Uptake and Transport of Different Concentrations of PPCPs by Vegetables
Abstract
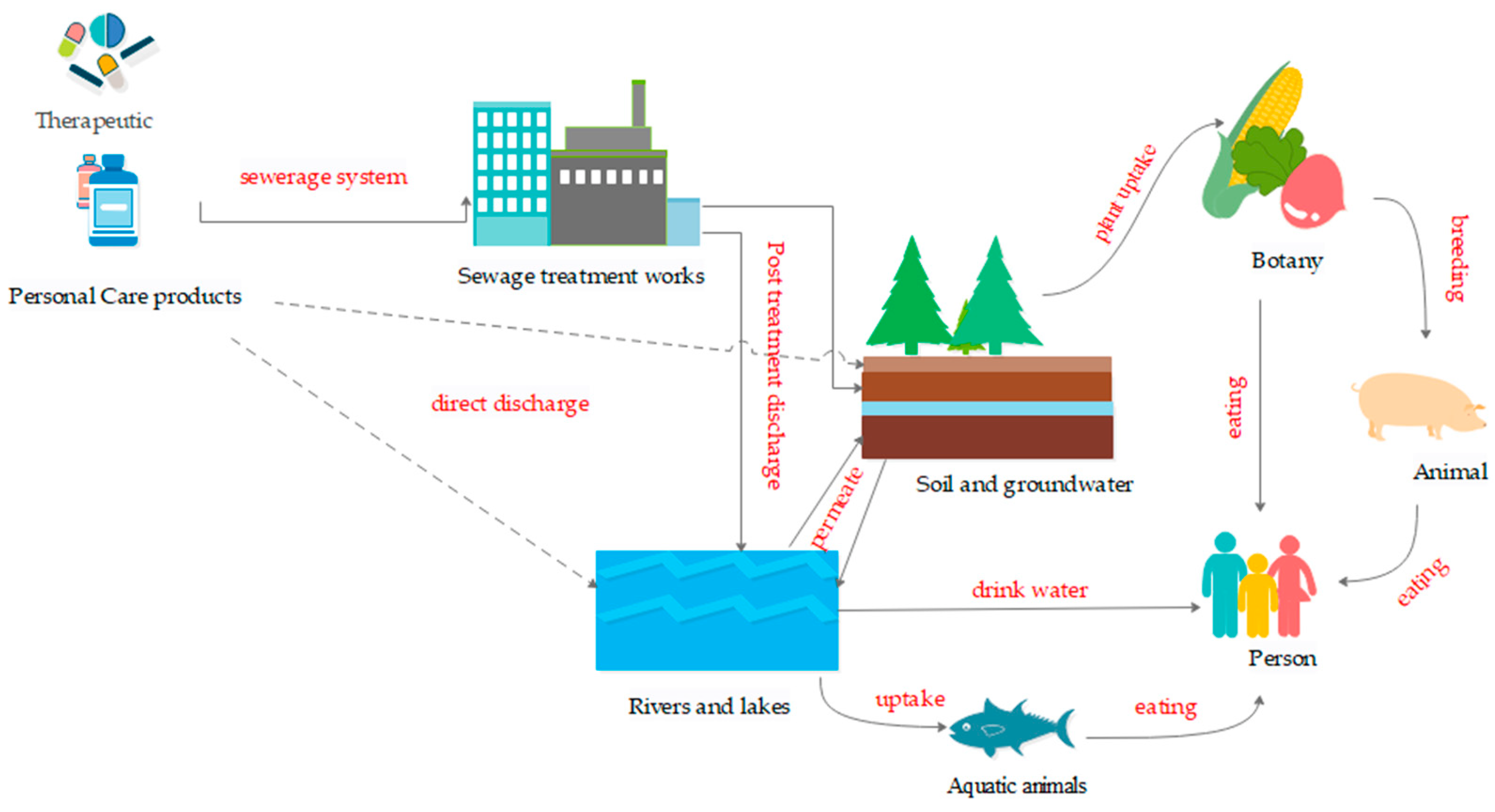
:1. Introduction
2. Methods and Materials
2.1. Reagents
2.2. Apparatus
2.3. Plant Cultivation and Treatment
2.4. Determination of PPCPs in Plants
2.4.1. Pre-Treatment of Plant Samples
2.4.2. Instrument Methods
2.4.3. Quantitative Analysis
3. Results and Discussion
3.1. Linearity Equation, Detection Limit, and Quantification Limit
3.2. Evaluation of Plant Growth Status
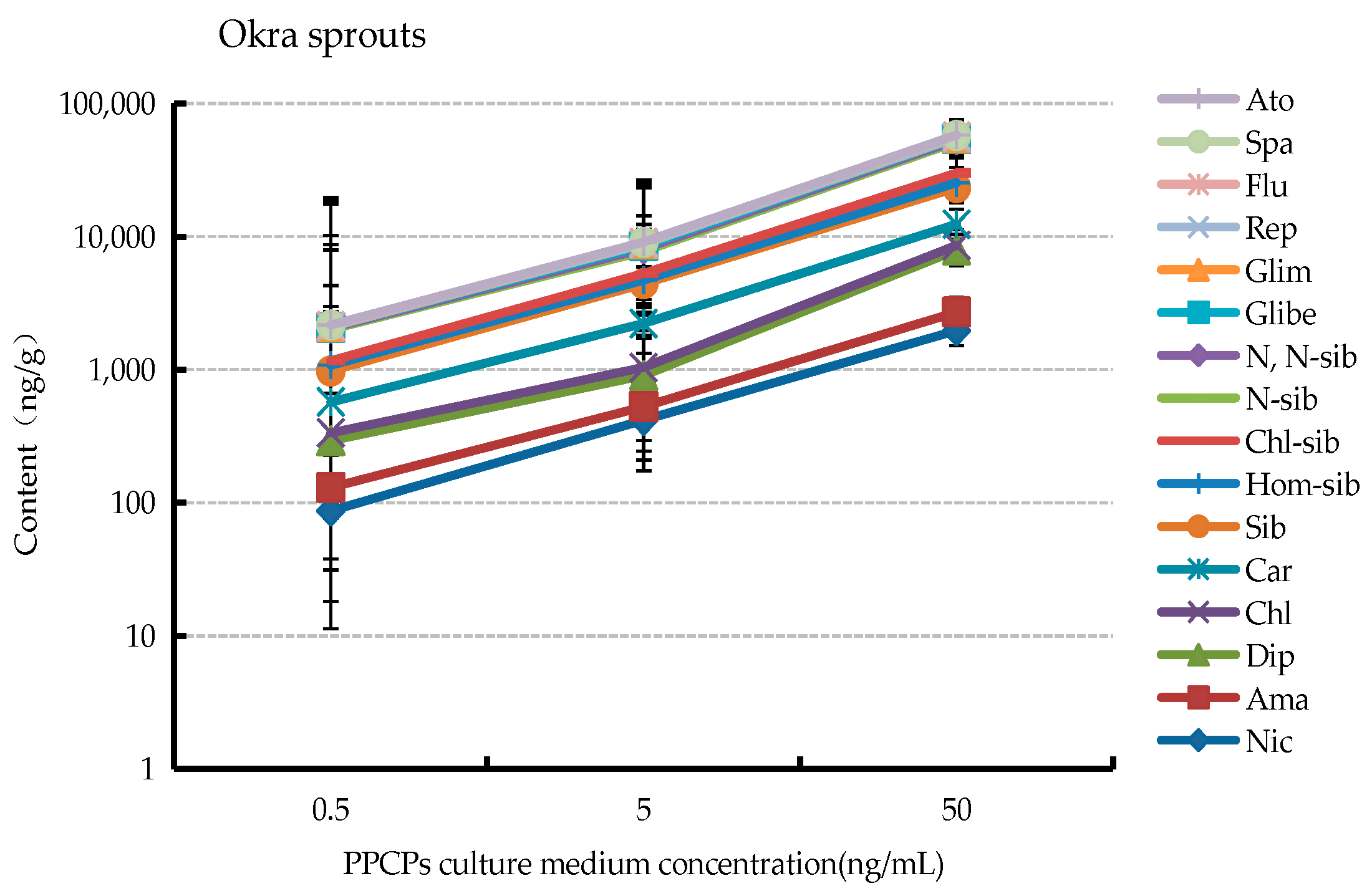
3.3. Uptake of Different Concentrations of PPCPs by Plants
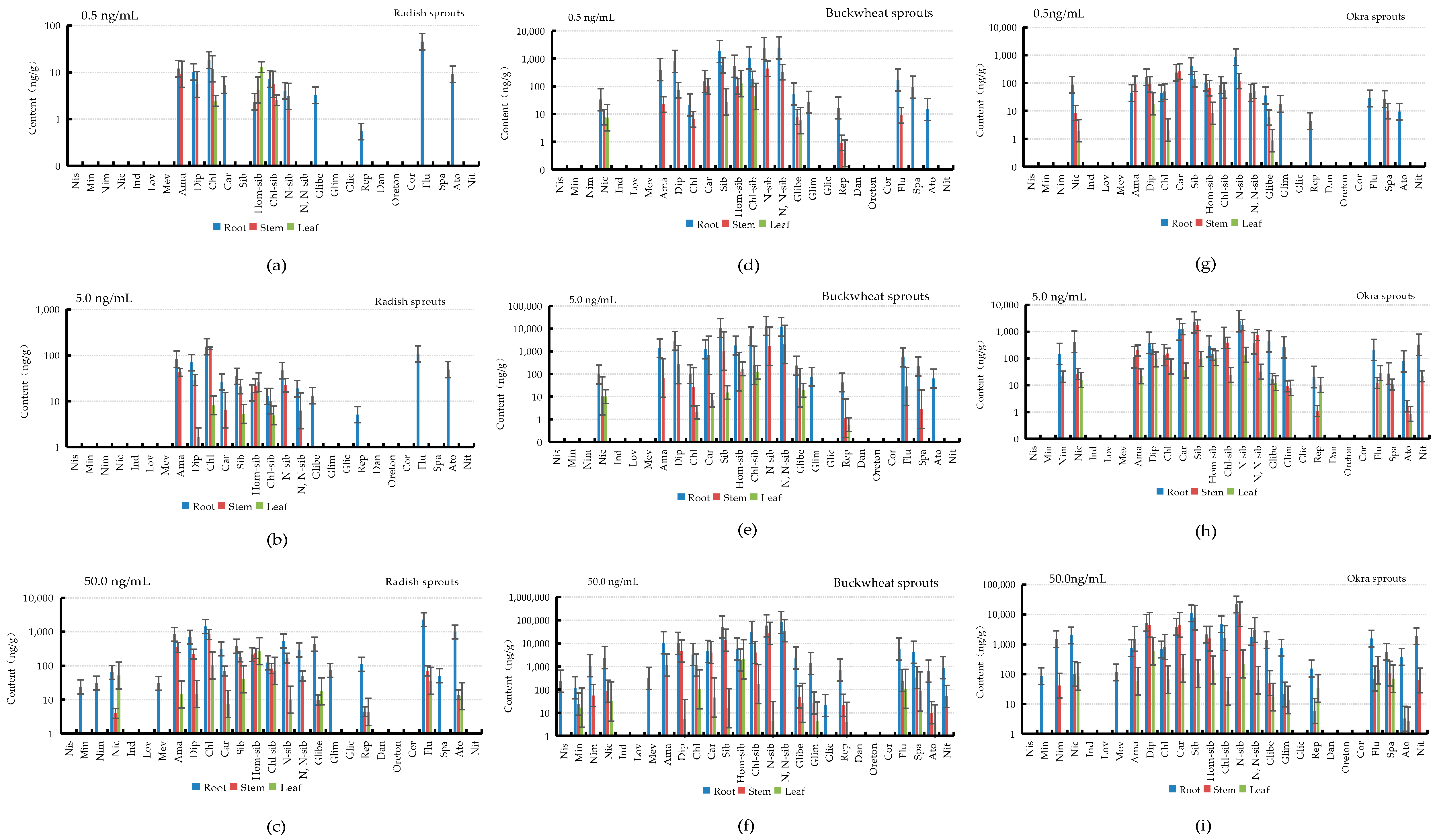
3.4. Accumulation and Distribution of PPCPs in Plants
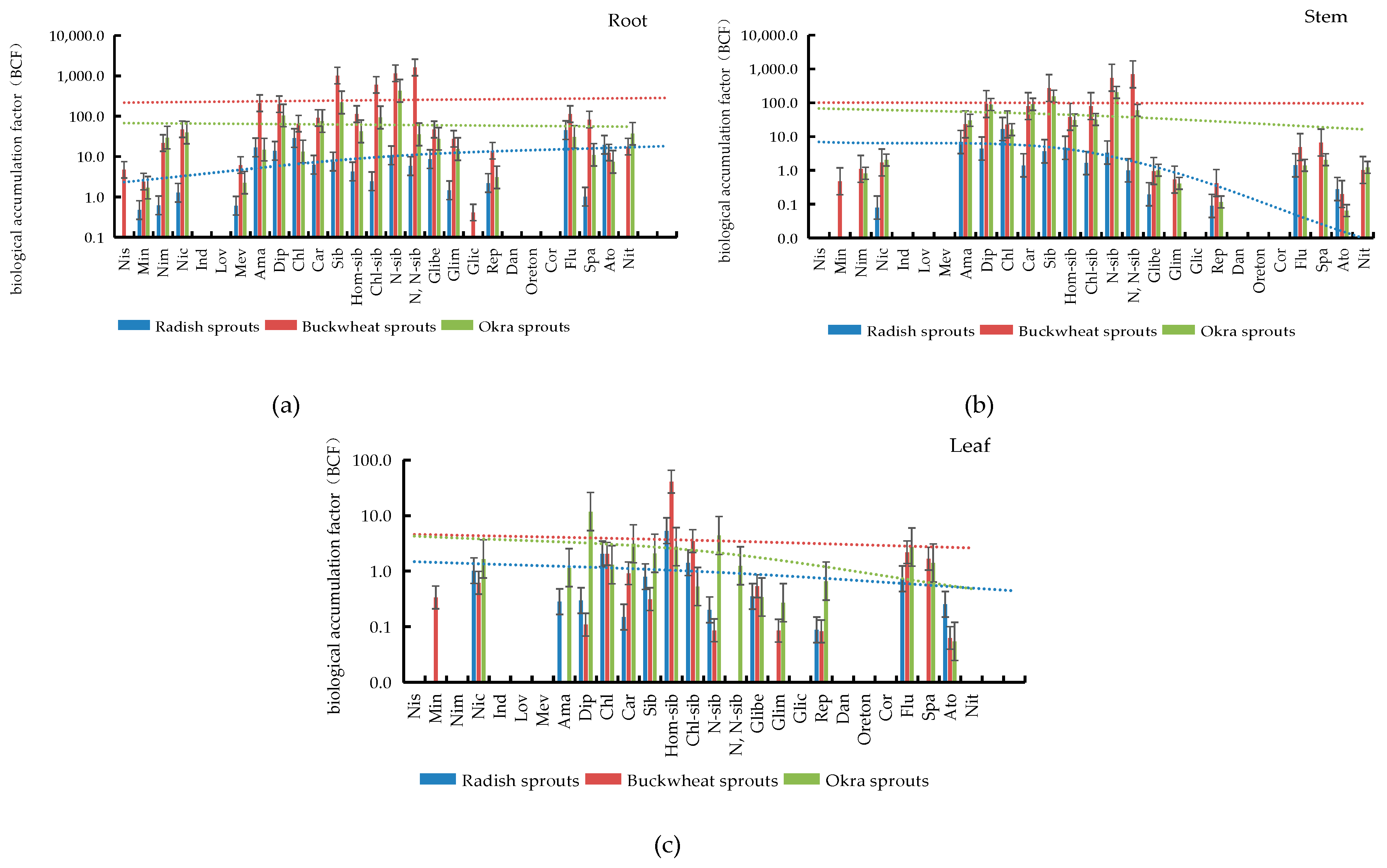
3.5. Bioconcentration Factor (BCF) Evaluation of PPCPs in Plant Tissues
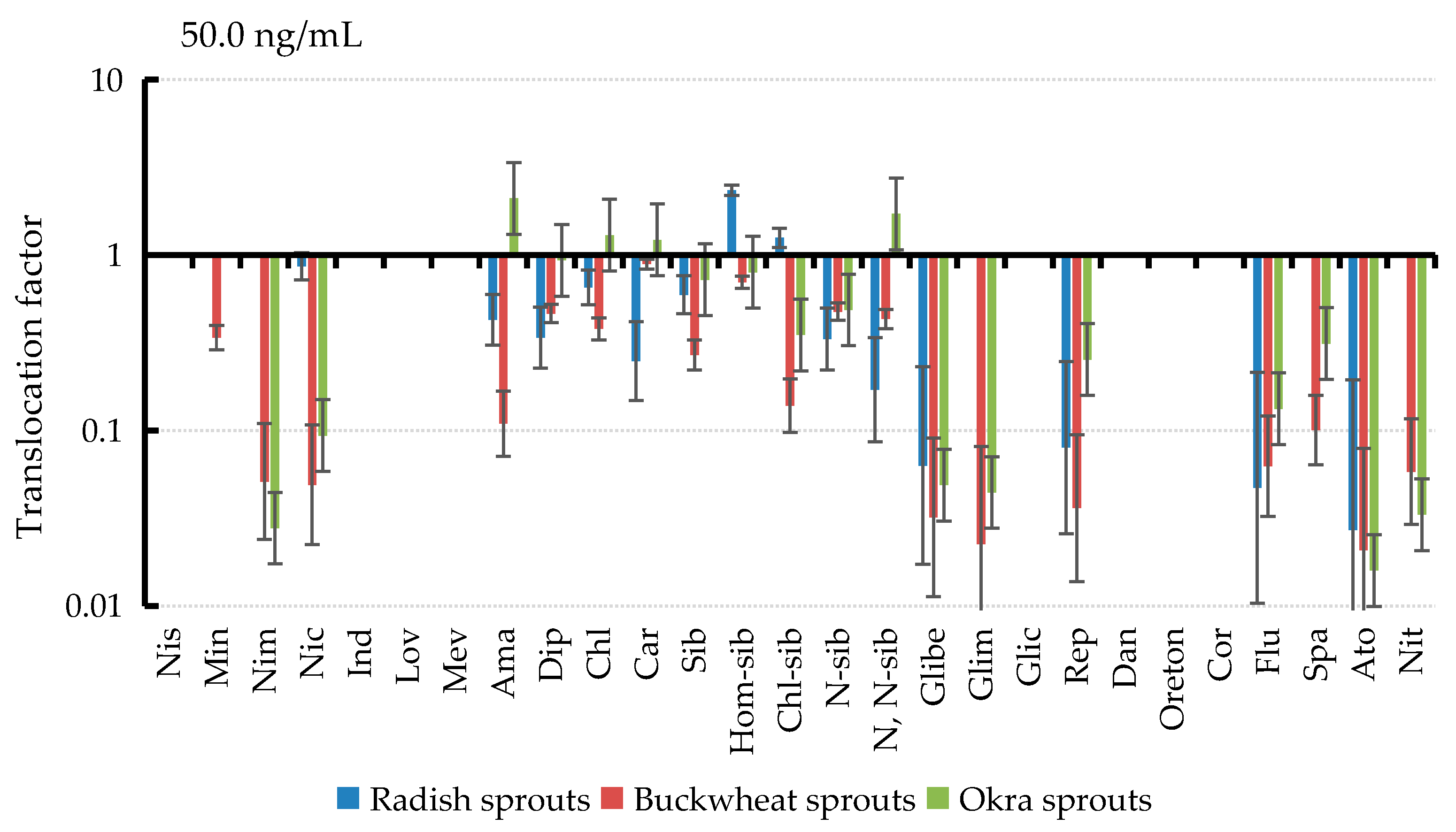
3.6. Translocation of PPCPs in Plant Tissues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Silke, J.; Isabelle, S.; Stefaan, D.H.; Yves, R.; Tanja, C.; Alice, T.; Martí, N.; António, M.; Wim, V. Marine environmental contamination: Public awareness, concern and perceived effectiveness in five European countries. Environ. Res. 2015, 143, 4–10. [Google Scholar]
- Martínez-Alcalá, I.; Guillén-Navarro, J.M.; Lahora, A. Occurrence and fate of pharmaceuticals in a wastewater treatment plant from southeast of Spain and risk assessment. J. Environ. Manag. 2021, 279, 111565. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G. Non-regulated water contaminants: Emerging research. Environ. Impact Assess. Rev. 2004, 24, 711–732. [Google Scholar] [CrossRef]
- An, W.; Duan, L.; Zhang, Y.; Zhou, Y.; Wang, B.; Yu, G. Pollution characterization of pharmaceutically active compounds (PhACs) in the northwest of Tai Lake Basin, China: Occurrence, temporal changes, riverine flux and risk assessment. J. Hazard. Mater. 2022, 422, 126889. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- Hamid, N.; Junaid, M.; Wang, Y.; Pu, S.; Jia, P.; Pei, D. Chronic exposure to PPCPs mixture at environmentally relevant concentrations (ERCs) altered carbohydrate and lipid metabolism through gut and liver toxicity in zebrafish. Environ. Pollut. 2021, 273, 116494. [Google Scholar] [CrossRef]
- Ali, A.M.; Rønning, H.T.; Sydnes, L.K.; Alarif, W.M.; Kallenborn, R.; Al-Lihaibi, S.S. Detection of PPCPs in marine organisms from contaminated coastal waters of the Saudi Red Sea. Sci. Total Environ. 2018, 621, 654–662. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Q.; Wang, Z.; Zhang, K.; Tang, C.; Cui, J.; Feng, J.; Peng, X. Occurrence and behavior of pharmaceuticals, steroid hormones, and endocrine-disrupting personal care products in wastewater and the recipient river water of the Pearl River Delta, South China. J. Environ. Monit. 2011, 13, 871–878. [Google Scholar] [CrossRef]
- Ali, A.M.; Rønning, H.T.; Al Arif, W.M.; Kallenborn, R.; Al Lihaibi, S.S. Occurrence of pharmaceuticals and personal care products in effluent-dominated Saudi Arabian coastal waters of the Red Sea. Chemosphere 2017, 175, 505–513. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, H.; Li, L.; Ren, M.; Qie, H.; Lin, A. A review of distribution and risk of pharmaceuticals and personal care products in the aquatic environment in China. Ecotox. Environ. Safe. 2021, 213, 112044. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.A.; Swarzenski, P.W.; Dinicola, R.S.; Reinhard, M. Occurrence of Herbicides and Pharmaceutical and Personal Care Products in Surface Water and Groundwater around Liberty Bay, Puget Sound, Washington. J. Environ. Qual. 2010, 39, 1173–1180. [Google Scholar] [CrossRef]
- Boxall, A.B.A. The environmental side effects of medication. EMBO Rep. 2004, 5, 1110–1116. [Google Scholar] [CrossRef]
- Wu, C.; Spongberg, A.L.; Witter, J.D.; Fang, M.; Czajkowski, K.P. Uptake of Pharmaceutical and Personal Care Products by Soybean Plants from Soils Applied with Biosolids and Irrigated with Contaminated Water. Environ. Sci. Technol. 2010, 44, 6157–6161. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Johnson, P.; Smith, E.J.; Sinclair, C.J.; Stutt, E.; Levy, L.S. Uptake of Veterinary Medicines from Soils into Plants. J. Agric. Food Chem. 2006, 54, 2288–2297. [Google Scholar] [CrossRef]
- Dolliver, H.; Kumar, K.; Gupta, S. Sulfamethazine Uptake by Plants from Manure-Amended Soil. J. Environ. Qual. 2007, 36, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Uptake of pharmaceuticals by plants grown under hydroponic conditions and natural occurring plant species: A review. Sci. Total Environ. 2018, 636, 477–486. [Google Scholar] [CrossRef]
- Aparicio, I.; Martín, J.; Abril, C.; Santos, J.L.; Alonso, E. Determination of household and industrial chemicals, personal care products and hormones in leafy and root vegetables by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 1533, 49–56. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef]
- Yang, X.; Flowers, R.C.; Weinberg, H.S.; Singer, P.C. Occurrence and removal of pharmaceuticals and personal care products (PPCPs) in an advanced wastewater reclamation plant. Water Res. 2011, 45, 5218–5228. [Google Scholar] [CrossRef] [PubMed]
- Fricke-Galindo, I.; Lerena, A.; Jung-Cook, H.; López-López, M. Carbamazepine adverse drug reactions. Expert Rev. Clin. Pharmacol. 2018, 11, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Poston, W.S.C.; Foreyt, J.P. Sibutramine and the management of obesity. Expert. Opin. Pharmacother. 2004, 5, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Alyu, F.; Olgar, Y.; Degirmenci, S.; Turan, B.; Ozturk, Y. Interrelated In Vitro Mechanisms of Sibutramine-Induced Cardiotoxicity. Cardiovasc. Toxicol. 2021, 21, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Preciado, D.; Jiménez-Cartagena, C.; Matamoros, V.; Bayona, J.M. Screening of 47 organic microcontaminants in agricultural irrigation waters and their soil loading. Water Res. 2011, 45, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S.; Bailey, J.L.; Vanden, H.B.; Kinney, C.A. Uptake of human pharmaceuticals and personal care products by cabbage (Brassica campestris) from fortified and biosolids-amended soils. J. Environ. Monit. 2012, 14, 3029–3036. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Wu, Q.; Huang, X.; Zhang, J.; Fang, H. Speciation and accumulation pattern of heavy metals from soil to rice at different growth stages in farmland of southwestern China. Environ. Sci. Pollut. Res. 2020, 27, 35675–35691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, Y.; Liu, Y.; Chang, H.; Li, Z.; Xue, J. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Huang, H.; Wen, B.; Christie, P. Partitioning of Phenanthrene by Root Cell Walls and Cell Wall Fractions of Wheat (Triticum aestivum L.). Environ. Sci. Technol. 2009, 43, 9136–9141. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, L. Sorption of Polycyclic Aromatic Hydrocarbons to Carbohydrates and Lipids of Ryegrass Root and Implications for a Sorption Prediction Model. Environ. Sci. Technol. 2009, 43, 2740–2745. [Google Scholar] [CrossRef]
- Al-Farsi, R.S.; Ahmed, M.; Al-Busaidi, A.; Choudri, B.S. Translocation of pharmaceuticals and personal care products (PPCPs) into plant tissues: A review. Emerg. Contam. 2017, 3, 132–137. [Google Scholar] [CrossRef]
- Wu, X.; Conkle, J.L.; Gan, J. Multi-residue determination of pharmaceutical and personal care products in vegetables. J. Chromatogr. A 2012, 1254, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Herklotz, P.A.; Gurung, P.; Vanden Heuvel, B.; Kinney, C.A. Uptake of human pharmaceuticals by plants grown under hydroponic conditions. Chemosphere 2010, 78, 1416–1421. [Google Scholar] [CrossRef]
- Stuer-Lauridsen, F.; Birkved, M.; Hansen, L.P.; Lutzhoft, H.C.; Halling-Sorensen, B. Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use. Chemosphere 2000, 40, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Simonich, S.L.; Hites, R.A. Organic pollutant accumulation in vegetation. Environ. Sci. Technol. 1995, 29, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Shenker, M.; Harush, D.; Ben-Ari, J.; Chefetz, B. Uptake of carbamazepine by cucumber plants—A case study related to irrigation with reclaimed wastewater. Chemosphere 2011, 82, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Winker, M.; Clemens, J.; Reich, M.; Gulyas, H.; Otterpohl, R. Ryegrass uptake of carbamazepine and ibuprofen applied by urine fertilization. Sci. Total Environ. 2010, 408, 1902–1908. [Google Scholar] [CrossRef]
- Martínez-Piernas, A.B.; Plaza-Bolaños, P.; Fernández-Ibáñez, P.; Agüera, A. Organic Microcontaminants in Tomato Crops Irrigated with Reclaimed Water Grown under Field Conditions: Occurrence, Uptake, and Health Risk Assessment. J. Agric. Food Chem. 2019, 67, 6930–6939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, L.; Chen, L.; Li, S.; Sun, L. Bioaccumulation of antibiotics in crops under long-term manure application: Occurrence, biomass response and human exposure. Chemosphere 2019, 219, 882–895. [Google Scholar] [CrossRef]
- Riemenschneider, C.; Al-Raggad, M.; Moeder, M.; Seiwert, B.; Salameh, E.; Reemtsma, T. Pharmaceuticals, Their Metabolites, and Other Polar Pollutants in Field-Grown Vegetables Irrigated with Treated Municipal Wastewater. J. Agric. Food Chem. 2016, 64, 5784–5792. [Google Scholar] [CrossRef]
- Snyder, S.A.; Westerhoff, P.; Yoon, Y.; Sedlak, D.L. Pharmaceuticals, Personal Care Products, and Endocrine Disruptors in Water: Implications for the Water Industry. Environ. Eng. Sci. 2003, 20, 449–469. [Google Scholar] [CrossRef]

| Drugs | Linear Equations | Linear Range (ng/mL) | R2 | LOD/(ng/g) | LOQ/(ng/g) | Average Recovery Rate (%) (n = 6) | ||
|---|---|---|---|---|---|---|---|---|
| 2 ng/mL | 20 ng/mL | 80 ng/mL | ||||||
| nisoldipine | y = 108034x − 15127.8 | 0.5–200 | 0.9997 | 1.8 | 5.5 | 93.6 | 101.3 | 96.1 |
| minoxidil | y = 473274x + 216871 | 0.1–150 | 0.9997 | 1.0 | 2.8 | 115.3 | 97.0 | 90.8 |
| nimodipine | y = 37211.8x + 47937.7 | 1.0–200 | 0.9999 | 2.4 | 7.2 | 86.0 | 84.5 | 90.7 |
| nicardipine hydrochloride | y = 109561x + 186323 | 0.1–200 | 0.9940 | 1.8 | 5.5 | 91.8 | 103.6 | 99.6 |
| indapamide | y = 71840.3x − 15690.5 | 0.5–200 | 0.9978 | 2.0 | 6.2 | 86.6 | 94.1 | 98.5 |
| lovastatin | y = 13679.3x + 3255.78 | 1.0–100 | 0.9970 | 2.8 | 8.4 | 95.7 | 92.1 | 91.1 |
| mevastatin | y = 27393.2x − 14427.6 | 0.5–100 | 0.9980 | 2.5 | 7.4 | 106.3 | 84.6 | 90.3 |
| amantadine | y = 120767x + 418807 | 0.1–200 | 0.9983 | 1.8 | 5.5 | 101.2 | 85.3 | 98.4 |
| diphenhydramine | y = 207602x − 1168800 | 1.0–300 | 0.9960 | 1.5 | 4.6 | 101.0 | 90.8 | 88.6 |
| chlorpheniramine maleate | y = 521211x + 473248 | 0.1–200 | 0.9971 | 0.9 | 2.8 | 94.8 | 90.8 | 105.1 |
| carbamazepine | y = 5579163x + 750344 | 0.1–100 | 0.9963 | 0.1 | 0.3 | 96.5 | 90.5 | 86.3 |
| sibutramine | y = 29292.6x + 1717.77 | 1.0–200 | 0.9980 | 2.5 | 7.4 | 120.4 | 94.9 | 110.1 |
| haemosibutramine | y = 778095x + 456360 | 0.5–150 | 0.9984 | 0.6 | 1.8 | 103.8 | 91.6 | 95.6 |
| chlorosibutramine | y = 173778x − 500933 | 1.0–100 | 0.9962 | 1.8 | 5.6 | 94.8 | 90.8 | 93.4 |
| N-monodesmethyl sibutramine | y = 8757.68x + 7416.21 | 0.5–100 | 0.9981 | 3.1 | 9.8 | 122.3 | 103.7 | 96.7 |
| N, N-bis-desmethyl sibutramine | y = 418080x + 110723 | 0.5–100 | 0.9997 | 1.0 | 3.0 | 81.7 | 96.2 | 96.3 |
| glibenclamide | y = 67746.8x + 3575.80 | 1.0–150 | 0.9963 | 2.0 | 6.2 | 97.6 | 93.5 | 102.3 |
| glimepiride | y = 29710.4x + 2040.04 | 1.0–150 | 0.9912 | 2.5 | 7.3 | 107.1 | 84.8 | 111.2 |
| gliclazide | y = 290295x + 4531.97 | 1.0–100 | 0.9999 | 1.5 | 4.6 | 97.7 | 100.6 | 89.6 |
| repaglinide | y = 533270x + 8743.73 | 0.1–100 | 0.9973 | 0.9 | 2.7 | 80.8 | 86.9 | 90.6 |
| danazo | y = 681958x + 14331.9 | 0.1–100 | 0.9964 | 0.8 | 2.5 | 93.6 | 106.2 | 93.8 |
| testosterone propionate | y = 28410.2x − 4312.44 | 0.5–100 | 0.9983 | 2.5 | 7.4 | 110.0 | 113.8 | 108.3 |
| cortisone | y = 235080x + 10535.7 | 0.5–100 | 0.9961 | 1.5 | 4.6 | 103.9 | 84.8 | 90.8 |
| flumequine | y = 23557x − 557.311 | 1.0–100 | 0.9983 | 2.7 | 8.1 | 86.9 | 81.1 | 95.3 |
| sparfloxacin | y = 79048.7x − 40180.1 | 0.5–200 | 0.9982 | 2.0 | 6.2 | 86.4 | 84.5 | 88.6 |
| atorvastatin calcium | y = 217752x − 455238 | 1.0–200 | 0.9970 | 1.5 | 4.6 | 95.3 | 99.6 | 94.6 |
| nicorandipine | y = 14159.6x + 11150.2 | 1.0–200 | 0.9998 | 2.8 | 8.4 | 103.3 | 94.3 | 90.5 |
| Drug | Radish Root/(ng/g) | Buckwheat Root/(ng/g) | Okra Root/(ng/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 ng/mL | 5 ng/mL | 50 ng/mL | 0.5 ng/mL | 5 ng/mL | 50 ng/mL | 0.5 ng/mL | 5 ng/mL | 50 ng/mL | |
| Ato | 9.1 ± 0.5 | 48.6 ± 2.0 | 9.8 × 102 ± 54.9 | 14.7 ± 0.9 | 63.8 ± 5.0 | 6.3 × 102 ± 34.7 | 9.5 ± 0.4 | 77.4 ± 1.7 | 3.7 × 102 ± 19.6 |
| Flu | 45.3 ± 1.5 | 106.7 ± 7.3 | 2.3 × 103 ± 163.3 | 1.7 × 102 ± 11.7 | 5.5 × 102 ± 29.2 | 5.7 × 103 ± 273.6 | 28.2 ± 0.7 | 2.1 × 102 ± 8.8 | 1.5 × 103 ± 48.0 |
| Rep | 4.9 ± 0.2 | 15.1 ± 0.5 | 1.1 × 102 ± 5.9 | 16.6 ± 0.8 | 42.5 ± 3.3 | 7.0 × 102 ± 30.8 | 4.3 ± 0.2 | 20.2 ± 0.6 | 1.5 × 102 ± 2.4 |
| Ama | 12 ± 0.9 | 82.1 ± 4.6 | 8.4 × 102 ± 33.6 | 4.0 × 102 ± 20.4 | 1.4 × 102 ± 10.4 | 1.1 × 104 ± 231.0 | 43.5 ± 1.0 | 1.1 × 102 ± 6.5 | 7.5 × 102 ± 48 |
| Dip | 10.2 ± 0.4 | 69.2 ± 3.5 | 7.0 × 102 ± 30.8 | 8.0 × 102 ± 33.6 | 2.9 × 103 ± 78.3 | 9.9 × 103 ± 336.6 | 1.6 × 102 ± 4.2 | 3.8 × 102 ± 19.8 | 5.2 × 103 ± 208.0 |
| Chl | 18.3 ± 0.3 | 1.5 × 102 ± 1.5 | 1.4 × 103 ± 75.6 | 21.3 ± 1.02 | 99.6 ± 6.5 | 3.3 × 103 ± 85.8 | 42.6 ± 1.7 | 1.3 × 102 ± 3.5 | 6.7 × 102 ± 36.2 |
| Car | 5.4 ± 0.5 | 26.3 ± 0.9 | 3.1 × 102 ± 22.3 | 1.5 × 102 ± 9.2 | 1.2 × 103 ± 69.6 | 4.5 × 103 ± 148.5 | 2.4 × 102 ± 7.2 | 1.2 × 103 ± 69.6 | 3.8 × 103 ± 68.4 |
| Hom-sib | 2.3 ± 0.1 | 15.2 ± 0.4 | 2.1 × 102 ± 4.6 | 5.3 × 102 ± 20.1 | 1.8 × 103 ± 66.6 | 5.6 × 103 ± 140.0 | 1.0 × 102 ± 2.3 | 2.8 × 102 ± 13.4 | 2.1 × 103 ± 157.5 |
| Chl-sib | 7.2 ± 0.7 | 12.8 ± 1.1 | 1.2 × 102 ± 11.9 | 1.1 × 103 ± 40.7 | 4.7 × 103 ± 197.4 | 3.0 × 104 ± 2430.0 | 84 ± 5.8 | 5.8 × 102 ± 129.6 | 4.7 × 103 ± 103.4 |
| Glibe | 3.2 ± 0.3 | 13.2 ± 0.6 | 4.3 × 102 ± 13.8 | 53.2 ± 3.2 | 236.3 ± 9.2 | 2.3 × 103 ± 131.1 | 35.9 ± 2.4 | 4.4 × 102 ± 18.9 | 1.4 × 103 ± 39.2 |
| N-sib | 4.0 ± 0.3 | 46.3 ± 4.4 | 5.4 × 102 ± 27.5 | 2.3 × 103 ± 112.7 | 1.3 × 104 ± 663.0 | 5.8 × 104 ± 3596 | 8.4 × 102 ± 62.2 | 2.5 × 103 ± 135.0 | 2.1 × 104 ± 1848 |
| N, N-sib | / | 18.7 ± 0.8 | 2.9 × 102 ± 22.3 | 2.5 × 103 ± 67.5 | 1.2 × 104 ± 744.0 | 8.1 × 104 ± 2673 | 44.4 ± 1.3 | 3.6 × 102 ± 7.56 | 1.8 × 103 ± 48.6 |
| Sib | / | 34.8 ± 1.5 | 3.8 × 102 ± 25.8 | 1.8 × 103 ± 55.8 | 1.1 × 104 ± 451.0 | 5.1 × 104 ± 2856 | 4.1 × 102 ± 33.6 | 2.2 × 103 ± 156.2 | 1.1 × 104 ± 484 |
| Glim | / | / | 73.0 ± 5.4 | 26.8 ± 2.1 | 76.2 ± 6.2 | 1.4 × 103 ± 96.6 | 17.9 ± 1.2 | 2.6 × 102 ± 15.3 | 7.7 × 102 ± 60.8 |
| Spa | / | / | 50.7 ± 2.1 | 93.9 ± 5.1 | 2.2 × 102 ± 11.4 | 4.1 × 103 ± 241.9 | 26.8 ± 1.6 | 27.1 ± 2.4 | 5.5 × 102 ± 37.4 |
| Nic | / | / | 63.6 ± 4.1 | 32.9 ± 1.7 | 95.0 ± 2.7 | 2.4 × 103 ± 172.8 | 86.7 ± 8.8 | 4.2 × 102 ± 22.3 | 2.0 × 103 ± 154.0 |
| Nim | / | / | 30.8 ± 1.0 | / | / | 1.1 × 103 ± 28.6 | / | 1.5 × 102 ± 10.2 | 1.5 × 103 ± 112.5 |
| Min | / | / | 23.8 ± 0.9 | / | / | 1.2 × 102 ± 10.0 | / | / | 84.8 ± 6.2 |
| Mev | / | / | 30.2 ± 2.5 | / | / | 3.1 × 102 ± 14.3 | / | / | 1.1 × 102 ± 7.15 |
| Nit | / | / | / | / | / | 8.8 × 102 ± 76.6 | / | 3.2 × 102 ± 17.9 | 1.8 × 103 ± 198.0 |
| Nis | / | / | / | / | / | 2.3 × 102 ± 11.0 | / | / | / |
| Glic | / | / | / | / | / | 20.6 ± 0.6 | / | / | / |
| Dan | / | / | / | / | / | / | / | / | / |
| Oreton | / | / | / | / | / | / | / | / | / |
| Cor | / | / | / | / | / | / | / | / | / |
| Ind | / | / | / | / | / | / | / | / | / |
| Lov | / | / | / | / | / | / | / | / | / |
| Drug | Radish Sprouts | Buckwheat Sprouts | Okra Sprouts | ||||||
|---|---|---|---|---|---|---|---|---|---|
| root/(L/kg) | stem/(L/kg) | leaf/(L/kg) | root/(L/kg) | stem/(L/kg) | leaf/(L/kg) | root/(L/kg) | stem/(L/kg) | leaf/(L/kg) | |
| Ato | 19.66 ± 1.10 | 0.28 ± 0.01 | 0.25 ± 0.01 | 12.59 ± 0.69 | 0.20 ± 0.01 | 0.06 ± 0.01 | 7.44 ± 0.39 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| Flu | 45.37 ± 3.22 | 1.41 ± 0.10 | 0.73 ± 0.04 | 113.13 ± 5.40 | 4.90 ± 0.43 | 2.19 ± 0.13 | 30.93 ± 0.99 | 1.41 ± 0.08 | 2.70 ± 0.29 |
| Rep | 2.21 ± 0.12 | 0.09 ± 0.01 | 0.09 ± 0.01 | 13.96 ± 0.62 | 0.42 ± 0.04 | 0.08 ± 0.01 | 3.06 ± 0.05 | 0.12 ± 0.01 | 0.66 ± 0.08 |
| Ama | 16.87 ± 0.68 | 6.92 ± 0.43 | 0.28 ± 0.02 | 210.94 ± 4.40 | 23.08 ± 1.74 | / | 14.92 ± 0.95 | 30.29 ± 2.29 | 1.15 ± 0.11 |
| Dip | 13.93 ± 0.61 | 4.42 ± 0.44 | 0.29 ± 0.03 | 198.13 ± 6.70 | 91.90 ± 11.13 | 0.11 ± 0.01 | 104.06 ± 4.20 | 89.05 ± 3.53 | 11.77 ± 1.70 |
| Chl | 28.77 ± 1.56 | 16.77 ± 0.89 | 2.04 ± 0.09 | 65.64 ± 1.70 | 22.88 ± 1.46 | 2.05 ± 0.13 | 13.39 ± 0.72 | 16.11 ± 0.59 | 1.30 ± 0.11 |
| Car | 6.24 ± 0.45 | 1.40 ± 0.07 | 0.15 ± 0.01 | 90.65 ± 2.99 | 79.62 ± 4.68 | 0.91 ± 0.06 | 76.11 ± 1.40 | 89.99 ± 4.59 | 3.10 ± 6.23 |
| Hom-sib | 4.25 ± 0.09 | 4.63 ± 0.31 | 5.32 ± 0.31 | 112.89 ± 2.80 | 38.19 ± 3.12 | 40.66 ± 2.28 | 42.13 ± 3.20 | 30.91 ± 1.73 | 2.74 ± 0.28 |
| Chl-sib | 2.44 ± 0.24 | 1.65 ± 0.13 | 1.41 ± 0.09 | 598.82 ± 48.50 | 79.47 ± 7.15 | 3.46 ± 0.12 | 93.07 ± 2.04 | 32.12 ± 1.46 | 0.53 ± 0.06 |
| Glibe | 8.64 ± 0.28 | 0.19 ± 0.01 | 0.35 ± 0.02 | 46.85 ± 2.67 | 0.96 ± 0.07 | 0.54 ± 0.05 | 27.61 ± 0.78 | 1.01 ± 0.02 | 0.34 ± 0.03 |
| N-sib | 10.70 ± 0.55 | 3.36 ± 0.13 | 0.20 ± 0.01 | 1154.50 ± 71.58 | 549.91 ± 25.10 | 0.09 ± 0.01 | 426.60 ± 37.54 | 203.64 ± 14.60 | 4.36 ± 0.27 |
| N, N-sib | 5.84 ± 0.45 | 1.00 ± 0.10 | / | 1611.96 ± 53.26 | 696.99 ± 80.30 | / | 35.48 ± 0.95 | 59.70 ± 4.90 | 1.25 ± 0.17 |
| Sib | 7.51 ± 0.51 | 3.66 ± 0.41 | 0.80 ± 0.08 | 1016.98 ± 56.95 | 273.28 ± 36.07 | 0.31 ± 0.01 | 218.84 ± 9.63 | 156.23 ± 9.70 | 2.09 ± 0.33 |
| Glim | 1.46 ± 0.11 | / | / | 27.57 ± 1.90 | 0.53 ± 0.05 | 0.08 ± 0.01 | 15.38 ± 1.21 | 0.41 ± 0.02 | 0.27 ± 0.03 |
| Spa | 1.01 ± 0.04 | / | / | 82.99 ± 4.89 | 6.68 ± 0.39 | 1.67 ± 0.07 | 11.06 ± 0.75 | 2.07 ± 0.09 | 1.40 ± 0.11 |
| Nic | 1.27 ± 0.08 | 0.08 ± | 1.02 ± 0.05 | 47.59 ± 3.43 | 1.72 ± 0.11 | 0.62 ± 0.03 | 39.28 ± 3.02 | 2.02 ± 0.07 | 1.65 ± 0.14 |
| Nim | 0.62 ± 0.02 | / | / | 21.60 ± 0.56 | 1.11 ± 0.06 | / | 29.53 ± 2.21 | 0.82 ± 0.04 | / |
| Min | 0.48 ± 0.02 | / | / | 2.40 ± 0.20 | 0.47 ± 0.03 | 0.34 ± 0.02 | 1.70 ± 0.12 | / | / |
| Mev | 0.60 ± 0.05 | / | / | 6.18 ± 0.29 | / | / | 2.26 ± 0.15 | / | / |
| Nit | / | / | / | 17.65 ± 1.54 | 1.03 ± 0.07 | / | 36.84 ± 4.05 | 1.22 ± 0.06 | / |
| Nis | / | / | / | 4.70 ± 0.23 | / | / | / | / | / |
| Glic | / | / | / | 0.41 ± 0.01 | / | / | / | / | / |
| Dan | / | / | / | / | / | / | / | / | / |
| Oreton | / | / | / | / | / | / | / | / | / |
| Cor | / | / | / | / | / | / | / | / | / |
| Ind | / | / | / | / | / | / | / | / | / |
| Lov | / | / | / | / | / | / | / | / | / |
| Drug | 0.5 ng/mL | 5 ng/mL | 50 ng/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Radish | Buckwheat | Okra | Radish | Buckwheat | Okra | Radish | Buckwheat | Okra | |
| Ama | 0.76 | 0.06 | 2.15 | 0.52 | 0.05 | 1.98 | 0.43 | 0.11 | 2.11 |
| Dip | 0.54 | 0.09 | 0.66 | 0.44 | 0.09 | 0.84 | 0.34 | 0.46 | 0.97 |
| Chl | 0.78 | 0.30 | 1.21 | 0.98 | 0.29 | 1.51 | 0.65 | 0.38 | 1.30 |
| Car | / | 0.67 | 1.09 | 0.24 | 0.54 | 1.07 | 0.25 | 0.89 | 1.22 |
| Sib | / | 0.33 | 0.34 | 0.75 | 0.10 | 0.83 | 0.59 | 0.27 | 0.72 |
| Hom-sib | 7.32 | 0.42 | 0.72 | 3.13 | 0.16 | 0.86 | 2.34 | 0.70 | 0.80 |
| Chl-sib | 1.12 | 0.21 | 0.64 | 1.17 | 0.08 | 0.69 | 1.25 | 0.14 | 0.35 |
| N-sib | 0.77 | 0.19 | 0.14 | 0.48 | 0.13 | 0.77 | 0.33 | 0.48 | 0.49 |
| N, N-sib | / | 0.13 | 1.18 | 0.33 | 0.17 | 2.13 | 0.17 | 0.43 | 1.72 |
| Glibe | / | 0.26 | 0.19 | / | 0.19 | 0.07 | 0.06 | 0.03 | 0.05 |
| Nic | / | 0.46 | 0.12 | / | 0.22 | 0.10 | 0.86 | 0.05 | 0.09 |
| Flu | / | 0.05 | / | / | 0.05 | 0.19 | 0.05 | 0.06 | 0.13 |
| Spa | / | / | 0.36 | / | 0.01 | 0.39 | / | 0.10 | 0.31 |
| Rep | / | 0.08 | / | / | 0.04 | 0.56 | 0.08 | 0.04 | 0.25 |
| Ato | / | / | / | / | / | 0.03 | 0.03 | 0.02 | 0.02 |
| Nit | / | / | / | / | / | 0.07 | / | 0.06 | 0.03 |
| Nim | / | / | / | / | / | 0.14 | / | 0.05 | 0.03 |
| Glim | / | / | / | / | / | 0.07 | / | 0.02 | 0.04 |
| Min | / | / | / | / | / | / | / | 0.34 | / |
| Glic | / | / | / | / | / | / | / | / | / |
| Nis | / | / | / | / | / | / | / | / | / |
| Dan | / | / | / | / | / | / | / | / | / |
| Oreton | / | / | / | / | / | / | / | / | / |
| Cor | / | / | / | / | / | / | / | / | / |
| Ind | / | / | / | / | / | / | / | / | / |
| Lov | / | / | / | / | / | / | / | / | / |
| Mev | / | / | / | / | / | / | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Zhang, Y.; Zhang, H.; Wang, J.; Lian, K.; Ai, L. Uptake and Transport of Different Concentrations of PPCPs by Vegetables. Int. J. Environ. Res. Public Health 2022, 19, 15840. https://doi.org/10.3390/ijerph192315840
Zeng Y, Zhang Y, Zhang H, Wang J, Lian K, Ai L. Uptake and Transport of Different Concentrations of PPCPs by Vegetables. International Journal of Environmental Research and Public Health. 2022; 19(23):15840. https://doi.org/10.3390/ijerph192315840
Chicago/Turabian StyleZeng, Yongfu, Yiming Zhang, Haichao Zhang, Jing Wang, Kaoqi Lian, and Lianfeng Ai. 2022. "Uptake and Transport of Different Concentrations of PPCPs by Vegetables" International Journal of Environmental Research and Public Health 19, no. 23: 15840. https://doi.org/10.3390/ijerph192315840





