GRP78 Activity Moderation as a Therapeutic Treatment against Obesity
Abstract
:1. Introduction
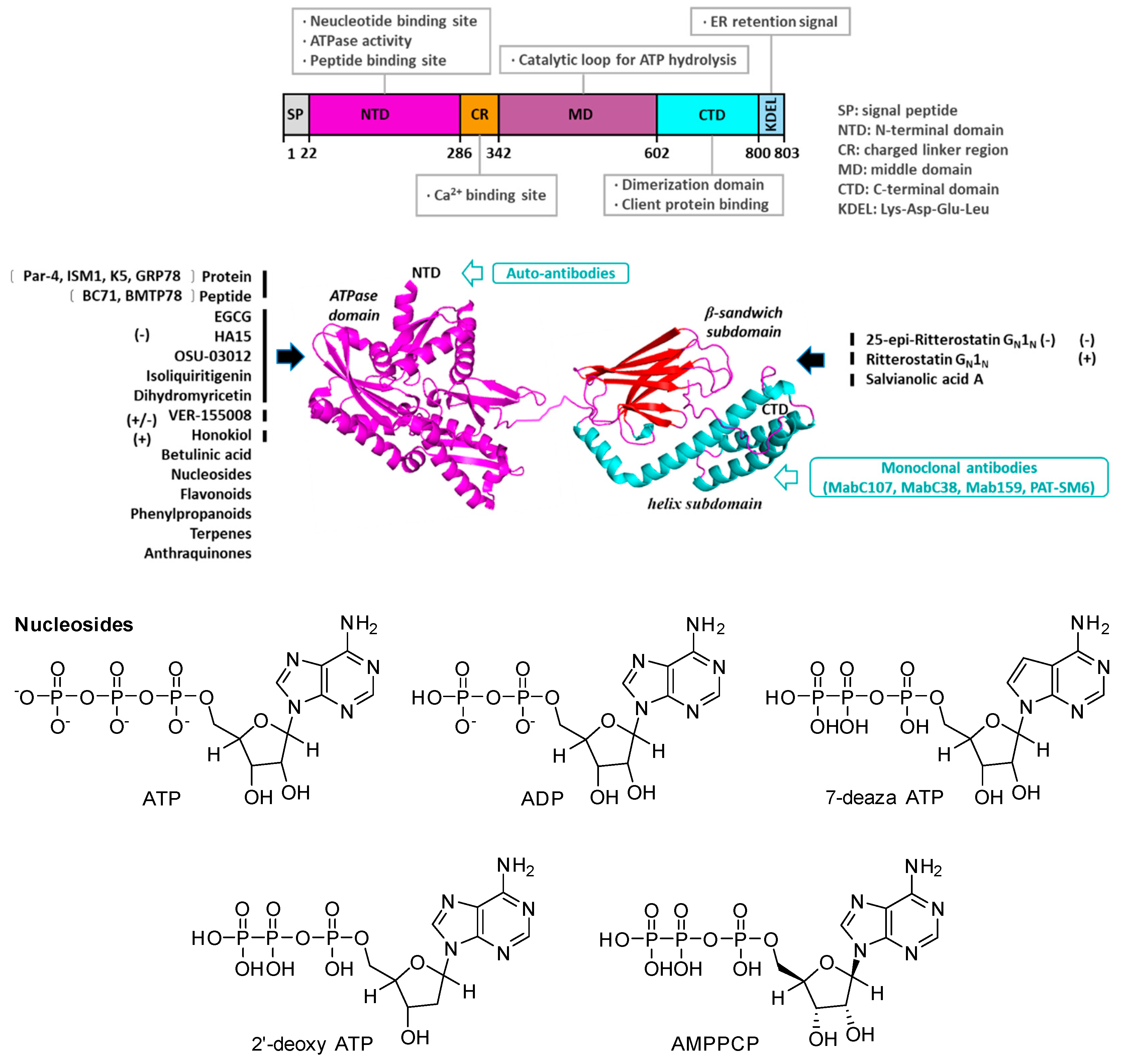
2. Structure, Function, and Subcellular Localization of GRP78
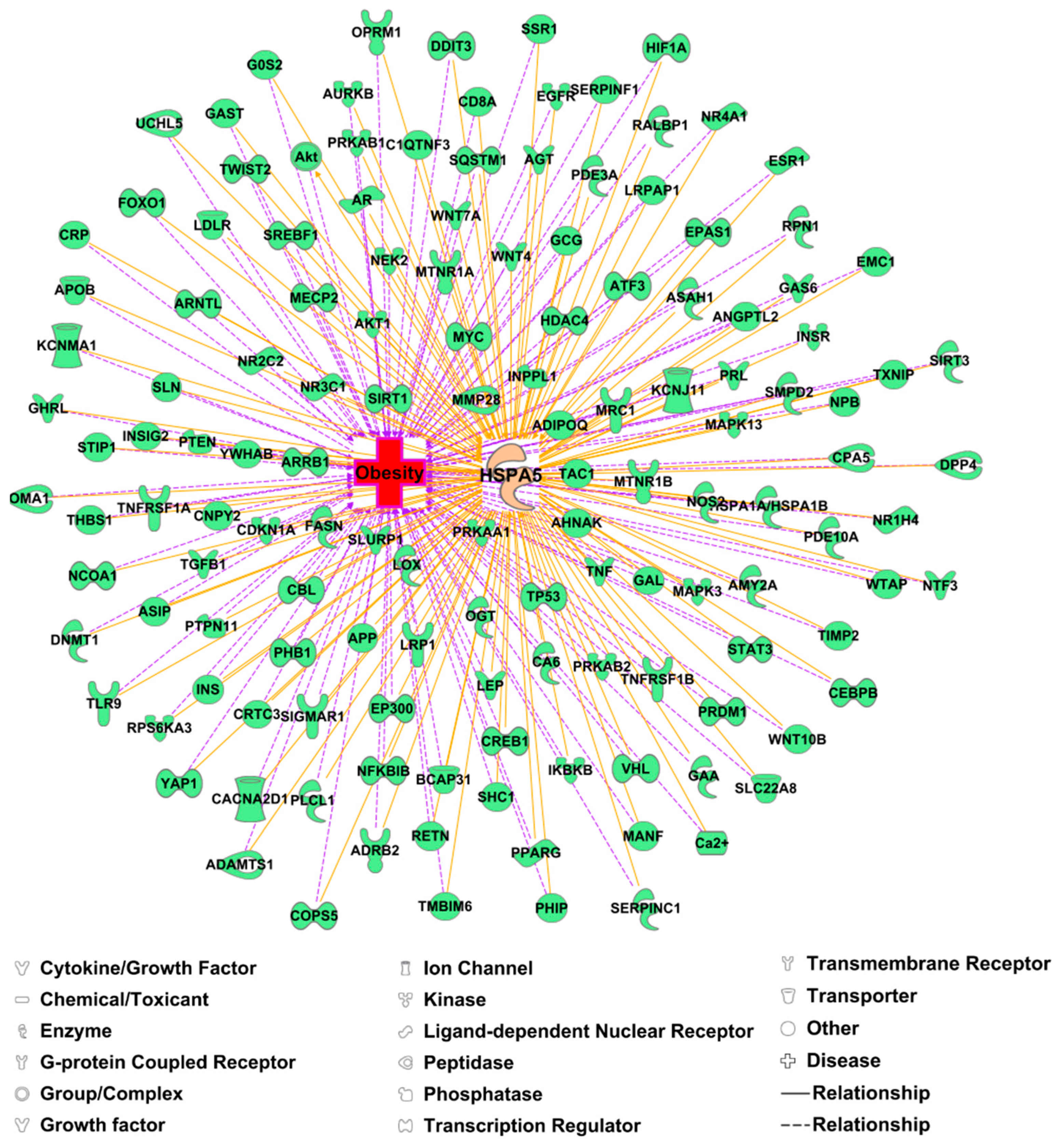
3. GRP78 Is Increased in Patients with Obesity and Is a Prognosis Marker
4. Roles of GRP78 in Regulating Lipid Metabolism
4.1. GRP78 Promotes Adipogenesis and Lipogenesis
4.2. GRP78 Promotes De Novo Formation of Lipid Droplets
4.3. GRP78 Negatively Regulates Mitochondrial Biosynthesis and Energy Balance
4.4. GRP78 Causes Insulin Resistance
4.5. GRP78 Can Eliminate Liver Lipotoxicity and then Improve Liver Steatosis
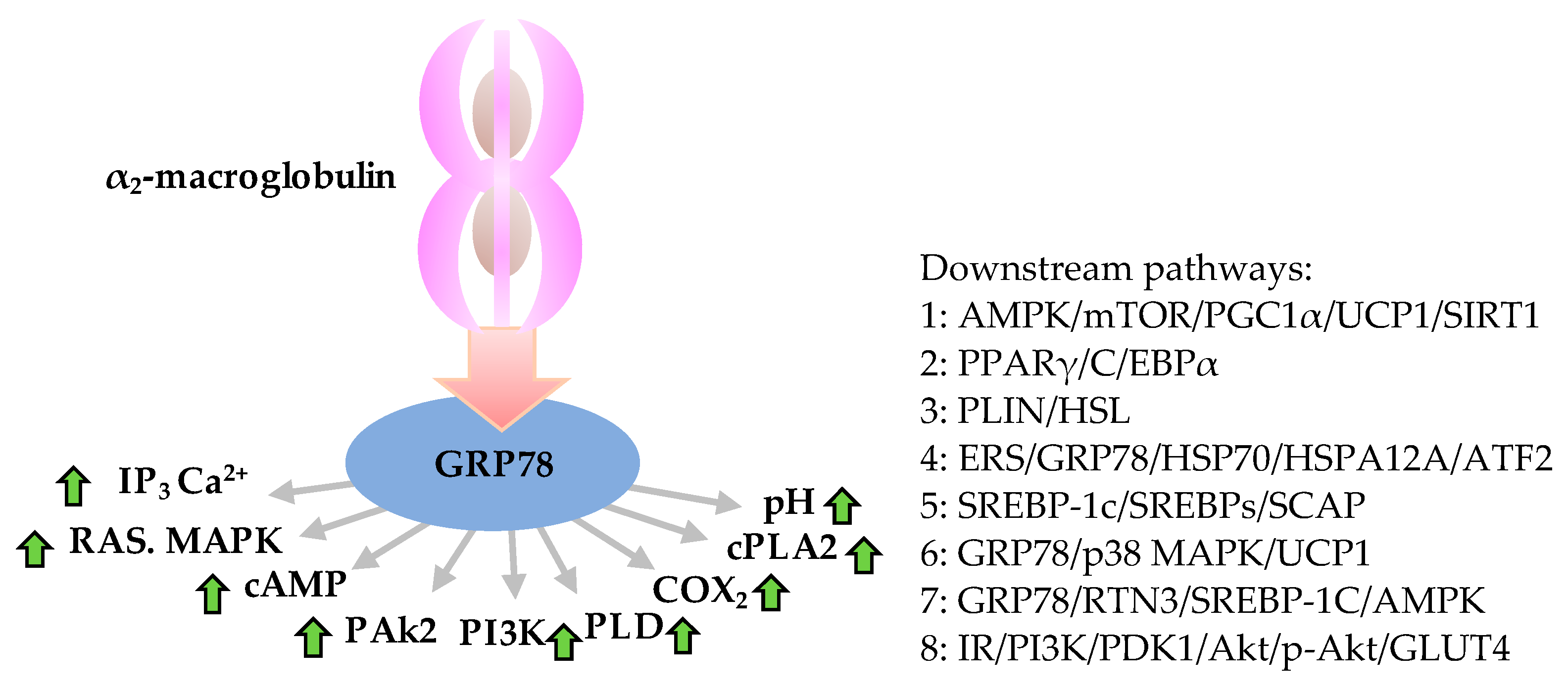
5. Molecular Mechanism of Action of GRP78 during Obesity Development
5.1. Proteins and Small Molecules Directly Bind GRP78
| No. | Protein | Binding Site and Interaction Mode | Downstream Signaling/Effect | Methods |
|---|---|---|---|---|
| 1 | PERK/IRE1/ATF6 | Calnexin and vimentin interact indirectly with annexin A2 via GRP78 | Initially activate PERK-eIF2A, IRE1-XBP1 and ATF6 signal pathways and trigger cell apoptosis | IP-MS; Bioinformatics analysis |
| 2 | PERK | Disinhibited by release from GRP78, and then phosphorylates eIF2α | Activated phosphorylation of eIF2α by PERK inhibits translation initiation, decreases protein synthesis and protein influx in the ER, then phosphorylation of ATF4 activates CHOP and apoptosis by PERK-eIF2α-ATF4-CHOP pathway | IHC |
| 3 | IRE1 | Disinhibited by release from GRP78 | Splicing mRNA encoding XBP1 triggers endoribonuclease activity of IRE1, and then targets genes in protein folding and ERAD by IRE1-TRAF2-JNK pathway | IHC |
| 4 | ATF6 | GRP78 directly activates ATF6 by binding to its luminal domain and inhibiting its Golgi localization signals; ATF6 is disinhibited by release from GRP78, then was cleaved and activated at Golgi apparatus | Active ATF6 moves to nucleus and upregulates proteins that promotes ER protein folding; ATF6 downstream target gene GRP78 | IFA; IP. |
| 5 | SREBP-1 | GRP78 retains SCAP/SREBP1 in the ER via direct interaction | Inhibit PI3K/Akt pathway; Positively regulates the transcription of ACC and FASN; SREBP-1 downstream targets involved in fatty acid synthesis, including FASN | Co-IP |
| 6 | CNPY2 | CNPY2 combines with GRP78 under normal conditions; After UPR inducer chlamycin treatment, CNPY2 disassociates from GRP78 and binds to PERK | Activate PERK-CHOP signaling; CNPY2 blocked the PERK-CHOP pathway of the unfolded protein response | Cnpy2 knockout mice fed a high-fat diet |
| 7 | p-Akt | Exocrine GRP78 | Activate PI3K/AKT signaling, then promote tumor cell proliferation and decrease the sensitivity of HCC cells to sorafenib | Co-IP |
| 8 | SR-A | SR-A binds to cytoplasmic GRP78 | GRP78 involved in SR-A mediates lipid endocytosis; Inhibits inflammatory cytokine expression (TNF-α, IL-1) through MAPK, PI3K-Akt and NF-κB signaling | IP; Indirect IFA; FRET |
| 9 | SCNN1B | SCNN1B interacts with GRP78 and induces GRP78 degradation via polyubiquitination. | Increase ubiquitin-mediated degradation of GRP78, subsequently trigger the unfolded protein response (UPR) | Tissue microarray analysis; SCNN1B ectopic expression and knockdown; IFA; IP–MS. |
| 10 | Anti-EGFR antibody | Anti-EGFR antibody combines and co-locates with GRP78 | Block the promotion of GRP78 to the invasion of cancer stem cells | Transwell; Confocal microscopy; WB |

| No. | Compound/Combination Therapy in GBM | Action Mechanism | Effects | Model |
|---|---|---|---|---|
| 1 | EGCG; EGCG+TMZ/5-fluorouracil/taxol/vinblastine/gemcitabine/TRAIL/doxorubicin/paclitaxel/IFN-α2b | GRP78 (NBD) | Impair GRP78 function; Enhance cytotoxicity when used with TMZ or others | Human cell lines, in vivo [51,52] |
| 2 | Honokiol; Honokiol+TMZ/fenretinide/bortezomib | GRP78 (NBD) | Interfere with GRP78 folding; Induce ER stress-mediated apoptosis with TMZ | Human cell lines [53,54,55] |
| 5 | NEO100 (Clinical trials); NEO100+TMZ/DMC/relfinavir | ER stress | Disrupt survival pathways; Induce more apoptosis with TMZ and others, reduce GBM invasion capacity, prolong survival | Human cell lines, in vivo [58,59,60] |
| 8 | EGF-SubA; EGF-SubA+radiation+TMZ | Cleave GRP78 | Correct the ATPase and protein binding domains; Delay tumor growth, enhance effects of TMZ and ionizing radiation | Human cell lines in vivo mouse models [64] |
| 3 | OSU-03012; radiotherapy+OSU-03012; | GRP78 (NBD) | PDK1 inhibition, GRP78 inhibition, PERK signaling inhibition; Enhance radiosensitivity; Prolong survival | Human cell lines, in vivo mouse models [52,56] |
| 4 | Celecoxib and bortezomib Celecoxib+bortezomib+GRP78 inhibition | ER stress | Augment ER stress; Induce ER stress-mediated apoptosis | Human cell lines [57] |
| 6 | HA15 | Bind and inhibit GRP78 | Disrupt GRP78 complexes with PERK/IRE1/ATF6; Induce apoptosis | Human cell lines, in vivo mouse models [61,62] |
| 7 | IT-139 | GRP78 | Involve transcriptional and post-transcriptional mechanisms; Decrease therapeutic resistance | Human cell lines, in vivo human xenograft studies [63] |
| 8 | EGF-SubA TMZ+radiation therapy+EGF-SubA [64] | GRP78; Cleave GRP78 | TMZ and ionizing radiation; Delay tumor growth, enhanc effects of TMZ and ionizing radiation | Human cell lines in vivo mouse models |
| 9 | Anti-GRP78 antibody; Ionizing radiation+anti-GRP78 antibody | Bind to surface GRP78 | Enhance effects of ionizing radiation via suppression of PI3K/AKT/mTOR signaling, and result in tumor delay | Human cell lines, in vivo mouse xenograft models [65] |
| 10 | RGD ligand-directed phage with GRP78 promoter | Bind GRP78 | RGD tumor homing ligand binds and improves expression of therapeutic transgenes with GRP78 promoter | Human cell lines in vivo [66] |
| 11 | TMZ-induced AAV phage with GRP78 promoter; TMZ+phage | RGD4C/AAV/phage/GRP78 binding | Activate therapeutic transgenes expression with GRP78 promoter; Permit dose escalation of TMZ | Human cell lines, mouse xenograft models [67] |
| 12 | GIRLPG; Radiation+phage | Bind GRP78 | Allow for adenovirus-mediated gene delivery to target tumor cells; Enhance radiation therapy and therapeutic transgenes expression | Human cell lines, mouse xenograft models [68] |
| No. | Chemicals | 2 mM MgCl2 | KD (M) a | 5 mM EDTA | Biologic Activity/Reference |
|---|---|---|---|---|---|
| Untreated b | |||||
| 1 | ATP | (4.5 ± 2.9) × 10−7 | (7.8 ± 7.1) × 10−7 | (9.8 ± 4.4) × 10−6 | [69] |
| 2 | ADP | (1.2 ± 0.9) × 10−8 | (2.7 ± 4.4) × 10−7 | (4.3 ± 7.7) × 10−5 | [69] |
| 3 | 7-deazaATP | (3.0 ± 2.0) × 10−8 | (1.5 ± 0.9) × 10−7 | (9.0 ± 5.4) × 10−7 | [69] |
| 4 | AMPPCP | (5.9 ± 1.2) × 10−5 | (5.2 ± 4.1) × 10−5 | >1 × 10−3 | [69] |
| 5 | 2′-deoxyATP | (7.5 ± 5.0) × 10−4 | >1 × 10−3 | [69] | |
| 6 | Honokiol | Bind GRP78 (NBD) using DSC and ITC [53,54,55,70] | |||
| 7 | Mangiferin | [71] | |||
| 8 | Isoliquiritigenin | [72] | |||
| 9 | OSU-03012 | [56,72,73] | |||
| 12 | Luteolin | [74] | |||
| 13 | DHM | 22 × 10−6 | Anti-adipogenesis, EC50 284 μM [24] | ||
| 14 | HA15 | Induce UPR and kill BRAF mutant melanomas [75] | |||
| 15 | EGCG | 6 × 10−6 | bind GRP78 (NBD); Anti-adipogenesis, EC50 103 μM [52,72,73,76] | ||
| 16 | Salvianolic acid A | Lysine 633 acetylation of GRP78 to block GRP78 secretion [72,77] | |||
| 17 | Salvianolic acid B | [74] | |||
| 18 | Salidroside | [74] | |||
| 19 | Salubrinal | [74] | |||
| 20 | Echinacoside | [74] | |||
| 21 | Betulinic acid | [72] | |||
| 22 | Capsaicin | [6] | |||
| 23 | Berberine | [6,23,47,50] | |||
| 24 | Naringin | Inhibit the expression of GRP78 [77] | |||
| 25 | Platycodon platycodon D | Up-regulate expression of GRP78 [77] | |||
| 26 | Diosmin | [77] | |||
| 27 | Isovitexin | [77] | |||
| 28 | Emodin | [6] | |||
| 29 | Curcumin | DARTs, directly targeting GRP78 [6] | |||
| 30 | Novolactone | Destabilize HER2 and EGFR in cancer cells [75] | |||
| 31 | Rifampicin | [74] | |||
| 32 | Puerarin | [6] | |||
| 33 | Hexachlorphene | Induce apoptosis and block autophagy in melanoma cell lines [78] | |||
| 34 | VER-155008 | 80 × 10−9 | [72] |
5.2. GRP78 Monomer/Heteromer and Conformational Changes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. About Diabetes; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Available online: https://www.worldobesity.org/news/world-obesity-day-all-countries-significantly-off-track-to-meet-2025-who-targets-on-obesity (accessed on 30 September 2022).
- Available online: http://test.health-china.com/c/2021-09-22/803749.shtml (accessed on 30 March 2022).
- Available online: https://www.fortunebusinessinsights.com/anti-obesity-drugs-market-104783 (accessed on 30 September 2022).
- Martínez-Rodríguez, O.P.; Thompson-Bonilla, M.D.R.; Jaramillo-Flores, M.E. Association between obesity and breast cancer: Molecular bases and the effect of flavonoids in signaling pathways. Crit. Rev. Food Sci. Nutr. 2020, 60, 3770–3792. [Google Scholar] [CrossRef]
- Garza, A.; Milagro, F.I.; Boque, N.; Campión, J.; Martínez, J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marycz, K.; Kornicka, K.; Szlapka-Kosarzewska, J.; Weiss, C. Excessive endoplasmic reticulum stress correlates with impaired mitochondrial dynamics, mitophagy and apoptosis, in liver and adipose tissue, but not in muscles in ems horses. Int. J. Mol. Sci. 2018, 19, 165. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Ye, Z.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Chitosan oligosaccharide ameliorated obesity by reducing endoplasmic reticulum stress in diet-induced obese rats. Food Funct. 2020, 11, 6285–6296. [Google Scholar] [CrossRef]
- Contreras, C.; Fondevila, M.F.; López, M. Hypothalamic GRP78, a new target against obesity? Adipocyte 2018, 7, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Takakuwa, J.; Nitika Knighton, L.; Truman, A. Oligomerization of Hsp70: Current perspectives on regulation and function. Front. Mol. Biosci. 2019, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Wieteska, L.; Shahidi, S.; Zhuravleva, A. Allosteric fine-tuning of the conformational equilibrium poises the chaperone BiP for post-translational regulation. eLife 2017, 6, e29430. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.C.; Larburu, N.; Durairaj, V.; Adams, C.J.; Ali, M.M.U. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat. Struct. Mol. Biol. 2019, 26, 1053–1062. [Google Scholar] [CrossRef]
- Jiang, H.; He, J.; Pu, S.; Tang, C.; Xu, G. Heat shock protein 70 is translocated to lipid droplets in rat adipocytes upon heat stimulation. Biochim. Biophys. Acta 2007, 1771, 66–74. [Google Scholar] [CrossRef]
- Girona, J.; Rodríguez-Borjabad, C.; Ibarretxe, D.; Vallvé, J.C.; Ferré, R.; Heras, M.; Rodríguez-Calvo, R.; Guaita-Esteruelas, S.; Martínez-Micaelo, N.; Plana, N.; et al. The circulating GRP78/BiP is a marker of metabolic diseases and atherosclerosis: Bringing endoplasmic reticulum stress into the clinical scenario. J. Clin. Med. 2019, 8, 1793. [Google Scholar] [CrossRef]
- Khadir, A.; Kavalakatt, S.; Abubaker, J.; Cherian, P.; Madhu, D.; Al-Khairi, I.; Abu-Farha, M.; Warsame, S.; Elkum, N.; Dehbi, M.; et al. Physical exercise alleviates ER stress in obese humans through reduction in the expression and release of GRP78 chaperone. Metabolism 2016, 65, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Girona, J.; Rodríguez-Borjabad, C.; Ibarretxe, D.; Vallvé, J.C.; Ferré, R.; Heras, M.; Rodríguez-Calvo, R.; Guaita-Esteruelas, S.; Martínez-Micaelo, N.; Plana, N.; et al. Circulating Grp78/Bip is increased in patients with obesity and related metabolic disorders and is associated with atherosclerosis. Atherosclerosis 2019, 287, e136. [Google Scholar] [CrossRef]
- Nourbakhsh, M.; Sharifi, R.; Heydari, N.; Nourbakhsh, M.; Ezzati-Mobasser, S.; Zarrinnahad, H. Circulating TRB3 and GRP78 levels in type 2 diabetes patients: Crosstalk between glucose homeostasis and endoplasmic reticulum stress. J. Endocrinol. Investig. 2022, 45, 649–655. [Google Scholar] [CrossRef]
- Zhu, G.; Ye, R.; Jung, D.Y.; Barron, E.; Friedline, R.H.; Benoit, V.M.; Hinton, D.R.; Kim, J.K.; Lee, A.S. GRP78 plays an essential role in adipogenesis and postnatal growth in mice. FASEB J. 2013, 27, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Camacho, A.; Segoviano-Ramírez, J.C.; Sánchez-Garcia, A.; de Jesus Herrera-de la Rosa, J.; García-Juarez, J.; Hernandez-Puente, C.A.; Calvo-Anguiano, G.; Maltos-Uro, S.R.; Olguin, A.; Gojon-Romanillos, G.; et al. Tyrphostin AG17 inhibits adipocyte differentiation in vivo and in vitro. Lipids Health Dis. 2018, 17, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, R.J.; Marcusson, E.G.; Koo, S.; Kang, X.; Kim, Y.; White, N.; Dean, N.M. Identification of novel PPARgamma target genes in primary human adipocytes. Gene 2006, 369, 90–99. [Google Scholar] [CrossRef]
- Nakachi, Y.; Yagi, K.; Nikaido, I.; Bono, H.; Tonouchi, M.; Schönbach, C.; Okazaki, Y. Identification of novel PPARgamma target genes by integrated analysis of ChIP-on-chip and microarray expression data during adipocyte differentiation. Biochem. Biophys. Res. Commun. 2008, 372, 362–366. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Qi, T.; Kong, Q.; Cheng, H.; Cao, X.; Li, Y.; Li, C.; Liu, L.; Ding, Z. HSPA12A is required for adipocyte differentiation and diet-induced obesity through a positive feedback regulation with PPARγ. Cell Death Differ. 2019, 26, 2253–2267. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, M.; Lang, H.; Zhou, Y.; Mi, M. Dihydromyricetin enhances glucose uptake by inhibition of MEK/ERK pathway and consequent down-regulation of phosphorylation of PPARγ in 3T3-L1 cells. J. Cell. Mol. Med. 2018, 22, 1247–1256. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Tan, D.; Pan, D.; Baker, M.R.; Liang, Z.; Wang, Z.; Lei, J.; Liu, S.; Hu, C.Y.; Li, Q.X. Dihydromyricetin imbues antiadipogenic effects on 3T3-L1 cells via direct interactions with 78-kDa glucose-regulated protein. J. Nutr. 2021, 151, 1717–1725. [Google Scholar] [CrossRef]
- Kammoun, H.L.; Chabanon, H.; Hainault, I.; Luquet, S.; Magnan, C.; Koike, T.; Ferré, P.; Foufelle, F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Investig. 2009, 119, 1201–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colgan, S.M.; Hashimi, A.A.; Austin, R.C. Endoplasmic reticulum stress and lipid dysregulation. Expert Rev. Mol. Med. 2011, 13, e4. [Google Scholar] [CrossRef]
- Lhoták, S.; Sood, S.; Brimble, E.; Carlisle, R.E.; Colgan, S.M.; Mazzetti, A.; Dickhout, J.G.; Ingram, A.J.; Austin, R.C. ER stress contributes to renal proximal tubule injury by increasing SREBP-2-mediated lipid accumulation and apoptotic cell death. Am. J. Physiol. Ren. Physiol. 2012, 303, F266–F278. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Jiang, X.; Dong, M.; Liu, X.; Shen, Q.; Huang, Y.; Zhang, H.; Ye, R.; Zhou, H.; Yan, C.; et al. Hepatokine pregnancy zone protein governs the diet-induced thermogenesis through activating brown adipose tissue. Adv. Sci. 2021, 8, e2101991. [Google Scholar] [CrossRef]
- Smolič, T.; Zorec, R.; Vardjan, N. Pathophysiology of Lipid Droplets in Neuroglia. Antioxidants 2021, 11, 22. [Google Scholar] [CrossRef]
- Wang, W.; Wei, S.; Li, L.; Su, X.; Du, C.; Li, F.; Geng, B.; Liu, P.; Xu, G. Proteomic analysis of murine testes lipid droplets. Sci. Rep. 2015, 5, 12070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, R.; Fan, L.L.; Huang, H.; Chen, Y.Q.; He, W.; Guo, S.; Li, J.J.; Jin, J.Y.; Du, R.; Yan, R.; et al. Increased reticulon 3 (RTN3) leads to obesity and hypertriglyceridemia by interacting with heat shock protein family A (Hsp70) member 5 (HSPA5). Circulation 2018, 138, 1828–1838. [Google Scholar] [CrossRef]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. Berbamine induced AMPK activation regulates mTOR/SREBP-1c axis and Nrf2/ARE pathway to allay lipid accumulation and oxidative stress in steatotic HepG2 cells. Eur. J. Pharmacol. 2020, 882, 173244. [Google Scholar] [CrossRef]
- Prasad, M.; Pawlak, K.J.; Burak, W.E.; Perry, E.E.; Marshall, B.; Whittal, R.M.; Bose, H.S. Mitochondrial metabolic regulation by GRP78. Sci. Adv. 2017, 3, e1602038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, X.; Fang, H.; Guo, F.; Li, F.; Chen, A.; Huang, S. Flavonoids as inducers of white adipose tissue browning and thermogenesis: Signalling pathways and molecular triggers. Nutr. Metab. 2019, 16, 47. [Google Scholar] [CrossRef]
- Zhang, Y.; Sowers, J.R.; Ren, J. Targeting autophagy in obesity: From pathophysiology to management. Nat. Rev. Endocrinol. 2018, 14, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Li, X. Relationship between mitophagy and browning of white adipose. Int. J. Endocrinol. Metab. 2019, 39, 87–90. [Google Scholar]
- Alan, P.; Vandevoorde, K.R.; Joshi, B.; Cardoen, B.; Gao, G.; Mohammadzadeh, Y.; Hamarneh, G.; Nabi, I.R. Gp78-mediated basal mitophagy promotes mitochondrial health and limits mitochondrial ROS production. bioRxiv 2021. [Google Scholar] [CrossRef]
- Leiva-Rodríguez, T.; Romeo-Guitart, D.; Herrando-Grabulosa, M.; Muñoz-Guardiola, P.; Polo, M.; Bañuls, C.; Petegnief, V.; Bosch, A.; Lizcano, J.M.; Apostolova, N.; et al. GRP78 overexpression triggers PINK1-IP3R-mediated neuroprotective mitophagy. Biomedicines 2021, 9, 1039. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, E.; Friedline, R.H.; Suk, S.; Jung, D.Y.; Dagdeviren, S.; Hu, X.; Inashima, K.; Noh, H.L.; Kwon, J.Y.; et al. Endoplasmic reticulum chaperone GRP78 regulates macrophage function and insulin resistance in diet-induced obesity. FASEB J. 2018, 32, 2292–2304. [Google Scholar] [CrossRef] [PubMed]
- Yung, H.W.; Charnock-Jones, D.S.; Burton, G.J. Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic reticulum stress modulates substrate specificity in a severity dependent manner. PLoS ONE 2011, 6, e17894. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Salinari, S.; Bertuzzi, A.; Iaconelli, A.; Mingrone, G. Metabolic surgery improves insulin resistance through the reduction of gut-secreted heat shock proteins. Commun. Biol. 2018, 1, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Lai, E.; Teodoro, T.; Volchuk, A. GRP78, but not protein-disulfide isomerase, partially reverses hyperglycemia-induced inhibition of insulin synthesis and secretion in pancreatic β-Cells. J. Biol. Chem. 2009, 284, 5289–5298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.T.; Zhu, G.; Pfaffenbach, K.; Kanel, G.; Stiles, B.; Lee, A.S. GRP78 as a regulator of liver steatosis and cancer progression mediated by loss of the tumor suppressor PTEN. Oncogene 2014, 33, 4997–5005. [Google Scholar] [CrossRef] [Green Version]
- Gentile, C.L.; Frye, M.; Pagliassotti, M.J. Endoplasmic reticulum stress and the unfolded protein response in nonalcoholic fatty liver disease. Antioxid. Redox Signal. 2011, 15, 505–521. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.; Szabo, G. Alcoholic and Non Alcoholic Fatty Liver Disease Pathogenesis of NAFLD and NASH; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Teodoro-Morrison, T.; Schuiki, I.; Zhang, L.; Belsham, D.D.; Volchuk, A. GRP78 overproduction in pancreatic beta cells protects against high-fat-diet-induced diabetes in mice. Diabetologia 2013, 56, 1057–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Tsung, K.; Attenello, F.J. Characterizing cell stress and GRP78 in glioma to enhance tumor treatment. Front. Oncol. 2020, 10, 608911. [Google Scholar] [CrossRef]
- Gray, M.J.; Mhawech-Fauceglia, P.; Yoo, E.; Yang, W.; Wu, E.; Lee, A.S.; Lin, Y.G. AKT inhibition mitigates GRP78 (glucose-regulated protein) expression and contribution to chemoresistance in endometrial cancers. Int. J. Cancer 2013, 133, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Li, E.; Chen, L.; Zhang, Y.; Wei, F.; Liu, J.; Deng, H.; Wang, Y. The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature 2015, 524, 243–246. [Google Scholar] [CrossRef]
- Qiao, Y.; Dsouza, C.; Matthews, A.A.; Jin, Y.; He, W.; Bao, J.; Jiang, F.; Chandna, R.; Ge, R.; Fu, L. Discovery of small molecules targeting GRP78 for antiangiogenic and anticancer therapy. Eur. J. Med. Chem. 2020, 193, 112228. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Wang, W.; Golden, E.B.; Thomas, S.; Sivakumar, W.; Hofman, F.M.; Louie, S.G.; Schönthal, A.H. Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett. 2011, 302, 100–108. [Google Scholar] [CrossRef]
- Gurusinghe, K.R.D.S.N.S.; Mishra, A.; Mishra, S. Glucose-regulated protein 78 substrate-binding domain alters its conformation upon EGCG inhibitor binding to nucleotide-binding domain: Molecular dynamics studies. Sci. Rep. 2018, 8, 5487. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Lamb, H.K.; Brady, C.; Lefkove, B.; Bonner, M.Y.; Thompson, P.; Lovat, P.E.; Arbiser, J.L.; Hawkins, A.R.; Redfern, C.P. Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78. Br. J. Cancer 2013, 109, 433–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chio, C.C.; Chen, K.Y.; Chang, C.K.; Chuang, J.Y.; Liu, C.C.; Liu, S.H.; Chen, R.M. Improved effects of honokiol on temozolomide-induced autophagy and apoptosis of drug-sensitive and -tolerant glioma cells. BMC Cancer 2018, 18, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chio, C.C.; Tai, Y.T.; Mohanraj, M.; Liu, S.H.; Yang, S.T.; Chen, R.M. Honokiol enhances temozolomide-induced apoptotic insults to malignant glioma cells via an intrinsic mitochondrion-dependent pathway. Phytomedicine 2018, 49, 41–51. [Google Scholar] [CrossRef]
- Booth, L.; Cazanave, S.C.; Hamed, H.A.; Yacoub, A.; Ogretmen, B.; Chen, C.S.; Grant, S.; Dent, P. OSU-03012 suppresses GRP78/BiP expression that causes PERK-dependent increases in tumor cell killing. Cancer Biol. Ther. 2012, 13, 224–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kardosh, A.; Golden, E.B.; Pyrko, P.; Uddin, J.; Hofman, F.M.; Chen, T.C.; Louie, S.G.; Petasis, N.A.; Schönthal, A.H. Aggravated endoplasmic reticulum stress as a basis for enhanced glioblastoma cell killing by bortezomib in combination with celecoxib or its non-coxib analogue, 2,5-dimethyl-celecoxib. Cancer Res. 2008, 68, 843–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, C.O.D.; Masini, M.; Futuro, D.; Caetano, R.; Gattass, C.R.; Quirico-Santos, T. Anaplastic oligodendroglioma responding favorably to intranasal delivery of perillyl alcohol: A case report and literature review. Surg. Neurol. 2006, 66, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Wang, W.; Jhaveri, N.; Torres, S.; Tseng, J.; Leong, M.N.; Lee, D.J.; Goldkorn, A.; Xu, T.; Petasis, N.A.; et al. Perillyl alcohol for the treatment of temozolomide-resistant gliomas. Mol. Cancer Ther. 2012, 11, 2462–2472. [Google Scholar] [CrossRef] [Green Version]
- Marín-Ramos, N.I.; Pérez-Hernández, M.; Tam, A.; Swenson, S.D.; Cho, H.Y.; Thein, T.Z.; Hofman, F.M.; Chen, T.C. Inhibition of motility by NEO100 through the calpain-1/RhoA pathway. J. Neurosurg. 2019, 133, 1020–1031. [Google Scholar] [CrossRef]
- Cerezo, M.; Lehraiki, A.; Millet, A.; Rouaud, F.; Plaisant, M.; Jaune, E.; Botton, T.; Ronco, C.; Abbe, P.; Amdouni, H.; et al. Compounds triggering ER stress exert anti-melanoma effects and overcome BRAF inhibitor resistance. Cancer Cell 2016, 29, 805–819. [Google Scholar] [CrossRef] [Green Version]
- Pinkham, K.; Park, D.J.; Hashemiaghdam, A.; Kirov, A.B.; Adam, I.; Rosiak, K.; da Hora, C.C.; Teng, J.; Cheah, P.S.; Carvalho, L.; et al. Stearoyl CoA desaturase is essential for regulation of endoplasmic reticulum homeostasis and tumor growth in glioblastoma cancer stem cells. Stem Cell Rep. 2019, 12, 712–727. [Google Scholar] [CrossRef] [Green Version]
- Bakewell, S.J.; Rangel, D.F.; Ha, D.P.; Sethuraman, J.; Crouse, R.; Hadley, E.; Costich, T.L.; Zhou, X.; Nichols, P.; Lee, A.S. Suppression of stress induction of the 78-kilodalton glucose regulated protein (GRP78) in cancer by IT-139, an anti-tumor ruthenium small molecule inhibitor. Oncotarget 2018, 9, 29698–29714. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, A.; Sarcar, B.; Kahali, S.; Shan, Y.; Chinnaiyan, P. Targeting the unfolded protein response in glioblastoma cells with the fusion protein EGF-SubA. PLoS ONE 2012, 7, e52265. [Google Scholar] [CrossRef] [Green Version]
- Dadey, D.Y.A.; Kapoor, V.; Hoye, K.; Khudanyan, A.; Collins, A.; Thotala, D.; Hallahan, D.E. Antibody targeting GRP78 enhances the efficacy of radiation therapy in human glioblastoma and non-small cell lung cancer cell lines and tumor models. Clin. Cancer Res. 2017, 23, 2556–2564. [Google Scholar] [CrossRef]
- Kia, A.; Przystal, J.M.; Nianiaris, N.; Mazarakis, N.D.; Mintz, P.J.; Hajitou, A. Dual systemic tumor targeting with ligand-directed phage and Grp78 promoter induces tumor regression. Mol. Cancer Ther. 2012, 11, 2566–2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przystal, J.M.; Waramit, S.; Pranjol, M.Z.I.; Yan, W.; Chu, G.; Chongchai, A.; Samarth, G.; Olaciregui, N.G.; Tabatabai, G.; Carcaboso, A.M.; et al. Efficacy of systemic temozolomide-activated phage-targeted gene therapy in human glioblastoma. EMBO Mol. Med. 2019, 11, e8492. [Google Scholar] [CrossRef]
- Kaliberov, S.A.; Kaliberova, L.N.; Yan, H.; Kapoor, V.; Hallahan, D.E. Retargeted adenoviruses for radiation-guided gene delivery. Cancer Gene Ther. 2016, 23, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Hughes, S.J.; Antoshchenko, T.; Chen, Y.; Lu, H.; Pizarro, J.C.; Park, H.W. Probing the ATP site of GRP78 with nucleotide triphosphate analogs. PLoS ONE 2016, 11, e0158256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.E.; Lai, Y.H.; Yang, K.C.; Lin, S.J.; Chen, C.L.; Tsai, P.S. Counteracting cisplatin-induced testicular damages by natural polyphenol constituent honokiol. Antioxidants 2020, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nie, Y.; Li, Y.; Hou, Y.; Zhao, W.; Deng, J.; Wang, P.G.; Bai, G. Identification of target proteins of mangiferin in mice with acute lung injury using functionalized magnetic microspheres based on click chemistry. J. Agric. Food Chem. 2015, 63, 10013–10021. [Google Scholar] [CrossRef]
- Bailly, C.; Waring, M.J. Pharmacological effectors of GRP78 chaperone in cancers. Biochem. Pharmacol. 2019, 163, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Devi, A.; Mishra, S. Molecular docking and molecular dynamics studies reveal structural basis of inhibition and selectivity of inhibitors EGCG and OSU-03012 toward glucose regulated protein-78 (GRP78) overexpressed in glioblastoma. J. Mol. Model. 2015, 21, 272. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Omoruyi, S.I.; Hiss, D.C.; Ekpo, O.E. GRP78/BIP/HSPA5 as a therapeutic target in models of parkinson’s disease: A mini review. Adv. Pharmacol. Sci. 2019, 2019, 2706783. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, A.J.; Chapman, E. Function, therapeutic potential, and inhibition of Hsp70 chaperones. J. Med. Chem. 2021, 64, 7060–7082. [Google Scholar] [CrossRef]
- Ermakova, S.P.; Kang, B.S.; Choi, B.Y.; Choi, H.S.; Schuster, T.F.; Ma, W.Y.; Bode, A.M.; Dong, Z. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006, 66, 9260–9269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y. Screening and Mechanism of Drugs for Intervention of Tumor Secretory GRP78. Master’s Thesis, Shanxi University, Taiyuan, China, 2019. [Google Scholar]
- Ambrose, A.J.; Zerio, C.J.; Sivinski, J.; Schmidlin, C.J.; Shi, T.; Ross, A.B.; Widrick, K.J.; Johnson, S.M.; Zhang, D.D.; Chapman, E. A high throughput substrate binding assay reveals hexachlorophene as an inhibitor of the ER-resident HSP70 chaperone GRP78. Bioorg. Med. Chem. Lett. 2019, 29, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Zoni, V.; Khaddaj, R.; Campomanes, P.; Thiam, R.; Schneiter, R.; Vanni, S. Lipid droplet biogenesis is driven by liquid-liquid phase separation. eLife 2020. [Google Scholar] [CrossRef]
- Shao, H.; Li, X.; Moses, M.A.; Gilbert, L.A.; Kalyanaraman, C.; Young, Z.T.; Chernova, M.; Journey, S.N.; Weissman, J.S.; Hann, B.; et al. Exploration of benzothiazole rhodacyanines as allosteric inhibitors of protein-protein interactions with heat shock protein 70 (Hsp70). J. Med. Chem. 2018, 61, 6163–6177. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, D.; Yang, Y.; Nong, A.; Tang, Z.; Li, Q.X. GRP78 Activity Moderation as a Therapeutic Treatment against Obesity. Int. J. Environ. Res. Public Health 2022, 19, 15965. https://doi.org/10.3390/ijerph192315965
Pan D, Yang Y, Nong A, Tang Z, Li QX. GRP78 Activity Moderation as a Therapeutic Treatment against Obesity. International Journal of Environmental Research and Public Health. 2022; 19(23):15965. https://doi.org/10.3390/ijerph192315965
Chicago/Turabian StylePan, Dongjin, Yunzhu Yang, Aihua Nong, Zhenzhou Tang, and Qing X. Li. 2022. "GRP78 Activity Moderation as a Therapeutic Treatment against Obesity" International Journal of Environmental Research and Public Health 19, no. 23: 15965. https://doi.org/10.3390/ijerph192315965
APA StylePan, D., Yang, Y., Nong, A., Tang, Z., & Li, Q. X. (2022). GRP78 Activity Moderation as a Therapeutic Treatment against Obesity. International Journal of Environmental Research and Public Health, 19(23), 15965. https://doi.org/10.3390/ijerph192315965






