ACL Reconstruction: Which Additional Physiotherapy Interventions Improve Early-Stage Rehabilitation? A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Design
2.2. Search Strategy
2.3. Study Selection and Eligibility Criteria
2.4. Data Extraction and Synthesis
2.5. Evaluation of Study Quality and Risk of Bias
3. Results
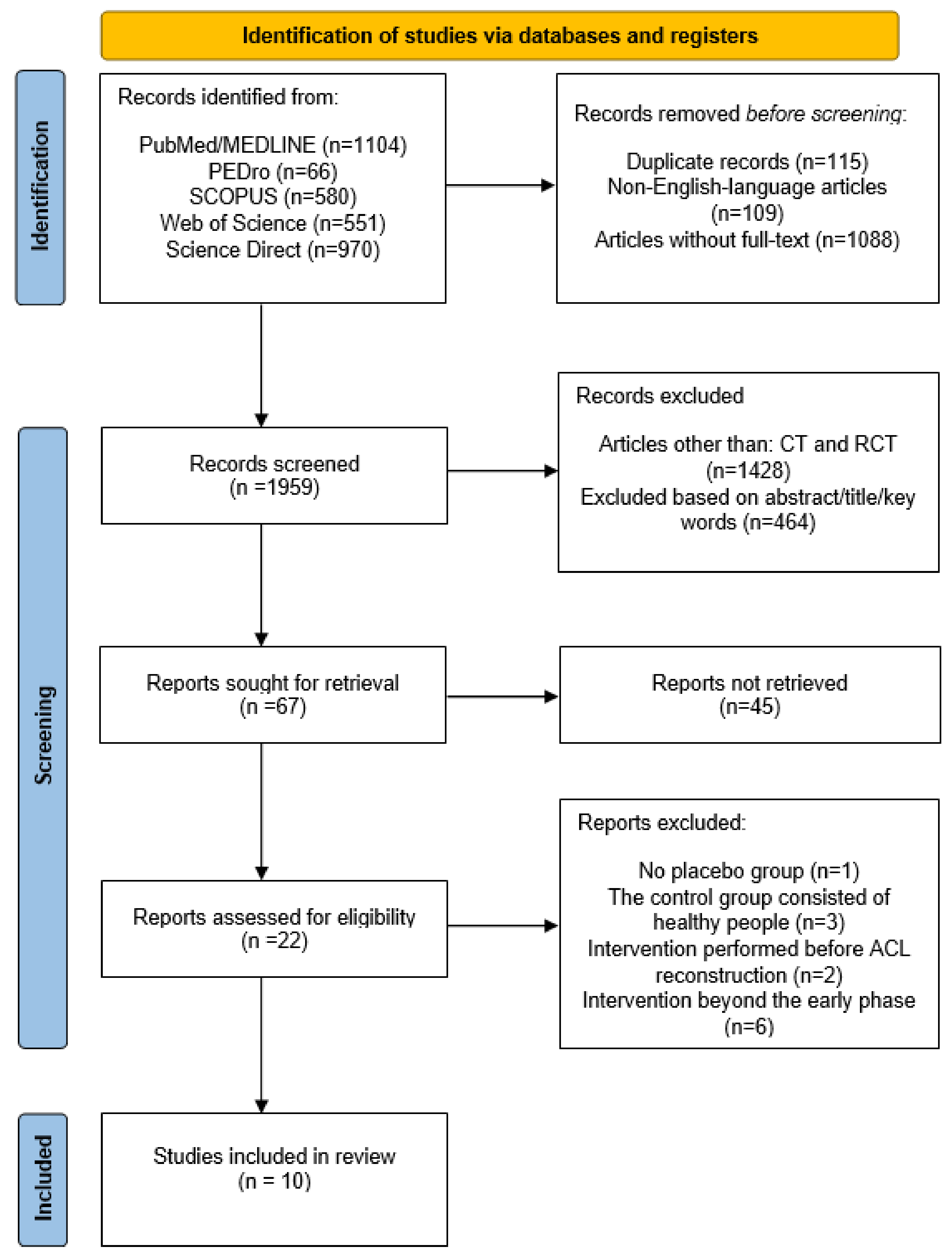
3.1. Selection of Included Studies
3.2. Characteristics of the Included Studies
3.3. Data Synthesis and Certainty of Evidence
3.4. Quality and Risk of Bias Assessment of the Included Studies
3.5. Participants
3.6. Intervention
3.7. Comparison
3.8. Outcomes
3.9. Summary of Reviewed Studies
3.10. Kinesio Taping
3.11. Vibration Training
3.12. Miscellaneous Interventions
4. Discussion
4.1. Practical Implications
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| PUBMED/MEDLINE: (ACL OR (anterior cruciate ligament) OR reconstruction OR (injury AND (repair OR surgery))) AND (rehabilitation OR physiotherapy OR (physical therapy) OR training OR exercise OR intervention OR treatment OR (standard rehabilitation program)) AND ((early phase) OR (acute phase) OR (initial phase)) AND (pain OR edema OR swelling OR (range of motion) OR ROM OR (muscle strength) OR (knee function) OR (knee activity)) WEB OF SCIENCE: (ACL OR (anterior cruciate ligament) OR reconstruction OR (injury AND (repair OR surgery))) AND (rehabilitation OR physiotherapy OR (physical therapy) OR training OR exercise OR intervention OR treatment OR (standard rehabilitation program)) AND ((early phase) OR (acute phase) OR (initial phase)) AND (pain OR edema OR swelling OR (range of motion) OR ROM OR (muscle strength) OR (knee function) OR (knee activity)) SCOPUS: (ACL OR (anterior cruciate ligament) OR reconstruction OR (injury AND (repair OR surgery))) AND (rehabilitation OR physiotherapy OR (physical therapy) OR training OR exercise OR intervention OR treatment OR (standard rehabilitation program)) AND ((early phase) OR (acute phase) OR (initial phase)) AND (pain OR edema OR swelling OR (range of motion) OR ROM OR (muscle strength) OR (knee function) OR (knee activity)) SCIENCE DIRECT: (ACL reconstruction) AND (rehabilitation OR physiotherapy) AND (early phase OR acute phase) AND (pain OR range of motion OR edema OR strength)))) |
References
- Diermeier, T.A.; Rothrauff, B.B.; Engebretsen, L.; Lynch, A.; Svantesson, E.; Hamrin Senorski, E.A.; Meredith, S.J.; Rauer, T.; Ayeni, O.R.; Paterno, M.; et al. Treatment after ACL Injury: Panther Symposium ACL Treatment Consensus Group. Br. J. Sport. Med. 2021, 55, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Van Melick, N.; van Cingel, R.E.H.; Brooijmans, F.; Neeter, C.; van Tienen, T.; Hullegie, W.; Nijhuis-van der Sanden, M.W.G. Evidence-Based Clinical Practice Update: Practice Guidelines for Anterior Cruciate Ligament Rehabilitation Based on a Systematic Review and Multidisciplinary Consensus. Br. J. Sport. Med. 2016, 50, 1506–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepulveda, F.; Sanchez, L.; Amy, E.; Micheo, W. Anterior Cruciate Ligament Injury: Return to Play, Function and Long-Term Considerations. Curr. Sport. Med. Rep. 2017, 16, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.G.; Kim, B.H.; Kang, T.K.; Jeong, H.S.; Lee, S.Y. Determination of the Strongest Factor and Component in a Relationship between Lower-Extremity Assessment Protocol and Patient-Oriented Outcomes in Individuals with Anterior Cruciate Ligament Reconstruction: A Pilot Study. Int. J. Environ. Res. Public. Health 2021, 18, 8053. [Google Scholar] [CrossRef] [PubMed]
- Czamara, A.; Krzemińska, K.; Widuchowski, W.; Dragan, S.L. The Muscle Strength of the Knee Joint after ACL Reconstruction Depends on the Number and Frequency of Supervised Physiotherapy Visits. Int. J. Environ. Res. Public. Health 2021, 18, 10588. [Google Scholar] [CrossRef]
- Martimbianco, A.L.C.; Gomes da Silva, B.N.; de Carvalho, A.P.V.; Silva, V.; Torloni, M.R.; Peccin, M.S. Effectiveness and Safety of Cryotherapy after Arthroscopic Anterior Cruciate Ligament Reconstruction. A Systematic Review of the Literature. Phys. Ther. Sport 2014, 15, 261–268. [Google Scholar] [CrossRef]
- Lindström, M.; Wredmark, T.; Wretling, M.-L.; Henriksson, M.; Felländer-Tsai, L. Post-Operative Bracing after ACL Reconstruction Has No Effect on Knee Joint Effusion. A Prospective, Randomized Study. Knee 2015, 22, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.C.; Villwock, M.; Wojtys, E.M.; Palmieri-Smith, R.M. Lower Extremity Muscle Strength After Anterior Cruciate Ligament Injury and Reconstruction. J. Athl. Train. 2013, 48, 610–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logerstedt, D.; Lynch, A.; Axe, M.J.; Snyder-Mackler, L. Pre-Operative Quadriceps Strength Predicts IKDC2000 Scores 6months after Anterior Cruciate Ligament Reconstruction. Knee 2013, 20, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Ribeiro, D.C.; Theis, J.-C.; Webster, K.E.; Sole, G. Movement Patterns of the Knee During Gait Following ACL Reconstruction: A Systematic Review and Meta-Analysis. Sport. Med. 2016, 46, 1869–1895. [Google Scholar] [CrossRef] [PubMed]
- Augustsson, J. Documentation of Strength Training for Research Purposes after ACL Reconstruction. Knee Surg. Sport. Traumatol. Arthrosc. 2013, 21, 1849–1855. [Google Scholar] [CrossRef]
- Perriman, A.; Leahy, E.; Semciw, A.I. The Effect of Open- Versus Closed-Kinetic-Chain Exercises on Anterior Tibial Laxity, Strength, and Function Following Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. J. Orthop. Sport. Phys. Ther. 2018, 48, 552–566. [Google Scholar] [CrossRef]
- Kruse, L.M.; Gray, B.; Wright, R.W. Rehabilitation After Anterior Cruciate Ligament Reconstruction: A Systematic Review. J. Bone Jt. Surg. 2012, 94, 1737–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewiss, D.; Ostman, C.; Smart, N. Open versus Closed Kinetic Chain Exercises Following an Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. J. Sport. Med. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Janssen, R.P.A.; van Melick, N.; van Mourik, J.B.A.; Reijman, M.; van Rhijn, L.W. ACL Reconstruction with Hamstring Tendon Autograft and Accelerated Brace-Free Rehabilitation: A Systematic Review of Clinical Outcomes. BMJ Open Sport Exerc. Med. 2018, 4, e000301. [Google Scholar] [CrossRef]
- Carter, H.M.; Littlewood, C.; Webster, K.E.; Smith, B.E. The Effectiveness of Preoperative Rehabilitation Programmes on Postoperative Outcomes Following Anterior Cruciate Ligament (ACL) Reconstruction: A Systematic Review. BMC Musculoskelet. Disord. 2020, 21, 647. [Google Scholar] [CrossRef] [PubMed]
- Giesche, F.; Niederer, D.; Banzer, W.; Vogt, L. Evidence for the Effects of Prehabilitation before ACL-Reconstruction on Return to Sport-Related and Self-Reported Knee Function: A Systematic Review. PLoS ONE 2020, 15, e0240192. [Google Scholar] [CrossRef]
- Lu, Y.; Patel, B.H.; Kym, C.; Nwachukwu, B.U.; Beletksy, A.; Forsythe, B.; Chahla, J. Perioperative Blood Flow Restriction Rehabilitation in Patients Undergoing ACL Reconstruction: A Systematic Review. Orthop. J. Sport. Med. 2020, 8, 232596712090682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckenrode, B.J.; Carey, J.L.; Sennett, B.J.; Zgonis, M.H. Prevention and Management of Post-Operative Complications Following ACL Reconstruction. Curr. Rev. Musculoskelet. Med. 2017, 10, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Loyd, B.J.; Stackhouse, S.; Dayton, M.; Hogan, C.; Bade, M.; Stevens-Lapsley, J. The Relationship between Lower Extremity Swelling, Quadriceps Strength, and Functional Performance Following Total Knee Arthroplasty. The Knee 2019, 26, 382–391. [Google Scholar] [CrossRef]
- Brown, D.W.; Curry, C.M.; Ruterbories, L.M.; Avery, F.L.; Anson, P.S. Evaluation of Pain After Arthroscopically Assisted Anterior Cruciate Ligament Reconstruction. Am. J. Sport. Med. 1997, 25, 182–186. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Ardern, C.L.; Büttner, F.; Andrade, R.; Weir, A.; Ashe, M.C.; Holden, S.; Impellizzeri, F.M.; Delahunt, E.; Dijkstra, H.P.; Mathieson, S.; et al. Implementing the 27 PRISMA 2020 Statement Items for Systematic Reviews in the Sport and Exercise Medicine, Musculoskeletal Rehabilitation and Sports Science Fields: The PERSiST (Implementing Prisma in Exercise, Rehabilitation, Sport Medicine and Sports Science) Guidance. Br. J. Sport. Med. 2022, 56, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without Meta-Analysis (SWiM) in Systematic Reviews: Reporting Guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulet-Pelletier, J.-C.; Cousineau, D. A Review of Effect Sizes and Their Confidence Intervals, Part I: The Cohen’s d Family. Quant. Methods Psychol. 2018, 14, 242–265. [Google Scholar] [CrossRef]
- Brakspear, L.; Boules, D.; Nicholls, D.; Burmester, V. The Impact of COVID-19-Related Living Restrictions on Eating Behaviours in Children and Adolescents: A Systematic Review. Nutrients 2022, 14, 3657. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Balki, S.; Göktaş, H.E.; Öztemur, Z. Kinesio Taping as a Treatment Method in the Acute Phase of ACL Reconstruction: A Double-Blind, Placebo-Controlled Study. Acta Orthop. Traumatol. Turc. 2016, 50, 628–634. [Google Scholar] [CrossRef]
- Laborie, M.; Klouche, S.; Herman, S.; Gerometta, A.; Lefevre, N.; Bohu, Y. Inefficacy of Kinesio-Taping® on Early Postoperative Pain after ACL Reconstruction: Prospective Comparative Study. Orthop. Traumatol. Surg. Res. 2015, 101, 963–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labianca, L.; Andreozzi, V.; Princi, G.; Princi, A.A.; Calderaro, C.; Guzzini, M.; Ferretti, A. The Effectiveness of Kinesio Taping in Improving Pain and Edema during Early Rehabilitation after Anterior Cruciate Ligament Reconstruction: A Prospective, Randomized, Control Study. Acta Biomed. Atenei Parm. 2022, 92, e2021336. [Google Scholar] [CrossRef]
- Berschin, G.; Sommer, B.; Behrens, A.; Sommer, H.-M. Whole Body Vibration Exercise Protocol versus a Standard Exercise Protocol after ACL Reconstruction: A Clinical Randomized Controlled Trial with Short Term Follow-Up. J. Sport. Sci. Med. 2014, 13, 580–589. [Google Scholar] [PubMed]
- Pistone, E.; Laudani, L.; Camillieri, G.; Cagno, A.; Tomassi, G.; Macaluso, A.; Giombini, A. Effects of Early Whole-Body Vibration Treatment on Knee Neuromuscular Function and Postural Control after Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Trial. J. Rehabil. Med. 2016, 48, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Coulondre, C.; Souron, R.; Rambaud, A.; Dalmais, É.; Espeit, L.; Neri, T.; Pinaroli, A.; Estour, G.; Millet, G.Y.; Rupp, T.; et al. Local Vibration Training Improves the Recovery of Quadriceps Strength in Early Rehabilitation after Anterior Cruciate Ligament Reconstruction: A Feasibility Randomised Controlled Trial. Ann. Phys. Rehabil. Med. 2022, 65, 101441. [Google Scholar] [CrossRef]
- Velázquez-Saornil, J.; Ruíz-Ruíz, B.; Rodríguez-Sanz, D.; Romero-Morales, C.; López-López, D.; Calvo-Lobo, C. Efficacy of Quadriceps Vastus Medialis Dry Needling in a Rehabilitation Protocol after Surgical Reconstruction of Complete Anterior Cruciate Ligament Rupture. Medicine 2017, 96, e6726. [Google Scholar] [CrossRef]
- Ogrodzka-Ciechanowicz, K.; Głąb, G.; Ślusarski, J.; Gądek, A. Quadriceps Muscle Strength Recovery with the Use of High Tone Power Therapy after Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Trial. BMC Musculoskelet. Disord. 2021, 22, 975. [Google Scholar] [CrossRef]
- Ogrodzka-Ciechanowicz, K.; Głąb, G.; Ciszek-Radwan, E.; Ślusarski, J.; Gądek, A. The Use of an Alternating Magnetic Field in the Resorption of Postoperative Joint Effusion Following Anterior Cruciate Ligament Reconstruction: A Randomized Double-Blind Controlled Trial. Medicine 2021, 100, e26572. [Google Scholar] [CrossRef]
- Clausen, J.-D.; Nahen, N.; Horstmann, H.; Lasch, F.; Krutsch, W.; Krettek, C.; Weber-Spickschen, T.S. Improving Maximal Strength in the Initial Postoperative Phase After Anterior Cruciate Ligament Reconstruction Surgery: Randomized Controlled Trial of an App-Based Serious Gaming Approach. JMIR Serious Games 2020, 8, e14282. [Google Scholar] [CrossRef] [Green Version]
- Costantino, C.; Bertuletti, S.; Romiti, D. Efficacy of Whole-Body Vibration Board Training on Strength in Athletes After Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Study. Clin. J. Sport Med. 2018, 28, 339–349. [Google Scholar] [CrossRef]
- Fu, C.L.A.; Yung, S.H.P.; Law, K.Y.B.; Leung, K.H.H.; Lui, P.Y.P.; Siu, H.K.; Chan, K.M. The Effect of Early Whole-Body Vibration Therapy on Neuromuscular Control After Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Trial. Am. J. Sport. Med. 2013, 41, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Ong, M.T.-Y.; Leong, H.T.; He, X.; Fu, S.-C.; Yung, P.S.-H. Effects of Whole-Body Vibration Therapy on Quadriceps Function in Patients With Anterior Cruciate Ligament Reconstruction: A Systematic Review. Sport. Health Multidiscip. Approach 2022, 14, 216–226. [Google Scholar] [CrossRef]
- Seixas, A.; Sañudo, B.; Sá-Caputo, D.; Taiar, R.; Bernardo-Filho, M. Whole-Body Vibration for Individuals with Reconstructed Anterior Cruciate Ligament: A Systematic Review. BioMed Res. Int. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Park, S.; Jee, Y.-S. Rehabilitation Program Combined with Local Vibroacoustics Improves Psychophysiological Conditions in Patients with ACL Reconstruction. Medicina (Mex.) 2019, 55, 659. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Cebrian, S.; Luchini, N.; Whiteley, R. Dry Needling: Effects on Activation and Passive Mechanical Properties of the Quadriceps, Pain and Range during Late Stage Rehabilitation of ACL Reconstructed Patients. Phys. Ther. Sport 2016, 21, 57–62. [Google Scholar] [CrossRef] [PubMed]
| Anterior Cruciate Ligament Reconstruction | Rehabilitation | Phase | Measure |
|---|---|---|---|
| ACL Reconstruction ACLR Repair Surgery | Physiotherapy Physical therapy Training Exercise Intervention Treatment Standard rehabilitation program | Acute phase Early phase Initial postoperative phase | Pain Effusion Edema Swelling Range of motion ROM AROM Muscle strength Knee function Knee activity |
| Study | Intervention | Outcome | |||||
|---|---|---|---|---|---|---|---|
| Pain | Edema | ROM | Knee Function | Strength of Flexors | Strength of Extensors | ||
| Balki et al. (2016) [30]; RCT | KT | + | + | + (l) | - | + (l) | - |
| Laborie et al. (2015) [31]; nRCT | KT | - | |||||
| Labianca et al. (2022) [32]; RCT | KT | + (s) | + (l, t) | - | - | ||
| Berschin et al. (2014) [33]; RCT | WBV | - | - | - | - | ||
| Pistone et al. (2016) [34]; RCT | WBV | + (l) | + | - | |||
| Coulondre et al. (2021) [35]; RCT | LVT | - | + (l) | ||||
| Velázquez-Saornil et al. (2017) [36]; RCT | TrPDN | * (l) | + (s, l) | + (m, l) | |||
| Ogrodzka-Ciechanowicz et al. I (2021) [37]; RCT | HiToP | - | + (l) | + (l) | + (l) | + (l) | |
| Ogrodzka-Ciechanowicz et al. II (2021) [38]; RCT | Alternating magnetic field | - | - | ||||
| Clausen et al. (2020) [39]; RCT | App-based serious gaming | - | - | - | + (m) | ||
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Impercision | Certainty |
|---|---|---|---|---|---|---|
| Pain (assessed with: VAS) | ||||||
| 5 | randomised trials | Not Serious | Serious | Serious | Serious | ⨁◯◯◯ Very low |
| Edema (assessed with: tailor’s tape) | ||||||
| 5 | randomised trials | Not Serious | Serious | Serious | Serious | ⨁◯◯◯ Very low |
| Range of motion—ROM (assessed with: goniometer) | ||||||
| 6 | randomised trials | Serious | Serious | Serious | Serious | ⨁◯◯◯ Very low |
| Knee function (assessed with: Lysholm test/WOMAC/Tegner scale/KOOS) | ||||||
| 8 | randomised trials | Serious | Serious | Serious | Serious | ⨁◯◯◯ Very low |
| Strength of flexors (assessed with: Dynamometer/force transducer) | ||||||
| 3 | randomised trials | Serious | Not Serious | Serious | Serious | ⨁◯◯◯ Very low |
| Strength of extensors (assessed with: Dynamometer/force transducer) | ||||||
| 6 | randomised trials | Serious | Serious | Serious | Serious | ⨁◯◯◯ Very low |
| Pain (assessed with: VAS) | ||||||
| 1 | nRCT | Serious | Serious | Serious | Serious | ⨁◯◯◯ Very low |
| Balki et al. (2016) [30] | Laborie et al. (2015) [31] | Labianca et al. (2022) [32] | Berschin et al. (2014) [33] | Pistone et al. (2016) [34] | Coulondre et al. (2021) [35] | Velázquez-Saornil et al. (2017) [36] | Ogrodzka-Ciechanowicz et al. I (2021) [37] | Ogrodzka-Ciechanowicz et al. II (2021) [38] | Clausen et al. (2020) [39] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Eligibility Criteria * | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Randomly allocated | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Concealed Allocation | No | No | No | No | No | No | Yes | Yes | Yes | No |
| Similar groups at baseline | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Blinding of Subjects | Yes | No | No | No | No | No | No | No | Yes | No |
| Blinding of Therapists | No | No | No | No | No | No | No | Yes | Yes | No |
| Blinding of Assessors | Yes | No | No | No | No | No | Yes | No | No | No |
| Data from >85% of Subjects | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Intention-to-Treat Analysis | No | Yes | No | No | Yes | No | Yes | No | Yes | Yes |
| Statistical comparision | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Measures of Variability | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Final score | 7/10 | 5/10 | 4/10 | 5/10 | 6/10 | 4/10 | 8/10 | 7/10 | 9/10 | 6/10 |
| Risk of Bias for Included Randomized Trials (RoB 2) | ||||||
|---|---|---|---|---|---|---|
| Study | Randomization Process | Deviations from the Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall |
| Balki et al. (2016) [30] | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Labianca et al. (2022) [32] | Low risk | Some concerns | Low risk | Some concerns | Low risk | Some concerns |
| Berschin et al. (2014) [33] | Low risk | Some concerns | Low risk | Some concerns | Low risk | Some concerns |
| Pistone et al. (2016) [34] | Low risk | Low risk | Low risk | Some concerns | Low risk | Some concerns |
| Coulondre et al. (2022) [35] | Low risk | Some concerns | High risk | Some concerns | Low risk | High risk |
| Velázquez-Saornil et al. (2017) [36] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ogrodzka-Ciechanowicz et al. I (2021) [37] | Low risk | Some concerns | Low risk | Some concerns | Low risk | Some concerns |
| Ogrodzka-Ciechanowicz et al. II (2021) [38] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Clausen et al. (2020) [39] | Low risk | Some concerns | Low risk | Some concerns | Low risk | Some concerns |
| Risk of Bias Included Nonrandomized Trials (ROBINS-I) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Confounding | Selection of Participants | Classification of Interventions | Deviations from Intended Interventions | Missing Data | Measurement of Outcomes | Selective Reporting | Overall |
| Laborie et al. (2015) [31] | Low | Low | Moderate | Low | Moderate | Moderate | Low | Moderate |
| Author | Balki et al. (2016) [30] RCT | Laborie et al. (2015) [31] CT | Labianca et al. 2022 [32] RCT |
|---|---|---|---|
| Method of randomization | Randomization table | No randomization | Online randomizer tool |
| Study population | N = 30; Experimental group N = 15 (15M; aged 22–37; mean age 28.60 ± 4.50), Control group N = 15 (15M; aged 18–39; mean age 27.66 ± 7.45). | N = 57 (44M, 13F); Experimental group N = 28 (21M, 7F; mean age 29.2 ± 8.6); Control group N = 29 (23M, 6F; mean age 32.6 ± 9.1) | N = 52 (M; aged 18–45); Experimental group N = 26 (26M; mean age 28.5 ± 5.3); Control group N = 26 (26M; mean age 29.2 ± 4.6) |
| ACLR method | Hamstring tendon autograft and tibialis posterior or peroneus longus allograft. | Hamstring tendon graft | Gracilis and semitendinosus tendon autograft |
| Enrollment time | 4th day postoperatively | Immediately postoperatively | 2nd day postoperatively |
| Intervention | 2 muscle and lymphatic KT techniques every 5 days during a 10-day period plus 6 week rehabilitation program vs. 2 placebo KT techniques every 5 days during a 10-day period plus 6 week rehabilitation program | 3 days of muscle/lymphatic KT technique plus anesthesia-analgesia protocol vs. 3 days of anesthesia-analgesia protocol | Muscle and lymphatic KT application every 5 days for 4 weeks plus standard 4 week rehabilitation program vs. standard 4 week rehabilitation program |
| Outcome measures | On 4th day (baseline; before KT application) and after 5 and 10 days after KT application: Pain (VAS); swelling (circumferences; tailor’s tape); ROM of knee flexion and extension (goniometer); hamstring and quadriceps strength (dynamometer). Follow up after 1 and 3 months: subjective functions (modified Cincinnati (30-point), Lysholm and Tegner tests). | At baseline (in the evening and at night) and on days: 1, 2, 3 after KT application: knee pain intensity (VAS); analgesia intake, awakening due to pain, postoperative discomfort, allergic reaction to KT, overall patient satisfaction (online self-assessment survey). | At the end of 2nd and 4th postoperative weeks: pain (VAS scale), edema (girth; measured at the mid patella), muscle mass (thigh circumference; measured 10 cm above the upper edge of the patella), passive knee ROM and knee function (Tegner-Lysholm Knee Scale and Knee Injury and Osteoarthritis Outcome Score (KOOS)) |
| Main outcomes | Significant improvements in experimental vs. control group in knee swelling, pain and hamstring muscle strength (6.33 ± 1.54 vs. 5.13 ± 1.40) on the 5th day (p < 0.05); in knee flexion ROM (76.80 ± 14.85 vs. 60.13 ± 8.79), night pain, knee swelling and hamstring muscle strength (9.86 ± 2.32 vs. 7.53 ± 2.16) on the 10th day (p < 0.05) No significant improvement in experimental vs. control group in knee function, extension and extensor muscle strength (p > 0.05). | No significant difference in experimental vs. control group for knee pain intensity, evolution of pain and overall satisfaction (p > 0.05). | Significant improvement in experimental vs. control group in pain intensity at week 2 (3.2 ± 1.6 vs. 4.7 ± 1.9; p = 0.029) and edema reduction at week 2 (−6.0 ± 2.2 vs. −0.75 ± 0.5; p = 0.007) and week 4 (−7.6 ± 2.9 vs. −2.75 ± 1.4; p = 0.006). No significant improvement in experimental vs. control group in thigh circumference, ROM recovery, and knee function (p > 0.05) |
| Author | Berschin et al. (2014) [33] RCT | Pistone et al. 2016 [34] RCT | Coulondre et al. 2021 [35] RCT |
|---|---|---|---|
| Method of randomization | Computer generated numbers | Block randomization program | REDCap Web application |
| Study population | N = 40 (29M and 11F). Experimental group N = 20 (14M, 6F; aged 25–29; mean age 27 ± 4,2), Control group N = 20 (15M, 5F; aged 25–39; mean age 28 ± 6,8). | N = 34 Experimental group N = 17 (mean age 29 ± 7), Control group N = 17 (mean age 27 ± 7). | N = 23 (13M and 10F; aged 18–50). Experimental group N = 11 (6M, 5F; mean age 30 ± 10); Control group N = 12 (7M, 5F; mean age 29 ± 9) |
| ACLR method | Patellar tendon graft | hamstring tendon autograft | hamstring or patellar tendon autograft |
| Enrollment time | 2nd week postoperatively | 1 month postoperatively | Individually; within 1st week postoperatively |
| Intervention | 8 weeks of WBV protocol training vs. 8 weeks standard rehabilitation program | 4 weeks of WBV training plus standard 4-week rehabilitation program vs. standard 4-week rehabilitation program | 24 LVT sessions plus 24 sessions of standard rehabilitation program (ca. 10 weeks) vs. 24 sessions of standard rehabilitation program (ca. 10 weeks). |
| Outcome measures | At 2nd (baseline), 5th, 8th and 11th week postoperatively: active ROM (goniometer), isometric and isokinetic muscle strength of knee flexors and extensors (dynamometer), postural control (stability platform), knee function (Lysholm Score) Immediately postoperatively and at 11th week postoperatively: knee joint laxity (arthrometer) | At 1st (baseline), 2nd and 3rd month postoperatively: isometric strength of knee flexors and extensors (limb symmetry index; dynamometer); balance (stability platform); knee function (Lysholm Score). | Assessment time was individual for each participant (1st assessment on enrollment, within 1st week; 2nd after last session; ca. at 11th week): knee extensors isometric strength and limb symmetry (force transducer); functional performance (TUG test and 6MWT). |
| Main outcomes | Significant improvement in experimental vs. control group in postural control (at week 8: 3.3 ± 1.5 vs. 4.9 ± 2.4 (p = 0.02); at week 11: 3.1 ± 1.3 vs. 4.7 ± 2.8 (p = 0.01)). No difference in experimental vs. control group in ROM, knee laxity, muscle strength or knee function (p > 0.05). | Significant improvement in experimental vs. control group in strength symmetry of the knee flexors at 2nd (mean 66% ± 15 vs. 58% ± 13; p < 0.05) and 3rd month (77% ± 15 vs. 64% ± 15; p < 0.05); knee function at 2nd (mean 11.5 ± 2.8 vs. 7.0 ± 2.7; p < 0.001) and 3rd month (23.7 ± 3.4 vs. 16.3 ± 3.1; p < 0.001). No difference in experimental vs. control group in strength symmetry of the knee extensors and balance (p > 0.05). | Significant difference in experimental vs. control group in PRE–POST extensor muscle strength changes (lower for LVT group; p = 0.0049). No difference in experimental vs. control group in limb strength symmetry (p < 0.05) and functional tests (p < 0.05). |
| Author | Velázquez-Saornil et al. (2017) [36] RCT | Ogrodzka-Ciechanowicz et al. I (2021) [37] RCT | Ogrodzka-Ciechanowicz et al. II (2021) [38] RCT | Clausen et al. (2020) [39] RCT (Pilot Study) |
|---|---|---|---|---|
| Method of randomization | Opaque closed letter envelopes | Sealed envelopes method | Coin toss | Computer-based randomization |
| Study population | N = 44 (28M, 16F); Experimental group N = 22 (16M, 6F; aged 19–46; mean age 31.4 ± 8.3); Control group N = 22 (12M, 10 F; aged 19–51; mean age 34.4 ± 8.6). | N = 35 (35M; aged 21–50); Experimental group N = 17 (17M; mean age 30 ± 7.35); Control group (N = 18 (18M; mean age 30 ± 10.42) | N = 38 (28M, 10F, aged 18–40); Experimental group N = 19 (15M, 4F; aged 18–40; mean age 28.2 ± 8.1), Control group N = 19 (13M, 6F; aged 19–39; mean age 27.4 ± 7.8). | N = 26 (12M, 14F; mean age 25.19 ± 8.2) Experimental group N = 14 (6M, 8F; mean age 24.86 ± 9.71); Control group N = 12 (6M, 6F; mean age 25.58 ± 6.4) |
| ACLR method | Patellar or hamstring tendon grafts | Hamstring tendon graft | Semitendinosus tendon autograft; | Hamstring graft |
| Enrollment time | 12–19 days postoperatively | 11 days postoperatively | 1st day postoperatively | Immediately postoperatively |
| Intervention | one-time vastus medialis trigger point dry needling plus 5 weeks of rehabilitation program vs. 5 weeks of rehabilitation program | 6 months of High Tone Power Therapy duration 1h, after every rehabilitation session) plus rehabilitation program vs. 6 months of rehabilitation program | 10 days of magnetic field (each day, 30′) plus postoperative instructions vs. 10 days of placebo magnetic field (each day, 30′) plus postoperative instructions | 3 weeks of app-based serious gaming (5 times daily) plus rehabilitation program vs. rehabilitation program |
| Outcome measures | At baseline, immediately after intervention, 24 hours, 1 and 5 weeks after the first TrPDN intervention: Pain intensity (VAS), ROM (goniometer), and function (WOMAC). | 2 days before and 6 months after surgery: muscle strength (dynamometer), ROM (goniometer), knee and thigh circumference (tailor’s tape), knee function (Lysholm test) and pain (VAS). | At baseline, each time after therapy for 11 days: knee effusion (circumference; tape measure). At 1st and 11th day: AROM (goniometer). | Before operation and after 6 weeks postoperatively: knee function (IKDC, Lysholm Knee Score, Tegner scale, KOOS), maximum strength, pain (VAS), and knee circumferences (10 cm and 20 cm above knee) |
| Main outcomes | Significant difference in experimental vs. control group (p < 0.001) in pain (increased in TrPDN; after intervention 6.86 ± 0.9 vs. 6.57 ± 0.9), ROM (at 1st: 98.57 ± 8.5 vs. 89.52 ± 8.6; 2nd: 104.76 ± 9.8 vs. 90 ± 9.5; and 3rd measurement: 131.43 ± 11.5 vs. 113.81 ± 11.6), and knee function (at 2nd: 64.48 ± 3.7 vs. 67.75 ± 4.2; 3rd: 38.51 ± 9 vs. 46.18 ± 10.9; and 4th measurement: 14.08 ± 3.7 vs. 18.6 ± 3.9). | Significant improvement in experimental vs. control group in extensors strength (23.28 ± 0.7 vs. 20.19 ± 0.6; p = 0.028), knee (35.00 ± 1.80 vs. 38.00 ± 2.18; p = 0.043) and thigh circumference (46.00 ± 3.86 vs. 41.00 ± 3.11; p = 0.033), knee extension (0.00 ± 0.15 vs. 3.00 ± 0.99; p = 0.048), and function (94 ± 7.01 vs. 85 ± 8.71; p = 0.035). No difference in experimental vs. control group in pain (p > 0.05). | No difference in experimental vs. control group in knee joint effusion and AROM (p > 0.05). | Significant improvement in experimental vs. control group in relative change in maximum strength (1.7 ± 1.17 vs. 1 ± 0.13; p = 0.03). No significant changes in knee function and pain (p > 0.05). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochman, M.; Kasprzak, M.; Kielar, A. ACL Reconstruction: Which Additional Physiotherapy Interventions Improve Early-Stage Rehabilitation? A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 15893. https://doi.org/10.3390/ijerph192315893
Kochman M, Kasprzak M, Kielar A. ACL Reconstruction: Which Additional Physiotherapy Interventions Improve Early-Stage Rehabilitation? A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(23):15893. https://doi.org/10.3390/ijerph192315893
Chicago/Turabian StyleKochman, Maciej, Marta Kasprzak, and Aleksandra Kielar. 2022. "ACL Reconstruction: Which Additional Physiotherapy Interventions Improve Early-Stage Rehabilitation? A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 23: 15893. https://doi.org/10.3390/ijerph192315893
APA StyleKochman, M., Kasprzak, M., & Kielar, A. (2022). ACL Reconstruction: Which Additional Physiotherapy Interventions Improve Early-Stage Rehabilitation? A Systematic Review. International Journal of Environmental Research and Public Health, 19(23), 15893. https://doi.org/10.3390/ijerph192315893








