Microplastic Contamination and Ecological Status of Freshwater Ecosystems: A Case Study in Two Northern Portuguese Rivers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Description and Sampling Sites
2.2. Ecological Status Assessment
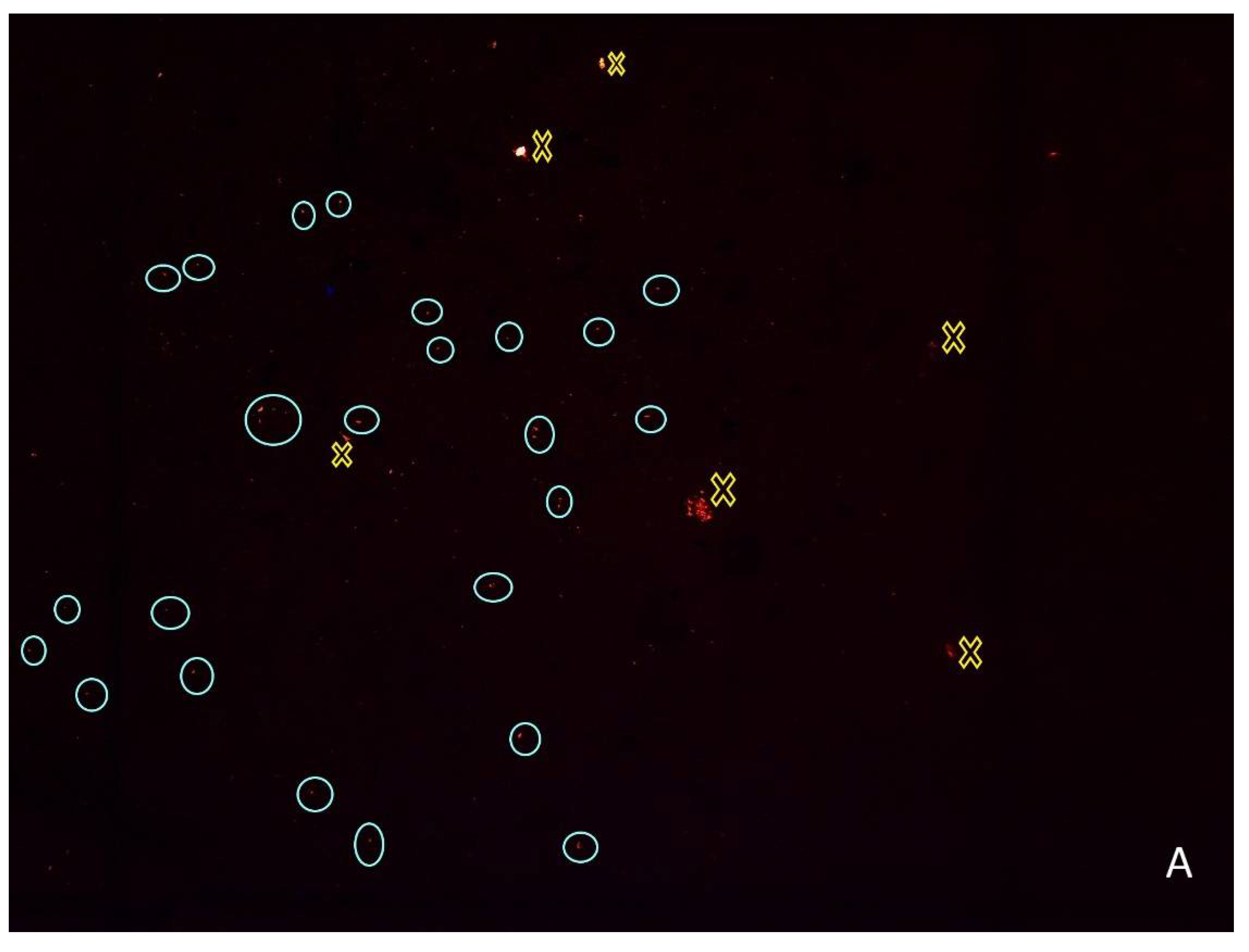
2.3. Microplastics Quantification
2.4. Quality Assurance and Quality Control
2.5. Statistical Analysis
3. Results
3.1. Ecological Status
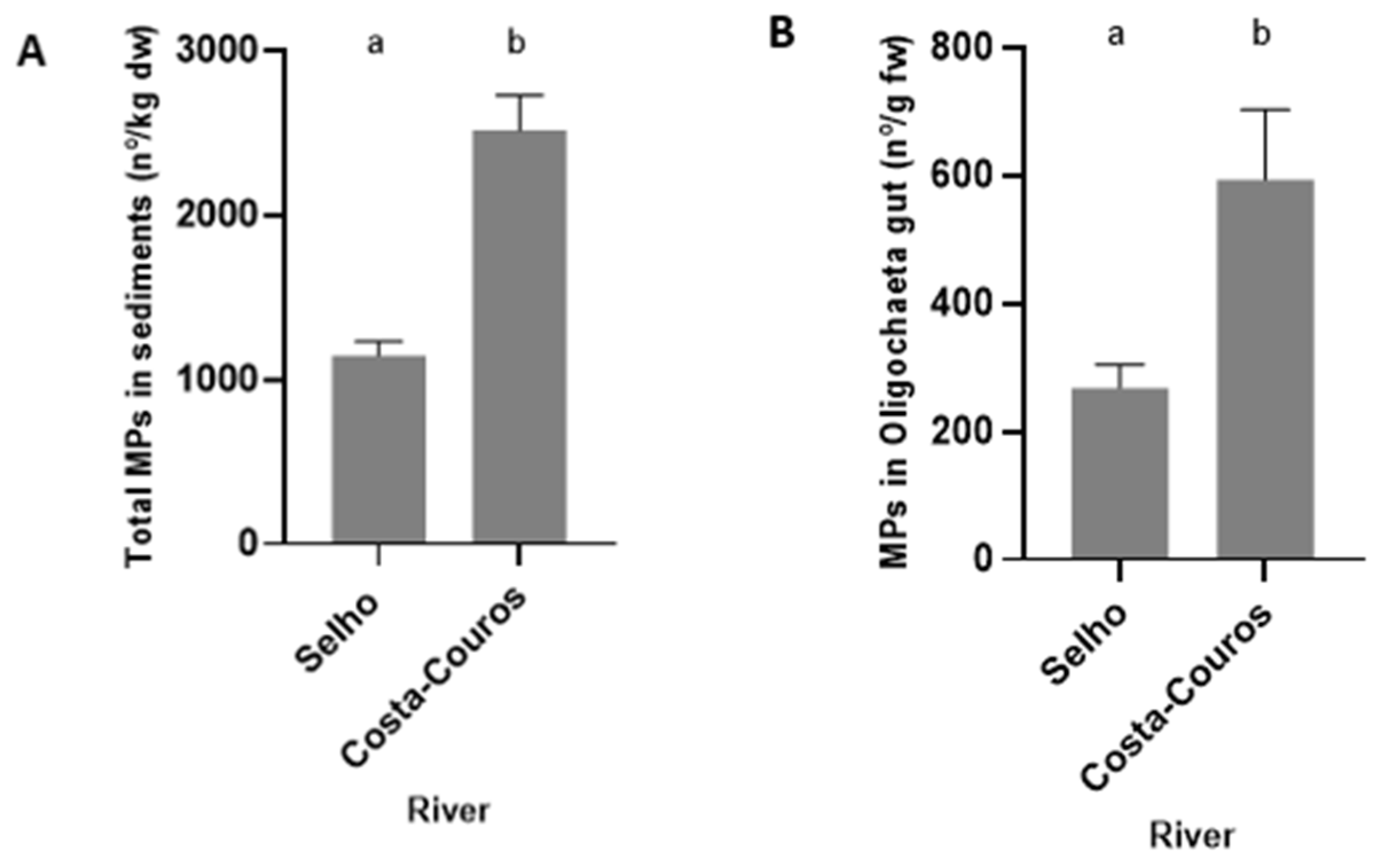
3.2. Analysis of Microplastics Contamination
4. Discussion
4.1. Ecological Status
4.2. Microplastic Contamination
4.3. Potential Linkage between Ecological Status and Microplastic Abundance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guerreiro, M.S.; Abreu, I.M.; Monteiro, Á.; Jesus, T.; Fonseca, A. Considerations on the monitoring of water quality in urban streams: A case study in Portugal. Environ. Monit. Assess. 2020, 192, 347. [Google Scholar] [CrossRef]
- Rodrigues, C.; Alves, P.; Bio, A.; Vieira, C.; Guimarães, L.; Pinheiro, C.; Vieira, N. Assessing the ecological status of small Mediterranean rivers using benthic macroinvertebrates and macrophytes as indicators. Environ. Monit. Assess. 2019, 191, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lemm, J.U.; Venohr, M.; Globevnik, L.; Stefanidis, K.; Panagopoulos, Y.; van Gils, J.; Posthuma, L.; Kristensen, P.; Feld, C.K.; Mahnkopf, J.; et al. Multiple stressors determine river ecological status at the European scale: Towards an integrated understanding of river status deterioration. Glob. Chang. Biol. 2020, 27, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- EC (European Comission). Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for Community Action in the Field of Water Policy; OJEC: Aberdeen, UK, 2000; Volume L327, pp. 1–73. [Google Scholar]
- EEA. WISE Water Framework Directive (Data Viewer); European Environment Agency: Copenhagen, Denmark, 2018; Available online: https://www.eea.europa.eu/data-and-maps/dashboards/wise-wfd (accessed on 10 August 2022).
- EEA. Floodplains: A Natural System to Preserve and Restore; EEA Report No 24/2019; European Environment Agency: Copenhagen, Denmark, 2020; Available online: https://www.eea.europa.eu/publications/floodplains-a-natural-system-to-preserve-and-restore (accessed on 10 August 2022).
- OECD. Diffuse Pollution, Degraded Waters: Emerging Policy Solutions. OECD Studies on Water; OECD Publishing: Paris, French, 2017. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; p. 43. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. M icroplastic—an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Thevenon, F.; Carroll, C.; Sousa, J. Plastic Debris in the Ocean: The Characterization of Marine Plastics and Their Environmental Impacts, Situation Analysis Report; IUCN: Gland, Switzerland, 2014. [Google Scholar]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Luís, L.G.; Ferreira, P.; Fonte, E.; Oliveira, M.; Guilhermino, L. Does the presence of microplastics influence the acute toxicity of chromium(VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat. Toxicol. 2015, 164, 163–174. [Google Scholar] [CrossRef]
- Santana, M.F.M.; Moreira, F.T.; Turra, A. Trophic transference of microplastics under a low exposure scenario: Insights on the likelihood of particle cascading along marine food-webs. Mar. Pollut. Bull. 2017, 121, 154–159. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemical from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Blair, R.M.; Waldron, S.; Phoenix, V.R.; Gauchotte-Lindsay, C. Microscopy and elemental analysis characterization of microplastics in sediment of a freshwater urban river in Scotland, UK. Environ. Sci. Pollut. Res. 2019, 26, 12491–12504. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Godoy, V.; da Costa, J.P.; Calero, M.; Martín-Lara, M.A.; Duarte, A.C.; Rocha-Santos, T. Microplastics and fibers from three areas under different anthropogenic pressures in Douro river. Sci. Total Environ. 2021, 776, 145999. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: Part Two of a Global Assessment; Kershaw, P.J., Rochman, C.M., Eds.; Report Studies GESAMP No. 93; IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2016; 220p. [Google Scholar]
- Lambert, S.; Wagner, M. Microplastics Are Contaminants of Emerging Concern in Freshwater Environment: An Overview. Freshw. Microplastics 2018, 58, 1–23. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef]
- Peng, J.; Wang, J.; Cai, L. Current understanding of microplastics in the environment: Occurrence, fate, risks, and what we should do. Integr. Environ. Assess. Manag. 2017, 13, 476–482. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.; de Carvalho, A.R.; Riem-Galliano, L.; Tudesque, L.; Albignac, M.; ter Halle, A.; Cucheroussest, J. Stable Isotope Insights into Microplastic Contamination within Freshwater Food Webs. Environ. Sci. Technol. 2021, 55, 1024–1035. [Google Scholar] [CrossRef]
- Sá, B.; Pais, J.; Antunes, J.; Pequeno, J.; Pires, A.; Sobral, P. Seasonal Abundance and Distribution Patterns of Microplastics in the Lis River, Portugal. Sustainability 2022, 14, 2255. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Berra, E.; Forcella, M.; Giacchini, R.; Marziali, L.; Rossaro, B.; Parenti, P. Evaluation of enzyme biomarkers in freshwater invertebrates from Taro and Ticino river, Italy. Ann. Limnol.-Int. J. Lim. 2004, 40, 169–180. [Google Scholar] [CrossRef]
- Nel, H.A.; Dalu, T.; Wasserman, R.J. Sinks and sources: Assessing microplastic abundance in river sediment and deposit feeders in an Austral temperate urban river system. Sci. Total Environ. 2018, 612, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.J.M.; Silva, A.L.P.; Gravato, C.; Pestana, J.L.T. Ingestion of small-sized and irregularly shaped polyethylene microplastics affect Chironomus riparius life-history traits. Sci. Total Environ. 2019, 672, 862–868. [Google Scholar] [CrossRef]
- Klein, K.; Piana, T.; Lauschke, T.; Schweyen, P.; Dierkes, G.; Ternes, T.; Schulte-Oehlmann, U.; Oehlmann, J. Chemicals associated with biodegradable microplastic drive the toxicity to the freshwater oligochaete Lumbriculus variegatus. Aquat. Toxicol. 2021, 231, 105723. [Google Scholar] [CrossRef]
- Baird, D.J.; Burton, G.A. Ecological Variability: Separating Natural from Anthropogenic Causes of Ecosystem Impairment; SETAC: Brussels, Belgium, 2001. [Google Scholar]
- Shumchenia, E.J.; Guarinello, M.L.; King, J.W. A Re-assessment of Narragansett Bay Benthic Habitat Quality Between 1988 and 2008. Estuaries Coast 2016, 39, 1463–1477. [Google Scholar] [CrossRef]
- Grizzetti, B.; Pistocchi, A.; Liquete, C.; Udias, A.; Bouraoui, F.; van de Bund, W. Human pressures and ecological status of European rivers. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Tibbetts, J.; Krause, S.; Lynch, I.; Smith, G.H.S. Abundance, Distribution, and Drivers of Microplastic Contamination in Urban River Environments. Water 2018, 10, 1597. [Google Scholar] [CrossRef]
- Ribeiro, A.C.C.; Correia, A.J.M.; Costa, F.D.S. O rio Selho: Contributo para uma proposta de requalificação ambiental. In Proceedings of the Congresso Nacional da Geografia Portuguesa—Territórios e Protagonistas, Guimarães, Portugal, 14–15 October 2004. [Google Scholar]
- Costa, F.D.S. PRIOS—Projecto de Reabilitação do Rio Selho: Um exemplo de intervenção na zona urbana de Guimarães. In Proceedings of the Actas do II Congresso Internacional de Engenharia Civil e Território “Água, Cultura e Sociedade”, Colegio Inge-nieros de Caminos, Canales y Puertos de Galacia, Vigo, Spain, 20–21 May 2013; pp. 495–506. [Google Scholar]
- Pereira, A.; Geraldes, P.; Lima-Fernandes, E.; Fernandes, I.; Cássio, F.; Pascoal, C. Structural and functional measures of leaf-associated invertebrates and fungi as predictors of stream eutrophication. Ecol. Indic. 2016, 69, 648–656. [Google Scholar] [CrossRef]
- Duarte, A.A.L.S.; Ferreira, C.V.; Ramísio, P.J.P.; Rodrigues, D.S. Modelação e avaliação da qualidade da água em sistemas hídricos urbanos. O caso da ribeira de Couros, em Guimarães (Portugal). In Proceedings of the 7º Congresso Luso-Brasileiro para o Planejamento Urbano, Regional, Integrado e Sustentável (Pluris 2016), Maceió, Brazil, 5–7 October 2016. [Google Scholar]
- INAG. Manual de Avaliação Biológica da Qualidade da água em Sistemas Fluviais Segundo a Diretiva Quadro da Água, Protocolo de Amostragem e Análise para os Macroinvertebrados Bentónicos; Ministério do Ambiente, do Ordenamento do Território e do Desenvolvimento Regional, Instituto da Água, I.P.: Lisbon, Portugal, 2008; Available online: https://www.apambiente.pt/dqa/assets/01-protocolo-de-amostragem-e-an%C3%A1lise-para-os-macroinvertebrados-bent%C3%B3nicos.pdf (accessed on 13 June 2022).
- Tachet, P.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés d’eau Douce: Systématique, Biologie, Écologie; CNRS: Paris, France, 2003; p. 487. [Google Scholar]
- INAG. Critérios para a Classificação do Estado das Massas de Água Superficiais—rios e Albufeiras; Ministério do Ambiente, do Ordenamento do Território e do Desenvolvimento Regional. Instituto da Água, I.P.: Lisbon, Portugal, 2009; Available online: https://www.apambiente.pt/dqa/assets/crit%C3%A9rios-classifica%C3%A7%C3%A3o-rios-e-albufeiras.pdf (accessed on 13 June 2022).
- EC (European Comission). Comission Decision of 20 September 2013 Establishing, Pursuant to Directive 2000/60/EC of the European Parliament and of the Council, the Values of the Member State Monitoring System Classifications as a Result of the Intercalibration Exercise and Repealing Decision 2008/915/EC; OJEU: Brussels, Belgium, 2013; Volume L266, pp. 1–47. [Google Scholar]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Technical Memorandum NOS-OR&R-48; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2015. [Google Scholar]
- Prata, J.C.; Alves, J.R.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Major factors influencing the quantification of Nile Red stained microplastics and improved automatic quantification (MP-VAT 2.0). Sci. Total Environ. 2020, 719, 137498. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Dias-Pereira, P.; Carvalho, A.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Microplastics in Internal Tissues of Companion Animals from Urban Environment. Animals 2022, 12, 1979. [Google Scholar] [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Shinde, S.E.; Pathan, T.S.; Raut, K.S.; Sonawane, D.L. Studies on the physico-chemical parameters and correlation coefficient of Harsool-savangi Dam, District Aurangabad, India. Middle East J. Sci. Res. 2011, 8, 544–554. [Google Scholar]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Alamri, S.A. Fertilizers and Their Contaminants in Soils, Surface and Groundwater. In The Encyclopedia of the Anthropocene; DellaSala, D.A., Goldstein, M.I., Eds.; Elsevier: Oxford, UK, 2018; Volume 5, pp. 225–240. [Google Scholar]
- Fernandes, D.T. An Integrated Approach of Landscape Design in the Rehabilitation of an Urban River Corridor: River Tinto. Proc. Fábos Conf. Landsc. Greenway Plan. 2013, 4, 679–686. [Google Scholar]
- Palanisamy, B.; Chui, T.F.M. Rehabilitation of concrete canals in urban catchments using low impact development techniques. J. Hydrol. 2015, 523, 309–319. [Google Scholar] [CrossRef]
- Bouraoui, F.; Grizzetti, B. Long term change of nutriente concentrations of rivers discharging in European seas. Sci. Total Environ. 2011, 409, 4899–4916. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.M.N.; Boaventura, R.; Pinto, F.T.; Lopes, H.; Gomes, F.V. Monitorização da Qualidade da Água durante uma Empreitada de Dragagem do porto de Leixões (Portugal). In Gestión Ambiental Integrada de áreas Costeras—Gestão Ambiental Integrada das áreas Costeiras; Mas-Pia, J., Zuppi, G.M., Eds.; Rubes Editorial: Barcelona, Spain, 2009; pp. 201–214. [Google Scholar]
- Kikuchi, R.M.; Uieda, V.S. Composição e distribuição dos macroinvertebrados em diferentes substratos de fundo de um riacho no município de Itatinga, São Paulo, Brasil. Entomol. Vectores 2005, 12, 193–231. [Google Scholar] [CrossRef]
- Sekudewicz, I.; Dąbrowska, A.M.; Syczewski, M.D. Microplastic pollution in surface water and sediments in the urban section of the Vistula River (Poland). Sci. Total Environ. 2021, 762, 143111. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and Spatial Distribution of Microplastics in River Shore Sediments of the Rhine-Main Area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef]
- Simon-Sanchéz, L.; Grelaud, M.; Garcia-Orellana, J.; Ziveri, P. River Deltas as hotspots of microplastic accumulation: The case study of the Ebro River (NW Mediterranean). Sci. Total Environ. 2019, 687, 1186–1196. [Google Scholar] [CrossRef]
- Schell, T.; Hurley, R.; Nizzetto, L.; Rico, A.; Vighi, M. Spatio-temporal distribution of microplastics in a Mediterranean river catchment: The importance of wastewater as an environmental pathway. J. Hazard. Mater. 2021, 420, 126481. [Google Scholar] [CrossRef]
- Bashir, S.M.; Kimiko, S.; Mak, C.W.; Fang, J.K.H.; Gonçalves, D. Personal Care and Cosmetic Products as a Potential Source of Environmental Contamination by Microplastics in a Densely Populated Asian City. Front. Mar. Sci. 2021, 8, 683482. [Google Scholar] [CrossRef]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in aquatic environments: Implications for Canadian ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Kalčikova, G.; Alič, B.; Skalar, T.; Bundschuh, M.; Gotvajn, A.Ž. Wastewater treatment plant effluents as source of cosmetic polyethylene microbeads to freshwater. Chemosphere 2017, 188, 25–31. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Castañeda, R.A.; Avlijas, S.; Simard, M.A.; Ricciardi, A. Microplastic pollution in St. Lawrence River sediments. Can. J. Fish. Aquat. Sci. 2014, 71, 1767–1771. [Google Scholar] [CrossRef]
- Govedich, F.R.; Bain, B.A.; Moser, W.E.; Gelder, S.R.; Davies, R.W.; Brinkhurst, R.O. Annelida (Clitellata): Oligochaeta, Branchiobdellida, Hirudinida, and Acanthobdellida. In Ecology and Classification of North American Freshwater Invertebrates, 3rd ed.; Thorp, J.H., Covich, A.P., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 385–436. [Google Scholar] [CrossRef]
- Silva, C.J.M.; Silva, A.L.P.; Campos, D.; Machado, A.L.; Pestana, J.L.T.; Gravato, C. Oxidative damage and decreased aerobic energy production due to ingestion of polyethylene microplastics by Chironomus riparius (Diptera) larvae. J. Hazard. Mater. 2021, 402, 123775. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.R.; Woodward, J.C.; Rothwell, J.J. Ingestion of Microplastics by Freshwater Tubifex Worms. Environ. Sci. Technol. 2017, 51, 12844–12851. [Google Scholar] [CrossRef] [PubMed]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Rauchschwalbe, M.-T.; Höss, S.; Haegerbaeumer, A.; Traunspurger, W. Long-term exposure of a free-living freshwater micro- and meiobenthos community to microplastic mixtures in microcosms. Sci. Total Environ. 2022, 827, 154207. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Kowalski, N.; Waniek, J.J. Effects of biofouling on the sinking behavior of microplastics. Environ. Res. Lett. 2017, 12, 124003. [Google Scholar] [CrossRef]
- Rodriguez, P.; Martinez-Madrid, M.; Arrate, J.A.; Navarro, E. Selective feeding by the aquatic oligochaete Tubifex Tubifex (Tubificidae, Clitellata). Hydrobiologia 2001, 463, 133–140. [Google Scholar] [CrossRef]
- Silva, C.J.M.; Beleza, S.; Campos, D.; Soares, A.M.V.M.; Silva, A.L.P.; Pestana, J.L.T.; Gravato, C. Immune response triggered by the ingestion of polyethylene microplastics in the dipteran larvae Chironomus riparius. J. Hazard. Mater. 2021, 414, 125401. [Google Scholar] [CrossRef] [PubMed]



| Site | Parish/Union of Parishes in the Left Bank (LB) and Right Bank (RB) | Coordinates | Land Use |
|---|---|---|---|
| S1 | LB and RB: Parish of S. Torcato | 41°29′11″ N, 8°15′16″ W | Forestry Agricultural Rural recreational area |
| S2 | LB: Parish of Aldão RB: Union of Parishes of Selho S. Lourenço and Gominhães | 41°27′52″ N, 8°16′51″ W | Artificial surfaces (housing) |
| S3 | LB: Union of Parishes of Candoso S. Tiago and Mascotelos RB: Parish of Creixomil | 41°26′14″ N, 8°19′21″ W | Agricultural Peri-urban recreational area |
| S4 | LB: Parish of Serzedelo RB: Parish of Gondar | 41°24′38″ N, 8°22′34″ W | Agricultural |
| C1 | LB and RB: Parish of Costa | 41°26′50″ N, 8°16′42″ W | Urban recreational area |
| C2 | LB and RB: Union of Parishes of Oliveira, S. Paio and S. Sebastião | 41°26′29″ N, 8°17′14″ W | Artificial surfaces (housing, commercial, transport, etc.) Urban recreational area |
| C3 | LB: Parish of Creixomil RB: Parish of Urgezes | 41°26′23″ N, 8°17′59″ W | Artificial surfaces (housing, commercial, transport, etc.) |
| C4 | LB and RB: Parish of Creixomil | 41°26′12″ N, 8°18′29″ W | Agricultural Peri-urban recreational area |
| Sampling Site | IPtIN (EQR) | Quality Class |
|---|---|---|
| S1 | 0.72 | II |
| S2 | 0.44 | III |
| S3 | 0.36 | IV |
| S4 | 1.65 | I |
| C1 | 0.79 | II |
| C2 | 0.45 | III |
| C3 | 0.27 | IV |
| C4 | 0.40 | IV |
| Sampling Site | Season/Year | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | Summer 2020 | Autumn 2020 | Winter 2021 | Spring 2021 | ||
| Selho river | Temp. | 12.94 ± 1.62 | 14.46 ± 1.54 | 16.08 ± 2.21 | 14.74 ± 2.59 | 15.69 ± 1.81 | 12.34 ± 0.48 | 13.66 ± 1.05 | 16.53 ± 1.80 |
| pH | 6.81 ± 0.53 | 6.71 ± 0.14 | 6.76 ± 0.22 | 6.99 ± 0.20 | 6.80 ± 0.36 | 7.05 ± 0.32 | 6.94 ± 0.06 | 6.51 ± 0.13 | |
| DO | 9.40 ± 1.62 | 9.14 ± 0.87 | 7.98 ± 1.37 | 9.16 ± 1.07 | 7.82 ± 1.42 | 8.81 ± 0.76 | 10.21 ± 0.94 | 8.84 ± 1.04 | |
| %DO | 92.18 ± 15.30 | 91.53 ± 7.78 | 84.37 ± 14.79 | 92.12 ± 7.92 | 81.05 ± 14.47 | 84.93 ± 6.27 | 100.20 ± 8.40 | 93.98 ± 7.56 | |
| Cond. | 51.50 ± 12.70 | 90.83 ± 23.96 | 141.8 ± 47.44 | 131.8 ± 39.6 | 136.50 ± 51.99 | 129.80 ± 49.84 | 70.75 ± 23.28 | 78.92 ± 25.58 | |
| Sal. | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.03 ± 0.01 | 0.04 ± 0.01 | |
| TDS | 26.00 ± 6.89 | 45.25 ± 11.99 | 71.00 ± 23.56 | 65.75 ± 19.59 | 68.17 ± 25.73 | 65.08 ± 24.51 | 35.25 ± 11.97 | 39.50 ± 12.76 | |
| NO3− | 10.49 ± 2.71 | 20.96 ± 2.41 | 21.10 ± 4.60 | 22.05 ± 3.69 | 23.35 ± 5.74 | 17.13 ± 5.52 | 15.47 ± 4.39 | 18.65 ± 5.01 | |
| NO2− | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.37 ± 0.31 | 0.18 ± 0.11 | 0.34 ± 0.10 | 0.17 ± 0.05 | 0.03 ± 0.01 | 0.05 ± 0.02 | |
| NH4+ | 0.06 ± 0.08 | 0.10 ± 0.10 | 0.90 ± 0.63 | 0.12 ± 0.09 | 0.48 ± 0.73 | 0.45 ± 0.49 | 0.07 ± 0.06 | 0.18 ± 0.21 | |
| P | 0.38 ± 0.08 | 0.33 ± 0.09 | 0.28 ± 0.06 | 0.19 ± 0.03 | 0.30 ± 0.12 | 0.25 ± 0.07 | 0.33 ± 0.09 | 0.31 ± 0.11 | |
| COD | 10.50 ± 13.81 | 8.50 ± 11.52 | 9.33 ± 9.60 | 14.08 ± 12.11 | 5.17 ± 4.13 | 8.25 ± 4.77 | 0.00 ± 0.00 | 29.00 ± 3.52 | |
| BOD5 | 3.47 ± 1.68 | 3.39 ± 0.77 | 2.75 ± 1.31 | 3.66 ± 1.15 | 2.58 ± 1.24 | 2.90 ± 0.66 | 4.90 ± 0.85 | 2.90 ± 0.75 | |
| C1 | C2 | C3 | C4 | Summer 2020 | Autumn 2020 | Winter 2021 | Spring 2021 | ||
| Costa-Couros river | Temp. | 14.72 ± 1.72 | 15.57 ± 2.35 | 15.96 ± 2.70 | 15.61 ± 1.86 | 18.52 ± 1.11 | 13.64 ± 0.22 | 13.54 ± 0.40 | 16.16 ± 0.57 |
| pH | 6.99 ± 0.34 | 6.95 ± 0.21 | 7.07 ± 0.13 | 7.04 ± 0.03 | 7.04 ± 0.10 | 7.18 ± 0.18 | 7.06 ± 0.08 | 6.77 ± 0.20 | |
| DO | 7.89 ± 0.71 | 8.23 ± 1.32 | 7.98 ± 1.56 | 6.69 ± 0.78 | 8.57 ± 1.54 | 7.74 ± 0.90 | 8.25 ± 0.19 | 6.24 ± 0.36 | |
| % DO | 81.18 ± 4.51 | 85.40 ± 15.65 | 84.45 ± 18.66 | 69.88 ± 5.70 | 94.44 ± 18.62 | 76.97 ± 9.03 | 80.52 ± 1.53 | 68.99 ± 3.46 | |
| Cond. | 142.80 ± 43.12 | 151.50 ± 38.90 | 73.92 ± 64.41 | 215.80 ± 68.58 | 173.80 ± 102.60 | 159.00 ± 98.10 | 113.20 ± 20.56 | 138.00 ± 20.00 | |
| Sal. | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.03 ± 0.03 | 0.10 ± 0.03 | 0.08 ± 0.05 | 0.08 ± 0.05 | 0.05 ± 0.01 | 0.06 ± 0.01 | |
| TDS | 71.25 ± 21.50 | 76.00 ± 19.50 | 37.00 ± 32.18 | 108.00 ± 34.15 | 89.11 ± 41.34 | 79.50 ± 49.06 | 56.58 ± 10.63 | 69.25 ± 9.75 | |
| NO3− | 19.07 ± 4.23 | 24.96 ± 5.38 | 18.16 ± 6.51 | 19.62 ± 12.67 | 29.72 ± 3.96 | 17.83 ± 5.12 | 12.52 ± 8.06 | 21.74 ± 1.35 | |
| NO2− | 0.16 ± 0.10 | 0.18 ± 0.07 | 0.30 ± 0.13 | 0.54 ± 0.21 | 0.48 ± 0.23 | 0.27 ± 0.17 | 0.15 ± 0.09 | 0.26 ± 0.16 | |
| NH4+ | 0.17 ± 0.23 | 0.29 ± 0.20 | 1.32 ± 0.81 | 2.43 ± 2.07 | 1.73 ± 1.91 | 1.78 ± 1.66 | 0.31 ± 0.14 | 0.40 ± 0.34 | |
| P | 0.20 ± 0.07 | 0.10 ± 0.07 | 0.35 ± 0.13 | 0.34 ± 0.22 | 0.28 ± 0.20 | 0.25 ± 0.18 | 0.19 ± 0.16 | 0.28 ± 0.14 | |
| COD | 8.67 ± 3.89 | 2.25 ± 2.18 | 15.92 ± 4.06 | 12.50 ± 8.99 | 8.00 ± 5.77 | 9.17 ± 8.64 | 12.50 ± 6.25 | 9.65 ± 8.40 | |
| BOD5 | 2.14 ± 1.09 | 3.00 ± 1.47 | 3.43 ± 1.69 | 1.82 ± 1.20 | 3.41 ± 1.84 | 2.36 ± 0.80 | 3.72 ± 0.04 | 0.88 ± 0.22 | |
| Selho River | Costa-Couros River | |||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C1 | C2 | C3 | C4 | |
| Min. (μm) | 20 | 20 | 10 | 20 | 20 | 30 | 40 | 20 |
| Max. (μm) | 60 | 70 | 70 | 60 | 70 | 70 | 90 | 40 |
| Mean (μm) | 42 | 35 | 34 | 36 | 44 | 47 | 53 | 31 |
| ±SEM | ±5.12 | ±6.01 | ±6.18 | ±4.99 | ±5.62 | ±3.96 | ±4.96 | ±3.15 |
| Selho River | Costa-Couros River | |||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C1 | C2 | C3 | C4 | |
| Min. (g) | 0.008 | 0.007 | 0.006 | 0.008 | 0.076 | 0.007 | 0.158 | 0.008 |
| Max. (g) | 0.169 | 0.021 | 0.017 | 0.215 | 0.134 | 0.520 | 0.744 | 0.019 |
| Mean (g) | 0.080 | 0.014 | 0.011 | 0.063 | 0.111 | 0.081 | 0.464 | 0.010 |
| ±SEM | ±0.013 | ±0.001 | ±0.001 | ±0.015 | ±0.005 | ±0.046 | ±0.045 | ±0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.; Gravato, C.; Cardoso, J.; Ribeiro, C.A.; Vieira, M.N.; Rodrigues, C. Microplastic Contamination and Ecological Status of Freshwater Ecosystems: A Case Study in Two Northern Portuguese Rivers. Int. J. Environ. Res. Public Health 2022, 19, 15956. https://doi.org/10.3390/ijerph192315956
Ribeiro A, Gravato C, Cardoso J, Ribeiro CA, Vieira MN, Rodrigues C. Microplastic Contamination and Ecological Status of Freshwater Ecosystems: A Case Study in Two Northern Portuguese Rivers. International Journal of Environmental Research and Public Health. 2022; 19(23):15956. https://doi.org/10.3390/ijerph192315956
Chicago/Turabian StyleRibeiro, Andreia, Carlos Gravato, João Cardoso, Carlos Alexandre Ribeiro, Maria Natividade Vieira, and Carolina Rodrigues. 2022. "Microplastic Contamination and Ecological Status of Freshwater Ecosystems: A Case Study in Two Northern Portuguese Rivers" International Journal of Environmental Research and Public Health 19, no. 23: 15956. https://doi.org/10.3390/ijerph192315956
APA StyleRibeiro, A., Gravato, C., Cardoso, J., Ribeiro, C. A., Vieira, M. N., & Rodrigues, C. (2022). Microplastic Contamination and Ecological Status of Freshwater Ecosystems: A Case Study in Two Northern Portuguese Rivers. International Journal of Environmental Research and Public Health, 19(23), 15956. https://doi.org/10.3390/ijerph192315956








