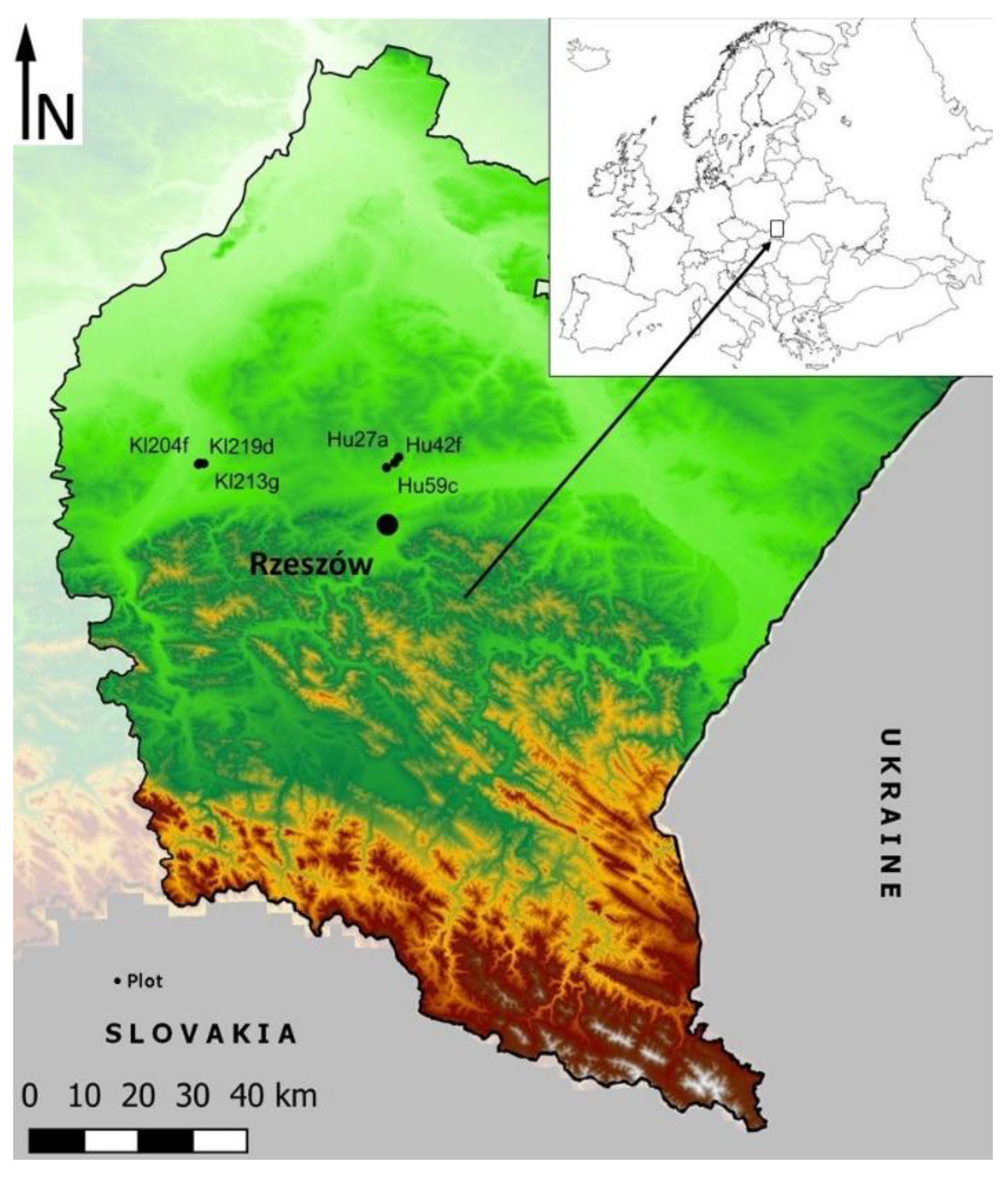


| Forest District Units | Coordinates (°) | Area (ha) | SC (%) | Admixture Species | Age (Years) | UU (%) | H (m) |
|---|---|---|---|---|---|---|---|
| Hu27a | N50.14072 E22.03605 | 7.51 | 100P | Q, Pi, A, F, C | 89 | 70 | 25 |
| Hu42f | N50.13173 E22.02485 | 8.53 | 100P | B | 77 | 20 | 21 |
| Hu59c | N50.12437 E22.00373 | 18.17 | 100P | Q, B | 78 | 30 | 22 |
| Kl204f | N50.14303 E21.52324 | 7.72 | 90F; 10Ac | Pi, C | 79 | 10 | 27 |
| Kl213g | N50.14227 E21.53394 | 2.50 | 70F; 30Q | - | 76 | 10 | 29 |
| Kl219d | N50.14136 E21.52017 | 4.29 | 80F; 20Q | Ac, C | 86 | 30 | 29 |
| Date | Forest District Units | Land Field Measurements | Data from Meteo Station Rzeszów-Jasionka | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T (°C) (avg.) | H (%) (avg.) | Ozone (avg.) * (µg·m−3) | Ozone (Min–Max) (µg·m−3) | AP (hPa) | T (°C) (avg.) | H (%) (avg.) | W (km/h) (avg.) | ||
| 17.06 | Hu27a | 21.1 | 75.1 | <LOQ | <LOQ | 1009 | 20 | 83 | 7 |
| Hu42f | 24.3 | 64.5 | <LOQ | <LOQ | 1008 | 22 | 73 | 7 | |
| Hu59c | 23.8 | 64.9 | <LOQ | 19–335 | 1008 | 23 | 69 | 15 | |
| 18.06 | Kl204f | 22.3 | 76.1 | <LOQ | 19–39 | 1008 | 19 | 94 | 6 |
| Kl213g | 20.7 | 82.9 | <LOQ | <LOQ | 1008 | 23 | 69 | 22 | |
| Kl219d | 23.9 | 71.3 | <LOQ | <LOQ | 1007 | 24 | 65 | 20 | |
| 15.07 | Hu27a | 20.5 | 51.6 | <LOQ | 19–270 | 1015 | 20 | 60 | 7 |
| Hu42f | 22.1 | 49.6 | 19 | 19–510 | 1014 | 22 | 50 | 13 | |
| Hu59c | 24.6 | 42.4 | <LOQ | 19–195 | 1014 | 24 | 41 | 0 | |
| Urban area | 26.0 | 36.5 | 19 | 19–622 | 1014 | 23 | 41 | 6 | |
| 16.07 | Kl219d | 20.3 | 69.4 | <LOQ | <LOQ | 1012 | 21 | 64 | 13 |
| Kl213g | 22.6 | 53.5 | <LOQ | <LOQ | 1012 | 23 | 53 | 20 | |
| Kl204f | 22.7 | 57.3 | <LOQ | 19–19 | 1012 | 23 | 61 | 22 | |
| Urban area | 24.3 | 52.9 | 19 | 19–409 | 1012 | 22 | 60 | 26 | |
| 11.08 | Hu27a | 24.2 | 69.9 | <LOQ | <LOQ | 1019 | 23 | 73 | 2 |
| Hu42f | 27.5 | 58.7 | <LOQ | <LOQ | 1019 | 27 | 54 | 6 | |
| Hu59c | 28.4 | 51.0 | <LOQ | <LOQ | 1018 | 27 | 45 | 9 | |
| 12.08 | Kl219d | 19.1 | 78.3 | <LOQ | <LOQ | 1020 | 20 | 68 | 4 |
| Kl213g | 21.5 | 68.7 | <LOQ | <LOQ | 1020 | 23 | 57 | 13 | |
| Kl204f | 23.1 | 61.7 | <LOQ | 19–158 | 1020 | 24 | 41 | 13 | |
| 22.09 | Hu27a | 15.7 | 70.6 | <LOQ | <LOQ | 1019 | 14 | 82 | 2 |
| Hu42f | 19.1 | 64.0 | <LOQ | <LOQ | 1018 | 20 | 56 | 13 | |
| Hu59c | 22.1 | 54.9 | <LOQ | <LOQ | 1018 | 23 | 50 | 13 | |
| 23.09 | Kl219d | 16.2 | 80.0 | <LOQ | <LOQ | 1012 | 17 | 72 | 4 |
| Kl213g | 20.8 | 62.4 | <LOQ | <LOQ | 1012 | 23 | 50 | 15 | |
| Kl204f | 23.5 | 51.3 | <LOQ | <LOQ | 1011 | 25 | 44 | 15 | |
| Chemical Compound | CAS No. | Formula | Range of LRI on DB-1 for Investigated Samples Based on GC-FID Analysis | Range of LRI on HP-1 MS for Investigated Samples Based on GC-MS Analysis | Range of LRI Based on Literature Data on Similar GC Column (a) |
|---|---|---|---|---|---|
| α-Pinene | 80-56-8 | C10H16 | 949–956 | 940–946 | 927–932 |
| Camphene | 79-92-5 | C10H16 | 969–975 | 955–960 | 940–946 |
| β-Pinene | 127-91-3 | C10H16 | 996–999 | 982–987 | 969–974 |
| 3-Carene | 13466-78-9 | C10H16 | 1025–1031 | 1014–1020 | 1002–1004 |
| Limonene | 5989-27-5 | C10H16 | 1039–1045 | 1031–1036 | 1024–1026 |
| γ-Terpinene | 99-85-4 | C10H16 | 1064–1070 | 1057–1062 | 1054–1056 |
| Terpinolene | 586-62-9 | C10H16 | 1101–1106 | 1082–1086 | 1085–1090 |
| Camphor | 464-49-3 | C10H16O | 1160–1166 | 1158–1063 | 1141–1148 |
| α-Terpineol | 98-55-5 | C10H18O | 1182–1188 | 1170–1176 | 1186–1194 |
| Carvone | 99-49-0 | C10H14O | 1248–1254 | 1231–1236 | 1239–1248 |
| Geranyl Acetate | 105-87-3 | C12H20O2 | 1361–1367 | 1363–1369 | 1379–1385 |
| Farnesene/Humulene | 6753-98-6 | C15H24 | 1474–1480 | 1443–1450 | 1440–1452 |
| Nerolidol/Farnesol | 7212-44-4 | C15H26O | 1531–1537 | 1527–1533 | 1531–1542 |
| Germacene B | 15423-57-1 | C15H24 | 1561–1568 | 1558–1563 | 1559–1565 |
| Sampling Date | TVOC (µg/m3) | ∑TERPENES (µg/m3) | Benzene (µg/m3) | Toluene (µg/m3) | Ethylbenzene (µg/m3) | p, m-Xylene (µg/m3) | ΣBTEX (µg/m3) | |
|---|---|---|---|---|---|---|---|---|
| Hu 27a | 17 June 2020 | 70.9 | 39.5 | 0.098 | 0.152 | <LOQ | <LOQ | 0.25 |
| Hu 27a | 15 July 2020 | 65.7 | 30.0 | <LOQ | 0.151 | <LOQ | 0.045 | 0.20 |
| Hu 27a | 11 August 2020 | 37.0 | 13.9 | <LOQ | 0.079 | <LOQ | <LOQ | 0.08 |
| Hu 27a | 22 September 2020 | 17.9 | 5.2 | 1.011 | 7.469 | <LOQ | 0.993 | 9.5 |
| Hu 42f | 17 June 2020 | 98.9 | 29.7 | 0.469 | 9.102 | <LOQ | <LOQ | 9.6 |
| Hu 42f | 15 July 2020 | 59.8 | 30.7 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Hu 42f | 11 August 2020 | 57.8 | 22.1 | 0.346 | 0.060 | <LOQ | <LOQ | 0.41 |
| Hu 42f | 22 September 2020 | 46.5 | 10.8 | 0.571 | 11.961 | 2.967 | 0.349 | 16 |
| Hu 59c | 17 June 2020 | 141.0 | 45.3 | 0.934 | 18.586 | <LOQ | <LOQ | 19 |
| Hu 59c | 15 July 2020 | 58.6 | 17.4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Hu 59c | 11 August 2020 | 79.7 | 22.8 | <LOQ | <LOQ | <LOQ | 0.192 | 0.19 |
| Hu 59c | 22 September 2020 | 50.8 | 15.5 | 0.358 | 0.037 | <LOQ | 0.843 | 1.2 |
| Kl 204f | 18 June 2020 | 64.8 | 30.9 | 2.094 | 1.983 | <LOQ | 2.295 | 6.4 |
| Kl 204f | 19 July 2020 | 56.0 | 25.1 | 0.623 | <LOQ | <LOQ | 0.306 | 0.93 |
| Kl 204f | 12 August 2020 | 49.1 | 16.0 | <LOQ | 2.297 | 1.343 | 1.390 | 5.0 |
| Kl 204f | 23 September 2020 | 89.8 | 16.5 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Kl 213g | 18 June 2020 | 201.5 | 44.4 | 2.141 | 0.937 | <LOQ | 2.961 | 6.0 |
| Kl 213g | 19 July 2020 | 311.8 | 27.4 | <LOQ | 0.910 | 0.369 | 1.053 | 2.3 |
| Kl 213g | 12 August 2020 | 137.2 | 20.9 | 0.965 | 0.184 | <LOQ | 1.870 | 3.0 |
| Kl 213g | 23 September 2020 | 250.1 | 27.3 | 2.712 | 0.011 | <LOQ | 1.834 | 4.56 |
| Kl 219d | 18 June 2020 | 263.6 | 73.7 | 0.237 | 0.646 | 0.641 | 0.344 | 1.87 |
| Kl 219d | 19 July 2020 | 117.9 | 59.3 | 0.563 | 0.084 | 0.040 | 0.519 | 1.21 |
| Kl 219d | 12 August 2020 | 46.0 | 18.2 | <LOQ | 0.209 | <LOQ | <LOQ | 0.21 |
| Kl 219d | 23 September 2020 | 49.5 | 26.8 | 0.517 | 0.074 | <LOQ | 0.094 | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, T.; Marć, M.; Zabiegała, B. Chemical Composition of Atmospheric Air in Nemoral Scots Pine Forests and Submountainous Beech Forests: The Potential Region for the Introduction of Forest Therapy. Int. J. Environ. Res. Public Health 2022, 19, 15838. https://doi.org/10.3390/ijerph192315838
Dudek T, Marć M, Zabiegała B. Chemical Composition of Atmospheric Air in Nemoral Scots Pine Forests and Submountainous Beech Forests: The Potential Region for the Introduction of Forest Therapy. International Journal of Environmental Research and Public Health. 2022; 19(23):15838. https://doi.org/10.3390/ijerph192315838
Chicago/Turabian StyleDudek, Tomasz, Mariusz Marć, and Bożena Zabiegała. 2022. "Chemical Composition of Atmospheric Air in Nemoral Scots Pine Forests and Submountainous Beech Forests: The Potential Region for the Introduction of Forest Therapy" International Journal of Environmental Research and Public Health 19, no. 23: 15838. https://doi.org/10.3390/ijerph192315838






