Effects of a Structured Multicomponent Physical Exercise Intervention on Quality of Life and Biopsychosocial Health among Chilean Older Adults from the Community with Controlled Multimorbidity: A Pre–Post Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Assessment and Variables
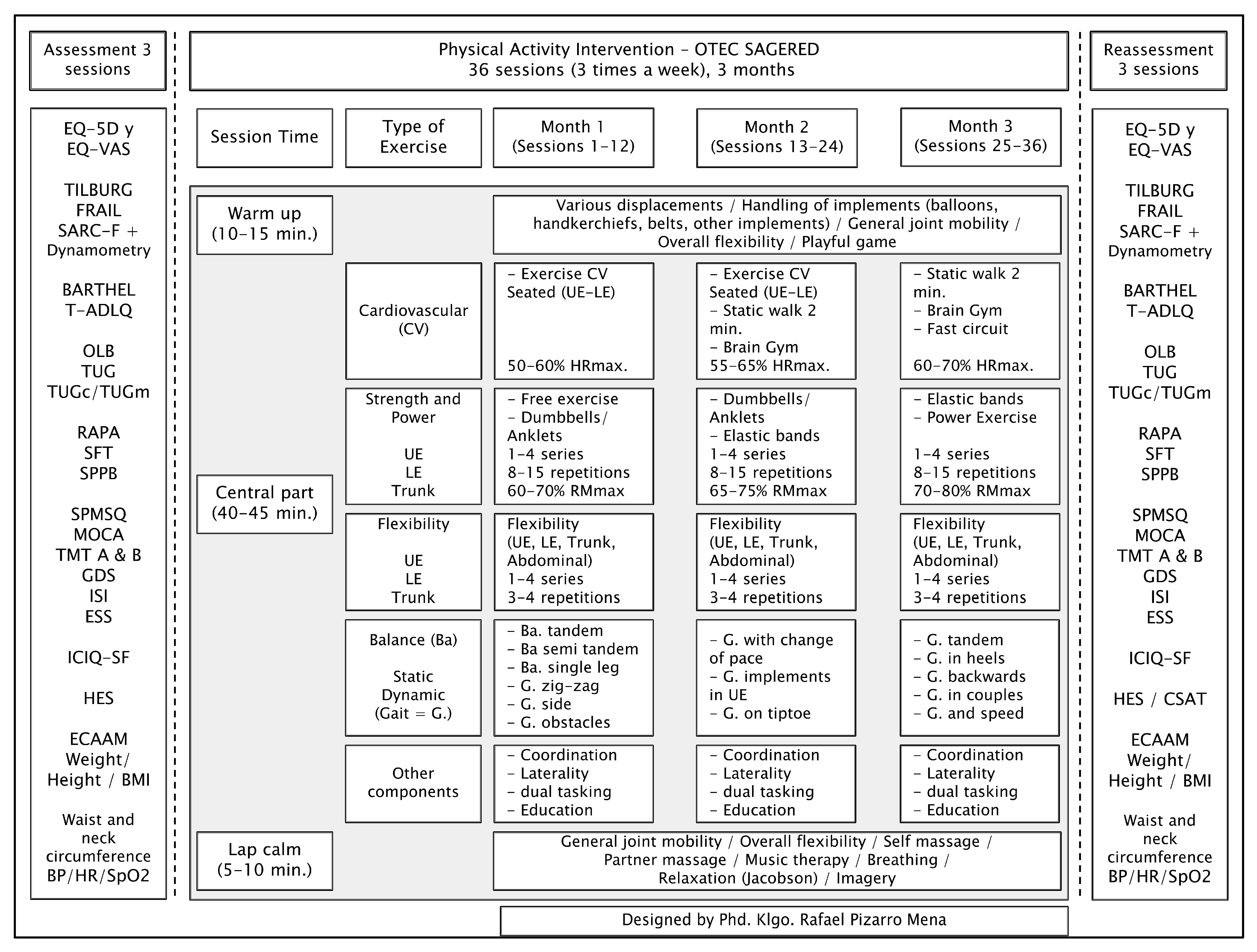
2.4. Intervention
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Implications for Future Practice
4.2. Implications for Future Research
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aging, Older People and the 2030 Agenda for Sustainable Development. Regional and Human Rights Perspective. Available online: https://repositorio.cepal.org/bitstream/handle/11362/44369/1/S1800629_es.pdf (accessed on 27 October 2022).
- Valenzuela, P.; Castillo, A.; Morales, J.; Izquierdo, M.; Serra, J.; Santos, A.; Lucia, A. Physical Exercise in the Oldest Old. Compr. Physiol. 2019, 9, 1281–1304. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.; Morley, J.; Anker, S.; Aprahamian, I.; Arai, H.; Aubertin, M.; Bernabei, R.; Cadore, E.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Figueiredo, D.; Teixeira, L.; Poveda, V.; Paúl, C.; Santos, A.; Costa, E. Physical inactivity among older adults across Europe based on the SHARE database. Age Ageing 2017, 46, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.; Roberts, C.; Thyfault, J.; Ruegsegger, G.; Toedebusch, R. Role of inactivity in chronic diseases: Evolutionary insight and pathophysiological mechanisms. Physiol. Rev. 2017, 97, 1351–1402. [Google Scholar] [CrossRef]
- Cristi, C.; Celis, C.; Ramírez, R.; Aguilar, N.; Álvarez, C.; Rodríguez, F. Sedentary behaviour and physical inactivity is not the same!: An update of concepts oriented towards the prescription of physical exercise for health. Rev. Med. Chil. 2015, 143, 1089–1090. [Google Scholar] [CrossRef][Green Version]
- Harvey, J.; Chastin, S.; Skelton, D. How Sedentary are Older People? A Systematic Review of the Amount of Sedentary Behavior. J. Aging Phys. Act. 2015, 23, 471–487. [Google Scholar] [CrossRef]
- Bouaziz, W.; Lang, P.; Schmitt, E.; Kaltenbach, G.; Geny, B.; Vogel, T. Health benefits of multicomponent training programmes in seniors: A systematic review. Int. J. Clin. Pract. 2016, 70, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Guise, J.; Chang, C.; Viswanathan, M.; Glick, S.; Treadwell, J.; Umscheid, C.; Whitlock, E.; Fu, R.; Berliner, E.; Paynter, R.; et al. Agency for Healthcare Research and Quality Evidence-based Practice Center methods for systematically reviewing complex multicomponent health care interventions. J. Clin. Epidemiol. 2014, 67, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, C.; Mesa-Gresa, P.; Redolat, R. What factors should be included in a multicomponent intervention aimed at maintaining brain health? Calid. Vida Salud 2017, 10, 23–40. [Google Scholar]
- De Resende, A.; Do Nascimento, M.; Aragão, J.; Oliveira, B.; Silva, A.; Pereira, D.; Mendes, R.; De Santana, J.; Da Silva, M. Effects of Multicomponent Training on Functional Fitness and Quality of Life in Older Women: A Randomized Controlled Trial. Int. J. Sport. Exerc. Med. 2019, 5, 126. [Google Scholar] [CrossRef]
- Pinheiro, M.; Oliveira, J.; Baldwin, J.; Hassett, L.; Costa, N.; Gilchrist, H.; Wang, B.; Kwok, W.; Albuquerque, B.; Pivotto, L.; et al. Impact of physical activity programs and services for older adults: A rapid review. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, L.; Carrasco, T.; Araya, M. Polypharmacy and deprescribing in older person. Rev. Médica Clínica Las Condes 2020, 31, 204–210. [Google Scholar] [CrossRef]
- Vásquez, E.; Solís, R.; Mahecha, S.; Zapata, R.; Cigarroa, I. Characteristics of Physical Exercise Programs for Older Adults in Latin America: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 2812. [Google Scholar] [CrossRef]
- Ministerio de Salud. Technical Guidance Program: More Self-Reliant Older Adults; Ministerio de Salud: Santiago, Chile, 2015; pp. 1–67. [Google Scholar]
- Servicio Nacional del Adulto Mayor. Operations Guide for Day Centers Community; Servicio Nacional del Adulto Mayor: Santiago, Chile, 2018; pp. 1–52. [Google Scholar]
- Bushman, B. Determining the i (Intensity) for a FITT-VP aerobic exercise prescription. ACSM’s Heal. Fit. J. 2014, 18, 4–7. [Google Scholar] [CrossRef]
- Menendez, M.; Brochier, R. The physical and psychomotor activity in the elderly: Their contributions to active, healthy andsuccessful aging. Textos Context. (Porto Alegre) 2011, 10, 179–192. [Google Scholar]
- Verdugo, C.; Pizarro, R. Effects of physical exercise on quality of life in older people. Literature review. Memorias del Inst. Investig. en Ciencias la Salud 2022, 20, 118–134. [Google Scholar] [CrossRef]
- Kelley, N. An Integrated Conceptual Model of Quality of Life for Older Adults Based on a Synthesis of the Literature. Appl. Res. Qual. Life 2009, 4, 259–282. [Google Scholar] [CrossRef]
- Freire, N.; Cabral, N.; Marchioni, D.; Vieira, S.; De Oliveira, C. Quality of life assessment instruments for adults: A systematic review of population-based studies. Health Qual. Life Outcomes 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Zurita, J.; Márquez, H.; Miranda, G.; Villasís, M. Experimental studies: Research designs for the evaluation of interventions in clinical settings. Rev. Alerg. México 2018, 65, 178–186. [Google Scholar] [CrossRef]
- Ministerio de Salud. Update of the Manual of Geriatrics for Physicians; Ministerio de Salud: Santiago, Chile, 2019; pp. 1–444. [Google Scholar]
- Zarate, V.; Kind, P.; Valenzuela, P.; Vignau, A.; Olivares-Tirado, P.; Munoz, A. Social Valuation of EQ-5D Health States: The Chilean Case. Value Health 2011, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Rosas, O.; Cruz, E.; Parra, L.; García, A.; Contreras, L.; Szlejf, C. Cross-Cultural Adaptation and Validation of the FRAIL Scale to Assess Frailty in Mexican Adults. J. Am. Med. Dir. Assoc. 2016, 17, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Vrotsou, K.; Machón, M.; Rivas, F.; Carrasco, E.; Contreras, E.; Mateo, M.; Güell, C.; Vergara, I. Psychometric properties of the Tilburg Frailty Indicator in older Spanish people. Arch. Gerontol. Geriatr. 2018, 78, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Parra, L.; Szlejf, C.; García, A.; Malmstrom, T.; Cruz, E.; Rosas, O. Cross-Cultural Adaptation and Validation of the Spanish-Language Version of the SARC-F to Assess Sarcopenia in Mexican Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2016, 17, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Lera, L.; Ángel, B.; Sánchez, H.; Picrin, Y.; Hormazabal, M.J.; Quiero, A.; Albala, C. Validation of cut points of skeletal muscle mass index for identifying sarcopenia in chilean older people. Nutr. Hosp. 2015, 31, 1187–1197. [Google Scholar] [CrossRef]
- Mahoney, F.; Barthel, D. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Muñoz-Neira, C.; López, O.; Riveros, R.; Nuñez-Huasaf, J.; Flores, P.; Slachevsky, A. The “Technology-Activities of Daily Living Questionnaire”: A version with a technology-related subscale. Dement. Geriatr. Cogn. Disord. 2012, 33, 361–371. [Google Scholar] [CrossRef]
- Vellas, B.J.; Wayne, S.J.; Romero, L.; Baumgartner, R.N.; Rubenstein, L.Z.; Garry, P.J. One-leg balance is an important predictor of injurious falls in older persons. J. Am. Geriatr. Soc. 1997, 45, 735–738. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Shumway, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and Validation of Criterion-Referenced Clinically Relevant Fitness Standards for Maintaining Physical Independence in Later Years. Gerontologist 2013, 53, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.; Simonsick, E.; Ferrucci, L.; Glynn, R.; Berkman, L.; Blazer, D.; Scherr, P.; Wallace, R. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.; Dueñas, R.; Onís, C.; Aguado, C.; Albert, C.; Luque, R. Cross-cultural adaptation and validation of Pfeiffer’s test (Short Portable Mental Status Questionnaire [SPMSQ]) to screen cognitive impairment in general population aged 65 or older. Med. Clin. 2001, 117, 129–134. [Google Scholar] [CrossRef]
- Delgado, C.; Araneda, A.; Behrens, M. Validation of the Spanish-language version of the Montreal Cognitive Assessment test in adults older than 60 years. Neurologia 2017, 34, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L.; Squillace, M.; Ferreres, A. Baremo del Trail Making Test para Capital Federal y Gran Buenos Aires. Rev. Argentina Ciencias del Comport. 2018, 3, 54–63. [Google Scholar]
- Martínez, J.; Onís, M.; Dueñas, R.; Albert, C.; Aguado, C.; Luque, R. The Spanish version of the Yesavage abbreviatedquestionnaire (GDS) to screen depressive dysfunc -tions in patients older than 65 years. Medifam 2002, 12, 26–40. [Google Scholar]
- Sierra, J.; Guillén, V.; Santos, P. Insomnia Severity Index: Some indicators about its reliability and validity on an older adults sample. Rev. Neurol. 2008, 47, 566–570. [Google Scholar] [PubMed]
- Chica, H.; Escobar, F.; Eslava, J. Validating the Epworth sleepiness scale. Rev. Salud Publica 2007, 9, 558–567. [Google Scholar]
- Durán, S.; Candia, P.; Pizarro, R. Content validity of Food Quality Survey of Elderly (ECAAM). Nutr. Hosp. 2017, 34, 1320–1327. [Google Scholar] [CrossRef]
- Serrani, D. Elders Health Empowerment Scale. Spanish adaptation and psychometric analysis. Colomb. Med. 2014, 45, 179–185. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; Technical Report Series; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Martínez, C.; Veiga, P.; Cobo, J.; Carbajal, A. Assessment of the nutritional status of a group of people older than 50 years by means of dietary and body composition parameters. Nutr. Hosp. 2011, 26, 1081–1090. [Google Scholar] [CrossRef]
- LaBerge, R.; Vaccani, J.; Gow, R.; Gaboury, I.; Hoey, L.; Katz, S. Inter- and intra-rater reliability of neck circumference measurements in children. Pediatr. Pulmonol. 2009, 44, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Gómez-León, A.; Morales, S.; Álvarez, C. Technique for a correct measurement of blood pressure in the outpatient. Rev. la Fac. Med. 2016, 59, 49–55. [Google Scholar]
- Agiovlasitis, S.; Riebe, D.; Ehrman, J.; Liguori, G.; Magal, M.; American College of Sports Medicina. ACSM Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Bull, F.; Al-Ansari, S.; Biddle, S.; Borodulin, K.; Buman, M.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Casas, Á.; Cadore, E.; Martínez, N.; Izquierdo, M. Physical exercise in the frail elderly: An update. Rev. Esp. Geriatr. Gerontol. 2015, 50, 74–81. [Google Scholar] [CrossRef]
- Izquierdo, M. Multicomponent physical exercise program: Vivifrail. Nutr. Hosp. 2019, 36, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.; Valencia, W. Exercise and Older Adults. Clin. Geriatr. Med. 2018, 34, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Leiva, A.; Troncoso, C.; Martínez, M.; Nazar, G.; Concha, Y.; Martorell, M.; Ramírez, K.; Petermann, F.; Cigarroa, I.; Díaz, X.; et al. Older people in Chile: The new social, economic and health challenge for the 21st century. Rev. Med. Chil. 2020, 148, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription, 9th Ed. 2014. J. Can. Chiropr. Assoc. 2014, 58, 328. [Google Scholar]
- Moreno, P.; Muñoz, C.; Pizarro, R.; Jiménez, S. Effects of physical exercise on sleep quality, insomnia, and daytime sleepiness in the elderly. A literature review. Rev. Esp. Geriatr. Gerontol. 2020, 55, 42–49. [Google Scholar] [CrossRef]
- National Institute on Aging. Exercise and Physical Activity; National Institute on Aging: Bethesda, MD, USA, 2010; pp. 1–132. [Google Scholar]
- Bigarella, L.; Ballotin, V.; Mazurkiewicz, L.; Ballardin, A.; Rech, D.; Bigarella, R.; Selistre, L. Exercise for depression and depressive symptoms in older adults: An umbrella review of systematic reviews and Meta-analyses. Aging Ment. Health 2022, 26, 1503–1513. [Google Scholar] [CrossRef]
- Jia, Y.; Yao, Y.; Zhuo, L.; Chen, X.; Yan, C.; Ji, Y.; Tao, J.; Zhu, Y. Aerobic Physical Exercise as a Non-medical Intervention for Brain Dysfunction: State of the Art and Beyond. Front. Neurol. 2022, 13, 862078. [Google Scholar] [CrossRef] [PubMed]
- Kameniar, K.; Mackintosh, S.; Van Kessel, G.; Kumar, S. The Psychometric Properties of the Short Physical Performance Battery to Assess Physical Performance in Older Adults: A Systematic Review. J. Geriatr. Phys. Ther. 2022. [Google Scholar] [CrossRef]
- Fishleder, S.; Petrescu, M.; Harris, J.; Leroux, B.; Bennett, K.; Helfrich, C.; Kohn, M.; Hannon, P. Predictors of Improvement in Physical Function in Older Adults in an Evidence-Based Physical Activity Program (EnhanceFitness). J. Geriatr. Phys. Ther. 2019, 42, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Criss, M.; Wingood, M.; Staples, W.; Southard, V.; Miller, K.; Norris, T.; Avers, D.; Ciolek, C.; Lewis, C.; Strunk, E. APTA Geriatrics’ Guiding Principles for Best Practices in Geriatric Physical Therapy: An Executive Summary. J. Geriatr. Phys. Ther. 2022, 45, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud. Technical Guidance: Physical Activity and Exercise according to Life Course and Comorbidity. Part 2; Ministerio de Salud: Santiago, Chile, 2021; pp. 1–34. [Google Scholar]
- Bjornsdottir, G.; Arnadottir, S.; Halldorsdottir, S. Physical Activity of Older Women Living in Retirement Communities: Capturing the Whole Picture through an Ecological Approach. J. Geriatr. Phys. Ther. 2021, 44, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Geriatría. Guide of Instruments Evaluation of Capacity Functional; Instituto Nacional de Geriatría: Mexico City, Mexico, 2022; pp. 1–227. [Google Scholar]
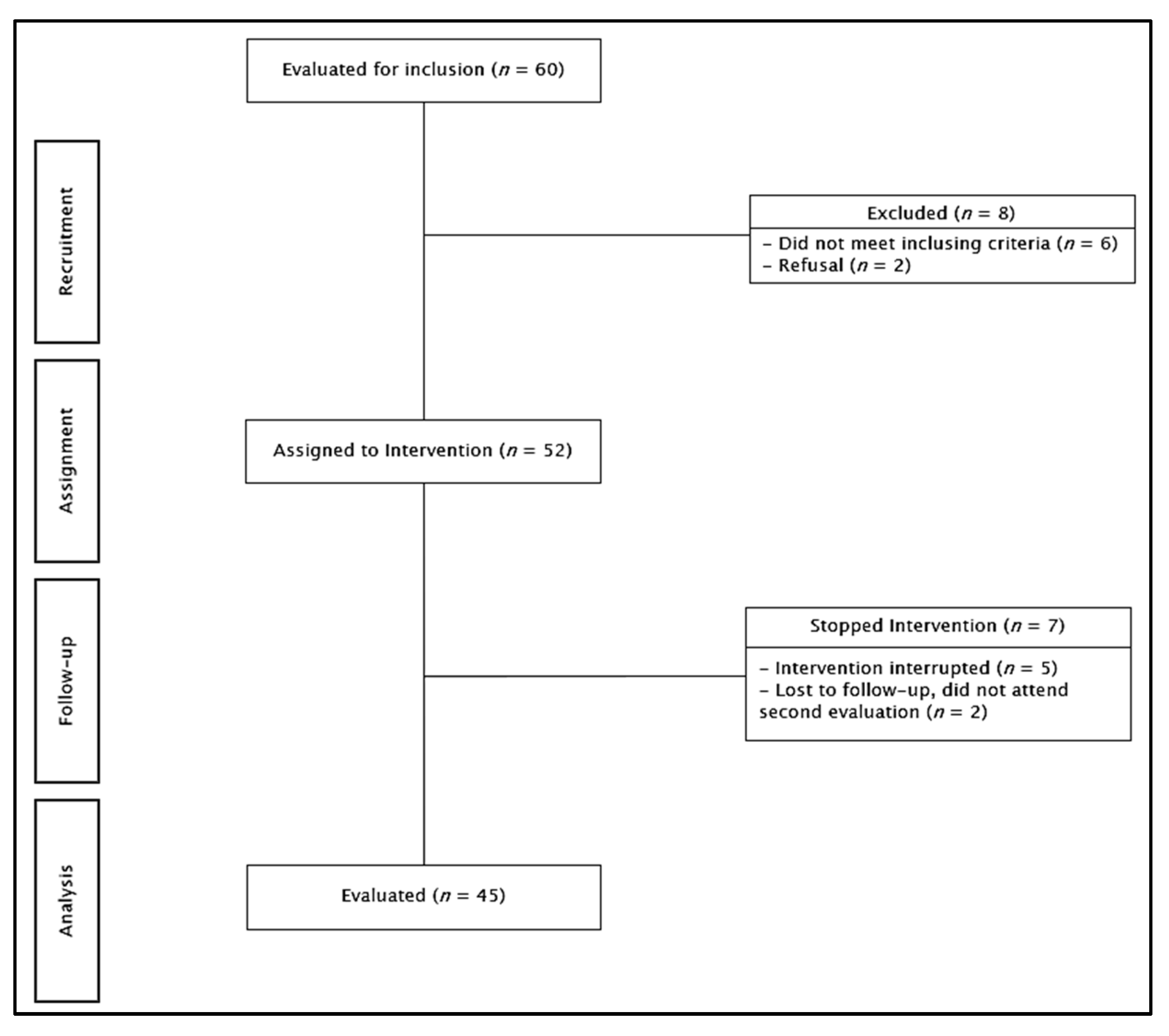
| Participants n = 45 | |||||
|---|---|---|---|---|---|
| n | Mean | SD | Min | Max | |
| Age | 45 | 70.74 | 7.7 | 60 | 87 |
| Gender | |||||
| Female (%) | 37 | 82.2 | |||
| Male (%) | 8 | 17.8 | |||
| Education | |||||
| Primary (%) | 6 | 13.3 | |||
| High school (%) | 13 | 28.9 | |||
| University (%) | 24 | 53.4 | |||
| No response | 2 | 4.4 | |||
| Marital Status | |||||
| Married (%) | 19 | 42.2 | |||
| Divorced (%) | 4 | 8.9 | |||
| Single (%) | 8 | 17.8 | |||
| Widow/widower (%) | 12 | 26.7 | |||
| No response | 2 | 4.4 | |||
| Use of cane | |||||
| Yes (%) | 1 | 2.2 | |||
| No (%) | 42 | 93.3 | |||
| No response | 2 | 4.5 | |||
| Number of comorbidities (list of 31) | 43 | 4.51 | 2.70 | 0 | 12 |
| No response | 2 | ||||
| Number of medications (list of 29) | 43 | 3.05 | 2.45 | 0 | 10 |
| No response | 2 | ||||
| Laterality | |||||
| Right | 42 | 93.33 | |||
| Left | 3 | 6.67 | |||
| Falls | |||||
| Number of falls in last year | 43 | 0.56 | 0.96 | 0 | 4 |
| No response | 2 | ||||
| Participants n = 45 | |||||
|---|---|---|---|---|---|
| Variables | Pre-Intervention Score | Post-Intervention Score | Change in Score | p-Value | Effect Size |
| Mean (SD) | Mean (SD) | ||||
| Quality of life (EQ-VAS) | 80.13 (21.31) | 87.93 (11.21) | 7.80 (3.22) | 0.020 * | 0.45 |
| Biopsychosocial frailty (TILBURG) | 4.36 (2.76) | 3.22 (2.19) | −1.13 (2.40) | 0.003 * | −0.45 |
| Sarcopenia (SARC-F) | 1.87 (1.74) | 1.22 (1.35) | −0.64 (1.28) | 0.002 * | −0.41 |
| Biological frailty (FRAIL) | 0.71 (0.94) | 0.36 (0.57) | −0.36 (0.96) | 0.017 * | −0.45 |
| Functionality: basic activities of daily living (BARTHEL) | 97.20 (0.75) | 99 (0.44) | 1.77 (0.83) | 0.037 * | 0.43 |
| Functionality: basic, instrumental, and advanced activities of daily living (T-ADLQ total) | 15.80 (10.43) | 13.8 (9.80) | −2.06 (5.62) | 0.021 * | −0.20 |
| Cognitive status (SPMSQ) | 8.91 (0.85) | 8.98 (0.97) | 0.07 (0.91) | 0.627 | |
| Cognitive status (MOCA) | 23.02 (4.70) | 24.41 (4.15) | 1.45 (3.43) | 0.007 * | 0.32 |
| Attention and psychomotor speed (TMT A) | 56.97 (20.69) | 56.36 (23.22) | −0.61 (20.31) | 0.841 | |
| Attention and psychomotor speed (TMT B) | 154.27 (97.21) | 143.73 (95.54) | −10.54 (42.90) | 0.106 | |
| Mood (GDS) | 2.02 (1.84) | 1.05 (1.28) | −0.98 (1.56) | 0.002 * | −0.61 |
| Sleep: insomnia (ISI) | 6.84 (5.11) | 6.31 (4.78) | −0.53 (4.67) | 0.448 | |
| Sleep: daytime sleepiness (ESS) | 4.44 (3.10) | 4.38 (3.28) | −0.07 (3.49) | 0.899 | |
| Health empowerment (HES) | 38.02 (2.33) | 38.64 (2.05) | 0.61 (3.13) | 0.199 | |
| Food quality (ECAAM total) | 77.67 (7.66) | 79.02 (6.93) | 1.35 (5.71) | 0.118 | |
| Participants n = 45 | |||||
|---|---|---|---|---|---|
| Variables | Pre-Intervention Score | Post-Intervention Score | Change in Score | p-Value | Effect Size |
| Mean (SD) | Mean (SD) | ||||
| Systolic blood pressure (seated) | 135.89 (17.47) | 124.16 (18.63) | −11.73 (15.42) | <0.001 * | −0.65 |
| Diastolic blood pressure (seated) | 75.33 (12.47) | 68.78 (10.29) | −6.56 (12.83) | 0.001 * | −0.57 |
| Systolic blood pressure (standing) | 131.78 (20.07) | 123.53 (17.29) | −8.24 (18.08) | 0.004 * | −0.44 |
| Diastolic blood pressure (standing) | 79.22 (9.44) | 77.11 (9.58) | −2.11 (8.50) | 0.103 | |
| Heart rate (seated) | 73.18 (11.61) | 75.56 (11.15) | 2.38 (10.49) | 0.136 | |
| Heart rate (standing) | 77.56 (13.09) | 80.60 (12.23) | 3.04 (10.25) | 0.053 | |
| Oxygen saturation (seated) | 96.13 (1.95) | 96.04 (1.72) | −0.09 (1.92) | 0.757 | |
| Oxygen saturation (standing) | 96.53 (1.71) | 96.07 (2.56) | −0.47 (2.38) | 0.195 | |
| Static balance (OLB-R (s)) | 24.72 (22.33) | 23.75 (19.20) | −0.97 (14.54) | 0.658 | |
| Static balance (OLB-L (s)) | 23.18 (20.06) | 25.17 (21.03) | 1.99 (16.19) | 0.413 | |
| Dynamic balance (TUG (s)) | 10.01 (2.56) | 9.42 (2.62) | −0.59 (1.97) | 0.052 | |
| Dynamic balance (TUGc: cognitive task (s)) | 14.27 (5.57) | 12.20 (3.76) | −2.07 (4.96) | 0.008 * | −0.44 |
| Dynamic balance (TUGm: functional task (s)) | 13.08 (3.48) | 12.21 (3.34) | −0.88 (2.92) | 0.051 | |
| Anthropometry | |||||
| Weight (kg) | 68.41 (11.11) | 67.50 (11.21) | −0.91 (2.47) | 0.018 * | −0.08 |
| Height (m) | 1.56 (0.08) | 1.56 (0.08) | 0.003 (0.01) | 0.193 | |
| BMI | 28.28 (4.23) | 27.79 (4.09) | −0.49 (1.20) | 0.008 * | −0.12 |
| Waist circumference (cm) | 94.08 (14.20) | 95.72 (11.92) | 1.64 (10.16) | 0.286 | |
| Neck circumference (cm) | 36.82 (10.36) | 35.23 (4.44) | −1.59 (7.94) | 0.186 | |
| Dynamometry | |||||
| Sarcopenia (grip strength—R) | 21.58 (6.37) | 21.86 (5.88) | 0.28 (2.85) | 0.514 | |
| Sarcopenia (grip strength—L) | 20.48 (5.93) | 20.26 (5.97) | −0.23 (3.48) | 0.661 | |
| Participants n = 45 | |||||
|---|---|---|---|---|---|
| Variables | Pre-Intervention Score | Post-Intervention Score | Change in Score | p-Value | Effect Size |
| Mean (SD) | Mean (SD) | ||||
| SPPB | |||||
| Side-by-side stand (s) | 10.00 (0.00) | 10.00 (0.00) | |||
| Semi-tandem stand (s) | 9.80 (0.90) | 10.00 (0.00) | 0.20 (0.90) | 0.135 | |
| Full tandem stand (s) | 8.24 (3.47) | 9.28 (2.13) | 1.04 (3.28) | 0.040 * | 0.36 |
| 4 m walking speed test (s) | 3.37 (0.72) | 3.36 (0.84) | −0.01 (0.87) | 0.913 | |
| Sit down and stand up 5 times test (s) | 14.05 (4.68) | 10.76 (3.28) | −3.29 (3.43) | <0.001 * | −0.81 |
| SPPB score (0–12) | 10.02 (0.29) | 11.13 (0.23) | 1.11 (0.25) | <0.001 * | 0.63 |
| SFT | |||||
| Chair stand test (n repetitions) | 10.58 (4.18) | 12.07 (3.12) | 1.49 (4.09) | 0.019 * | 0.40 |
| Arm curl test (n repetitions) | 13.51 (4.51) | 15.09 (3.70) | 1.58 (4.03) | 0.012 * | 0.38 |
| 2 min step test (n repetitions) | 72.09 (20.05) | 83.31 (20.15) | 11.22 (16.91) | <0.001 * | 0.55 |
| Chair sit and reach test—preferred LE (cm) | −6.76 (8.78) | −5.51 (8.98) | 1.24 (2.39) | 0.001 * | 0.14 |
| Back scratch test—preferred UE (cm) | −13.83 (10.87) | −11.65 (10.92) | 2.18 (5.50) | 0.011 * | 0.20 |
| 8 foot up and go test (s) | 6.97 (2.09) | 6.07 (1.90) | −0.90 (1.23) | <0.001 * | −0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizarro-Mena, R.; Duran-Aguero, S.; Parra-Soto, S.; Vargas-Silva, F.; Bello-Lepe, S.; Fuentes-Alburquenque, M. Effects of a Structured Multicomponent Physical Exercise Intervention on Quality of Life and Biopsychosocial Health among Chilean Older Adults from the Community with Controlled Multimorbidity: A Pre–Post Design. Int. J. Environ. Res. Public Health 2022, 19, 15842. https://doi.org/10.3390/ijerph192315842
Pizarro-Mena R, Duran-Aguero S, Parra-Soto S, Vargas-Silva F, Bello-Lepe S, Fuentes-Alburquenque M. Effects of a Structured Multicomponent Physical Exercise Intervention on Quality of Life and Biopsychosocial Health among Chilean Older Adults from the Community with Controlled Multimorbidity: A Pre–Post Design. International Journal of Environmental Research and Public Health. 2022; 19(23):15842. https://doi.org/10.3390/ijerph192315842
Chicago/Turabian StylePizarro-Mena, Rafael, Samuel Duran-Aguero, Solange Parra-Soto, Francisco Vargas-Silva, Sebastian Bello-Lepe, and Mauricio Fuentes-Alburquenque. 2022. "Effects of a Structured Multicomponent Physical Exercise Intervention on Quality of Life and Biopsychosocial Health among Chilean Older Adults from the Community with Controlled Multimorbidity: A Pre–Post Design" International Journal of Environmental Research and Public Health 19, no. 23: 15842. https://doi.org/10.3390/ijerph192315842
APA StylePizarro-Mena, R., Duran-Aguero, S., Parra-Soto, S., Vargas-Silva, F., Bello-Lepe, S., & Fuentes-Alburquenque, M. (2022). Effects of a Structured Multicomponent Physical Exercise Intervention on Quality of Life and Biopsychosocial Health among Chilean Older Adults from the Community with Controlled Multimorbidity: A Pre–Post Design. International Journal of Environmental Research and Public Health, 19(23), 15842. https://doi.org/10.3390/ijerph192315842








